CHAPTERS 89 NAMES FORMULAS AND OXIDATION NUMBERS Topics

CHAPTERS 8&9: NAMES, FORMULAS, AND OXIDATION NUMBERS

Topics in this unit: • Binary Ionic Compounds of Metals with Fixed Charges • Formulas of Ionic Compounds: The criss-cross method • Binary Compounds of Cations with Variable Charges: Stock System • Polyatomics • Names and Formulas of Covalent (Nonmetal) Compounds • Acids • Hydrates • Oxidation Numbers

Binary Compounds of Metals with Fixed Charges • Name the cation (metal) first, then the anion (nonmetal) • Use the cation’s name directly from the periodic table (eg. Ca+2 is Calcium) • Name the anion using the root of the element’s name plus the suffix “ide” (eg. S-2 would be Sulfide) • Examples: • Ca. S would be Calcium Sulfide • Na 2 O would be Sodium Oxide • Potassium Chloride would be KCl

Practice • Name the Following Compounds: • Ba. Cl 2 • Zn. F 2 • Ag 2 S • Al. Br 3

Practice • Name the Following Compounds: • Ba. Cl 2 = barium chloride • Zn. F 2 = zinc fluoride • Ag 2 S = silver sulfide • Al. Br 3 = aluminum bromide
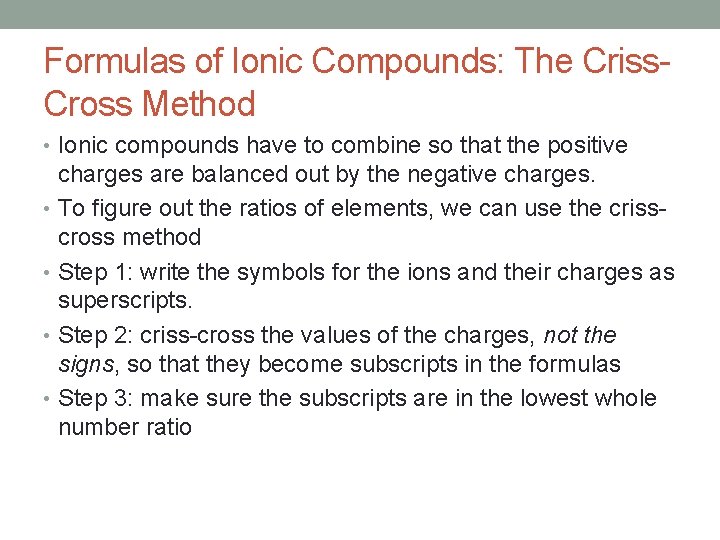
Formulas of Ionic Compounds: The Criss. Cross Method • Ionic compounds have to combine so that the positive charges are balanced out by the negative charges. • To figure out the ratios of elements, we can use the crisscross method • Step 1: write the symbols for the ions and their charges as superscripts. • Step 2: criss-cross the values of the charges, not the signs, so that they become subscripts in the formulas • Step 3: make sure the subscripts are in the lowest whole number ratio
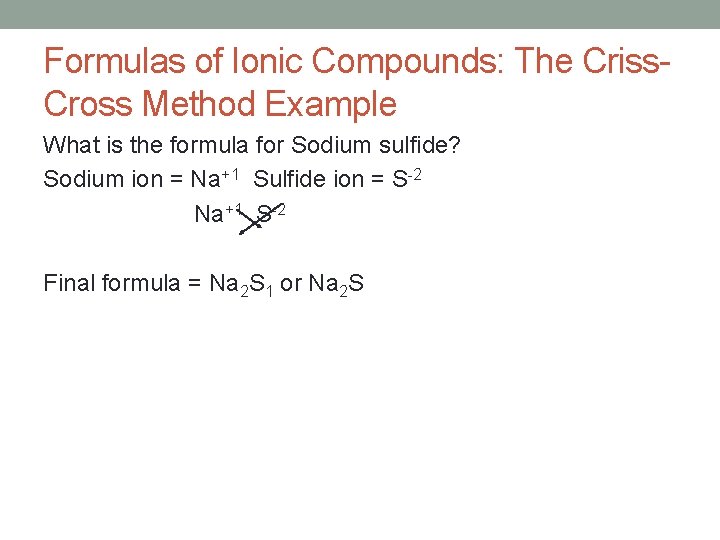
Formulas of Ionic Compounds: The Criss. Cross Method Example What is the formula for Sodium sulfide? Sodium ion = Na+1 Sulfide ion = S-2 Na+1 S-2 Final formula = Na 2 S 1 or Na 2 S

Practice Write the formulas of the following ionic compounds: • Magnesium chloride • Lithium Sulfide • Nickel Oxide • Tungsten Nitride • Sodium Fluoride

