Chapters 7 9 Chemical Composition Intrachemical Forces Intra





- Slides: 37

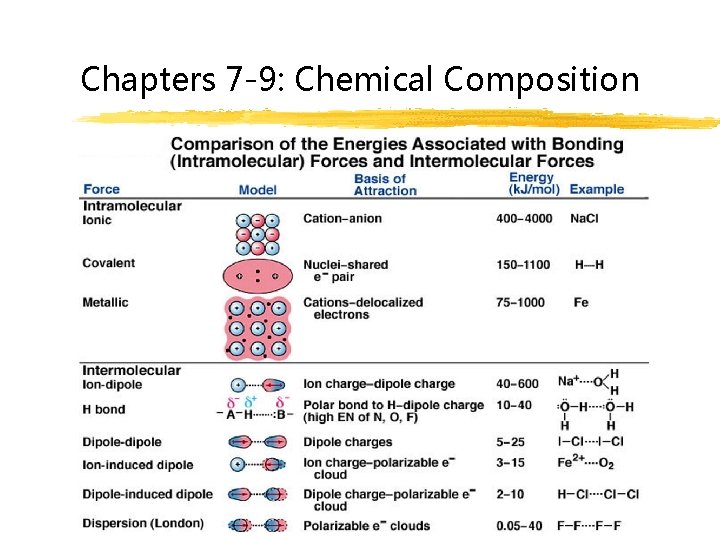
Chapters 7 -9: Chemical Composition

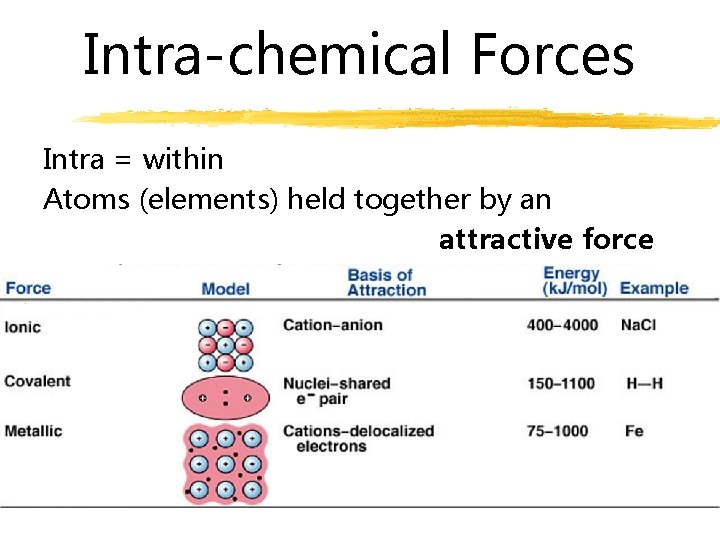
Intra-chemical Forces Intra = within Atoms (elements) held together by an attractive force

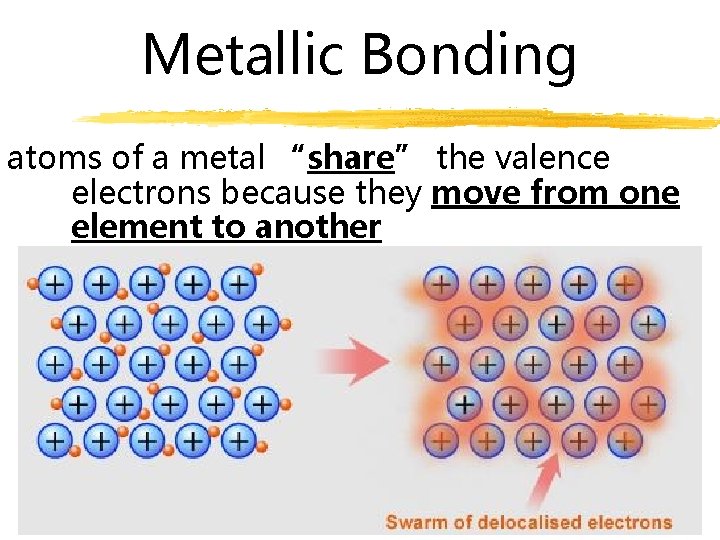
Metallic Bonding atoms of a metal “share” the valence electrons because they move from one element to another