Practice Write the formulas of the following ionic compounds: • Magnesium Chloride = Mg. Cl 2 • Lithium Sulfide = Li 2 S • Nickel Oxide = Ni. O • Tungsten Nitride = WN 2 • Sodium Fluoride = Na. F
Binary Compounds of Cations with Variable Charges: The Stock System • Named for German chemist Alfred Stock • Cations (metals) involved have multiple charges • Anion has fixed charge • Uses roman numerals in parentheses to show the cation’s charge • Example: Fe. Cl 2 is iron(II) chloride; Fe. Cl 3 is iron (III) chloride
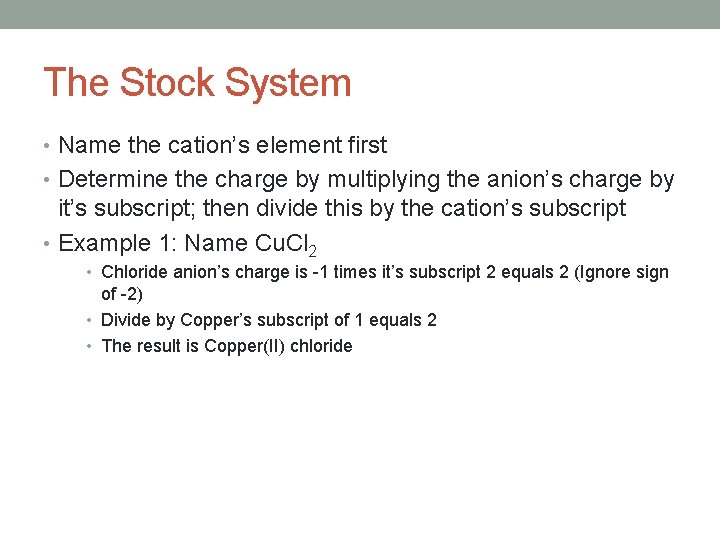
The Stock System • Name the cation’s element first • Determine the charge by multiplying the anion’s charge by it’s subscript; then divide this by the cation’s subscript • Example 1: Name Cu. Cl 2 • Chloride anion’s charge is -1 times it’s subscript 2 equals 2 (Ignore sign of -2) • Divide by Copper’s subscript of 1 equals 2 • The result is Copper(II) chloride
The Stock System • Example 2: Name Fe 2 O 3 • Oxide anion’s charge is -2 times it’s subscript 3 equals -6 • Divide by Iron’s subscript of 2 equals 3 • The result is iron(III) oxide • Example 3: Write the formula for manganese(IV) oxide • The cation’s charge is given from the formula +4 • Oxide’s charge is -2 • Using the criss-cross method we first get Mn 2 O 4, but this is not in the lowest ratio. So we divide each subscript by 2. • The result is Mn. O 2
Polyatomics • Polyatomic ions can bond with metals that have fixed or variable charges. • When more than one polyatomic ion is required, parentheses must be used around the polyatomic • Example 1: Fe(NO 3)2 • Decide if the cation has a variable charge (iron does so you must determine the roman numeral) • NO 3 has a -1 charge so iron must be +2 • The name is iron(II) nitrate
Polyatomics • Example 2: Fe(OH)3 • Determine the charge of iron: Hydroxide is -1 so iron must be +3 • The compound is iron(III) hydroxide • Example 3: Aluminum Phosphate • Aluminum is a fixed-charge of +3 • Phosphate ion is PO 4 -3 • The formula is Al. PO 4 • Example 4: Ammonium Sulfate • Ammonium is a polyatomic cation NH 4+ • Sulfate is SO 4 -2 • The formula is (NH 4)2 SO 4
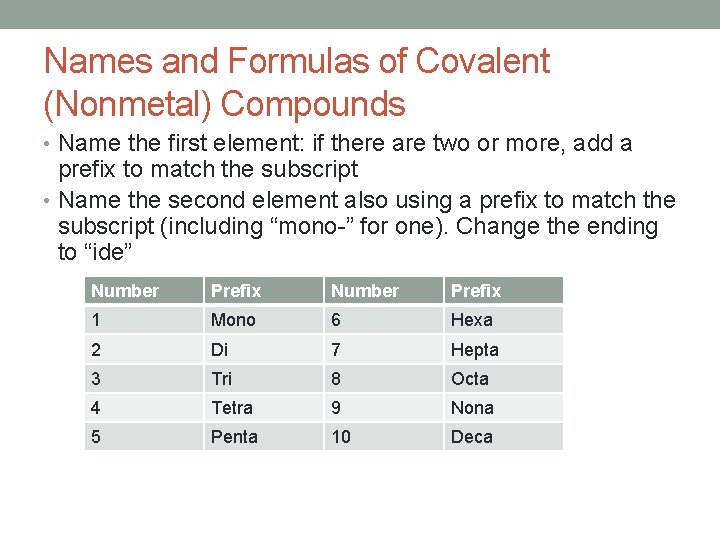
Names and Formulas of Covalent (Nonmetal) Compounds • Name the first element: if there are two or more, add a prefix to match the subscript • Name the second element also using a prefix to match the subscript (including “mono-” for one). Change the ending to “ide” Number Prefix 1 Mono 6 Hexa 2 Di 7 Hepta 3 Tri 8 Octa 4 Tetra 9 Nona 5 Penta 10 Deca
Names of Nonmetal Compounds: Examples • Example 1: N 2 O • Subscript for 2 is “di-” so it is dinitrogen • Subscript for O is 1 so the name is dinitrogen monoxide • Example 2: N 2 O 5 • Subscript for nitrogen is 2 so it is dinitrogen • Subscript is 5 for oxygen or pentaoxide • The name is dinitrogen pentaoxide (pentoxide is also acceptable) • Example 3: What is the formula for triphosphorus hexabromide? • Phosphorus is P and tri means there’s 3 P atoms • Bromine is Br and hexa means there’s 6 Br atoms • The formula is P 3 Br 6 (notice we don’t reduce the subscripts)

Practice: Covalent Compounds Name the following covalent compounds: • CO • P 2 O 4 • PCl 3 Write formulas for the following covalent compounds: • Antimony tribromide • Xenon hexafluoride • Diphosphorus decasulfide
Practice: Covalent Compounds Name the following covalent compounds: • CO = carbon monoxide • N 2 O 4 = dinitrogen tetroxide • PCl 3 = phosphorus trichloride Write formulas for the following covalent compounds: • Antimony pentabromide= Sb. Br 5 • Xenon hexafluoride = Xe. F 6 • Diphosphorus decasulfide = P 2 S 10

Acids • Acids are a special type of ionic compound that forms when certain polar molecules dissolve in water • The formulas typically have an H in front • Ex: HCl, H 3 PO 4 • The cation is always H+1, so the criss-cross rule still applies for writing formulas • Ex: What acid forms when hydrogen bonds with the sulfate ion? • Hydrogen ion = H+1 sulfate = SO 42 • The formula is H 2 SO 4 • There are two types of acids, binary and ternary (oxyacids)

Binary Acids • Binary acids are hydrogen with a non-metal (Examples: HCl, HF, HBr, HI) • “hydro-” STEM “-ic” is used (STEM comes from the anion) • The word acid is added at the end • Examples: • HCl is hydrochloric acid (STEM is chlor) • HF is hydrofluoric acid • HBr is hydrobromic acid
Oxy (Ternary) Acids • Contains hydrogen and a polyatomic ion • Change the “ate” ending to “ic” and the “ite” ending to “ous” and add the word acid • Polyatomic ion that ends in “ate” • STEM –ic acid • Example HCl. O 3 = chloric acid • H 2 SO 4 = sulfuric acid • Polyatomic ion that ends in “ite” • STEM –ous acid • Example HCl. O 2 = chlorous acid • H 3 PO 3 = phosphorous acid

Oxy (Ternary) Acids • Polyatomic ion with prefix “hypo” • Hypo- STEM –ous acid • Example HCl. O hypochlorous acid • Polyatomic ion with prefix “per” • Per- STEM –ic acid • Example HCl. O 4 perchloric acid
Hydrates • Ionic compounds that have water in them . • General Formula AB x. H 2 O • Name the compound (AB) and identify the number of water molecules present (x equals the coefficient) with a prefix • Examples: • Cu. SO 4. 5 H 2 O is copper(II) sulfate pentahydrate • Mg. SO 4. 9 H 2 O is magnesium sulfate nonahydrate
Oxidation Numbers: The Rules • All free uncombined elements have an oxidation number of zero (In diatomic elements like F 2, each fluorine’s oxidation number is 0) • Hydrogen has an oxidation number of +1 • Exception: when combined with metals, H’s oxidation number is -1 • Oxygen has an oxidation number of -2 • Exception #1: In peroxide (O 22 -) the oxidation number is -1 • Exception # 2: When oxygen bonds with fluorine (OF 2) it is +2 because fluorine is more electronegative

Oxidation Numbers: The Rules • The alkali metals (group 1 A) have an oxidation state of +1 • The alkaline earth metals (group 2 A) have an oxidation state of +2 • The oxidation number of Aluminum (Al) is always +3 • The halogens (group 7 A) have an oxidation state of − 1 (except when they are bonded to oxygen or with another halogen)
Oxidation Numbers: The Rules • In a neutral molecule, the sum of the oxidation numbers of all of the atoms must be zero. • Ex. In H 2 O, each hydrogen is +1 and the oxygen is -2. So, (2 x +1) + (-2) = 0. • In a polyatomic ion, the sum of oxidation numbers of all the elements in the ion must be equal the net charge of the ion. • Ex. In the polyatomic ion known as hydroxide (OH-), the oxygen is -2 and the hydrogen is +1. So, (-2) + (+1) = -1, the same as the charge on the hydroxide ion (OH-)
Oxidation Number Examples • What is the oxidation number of Na in Na 2 O? • O is -2; the two Na must be +1 each • What is the oxidation number of Cl in Cl. O¯? • The O is -2, but since a -1 must be left over, then the Cl is +1 • What is the oxidation number for each element in KMn. O 4? • K = +1; O = -2 Mn = +7. There are 4 oxygens for a total of -8, K is +1, so Mn = +7.
- Slides: 27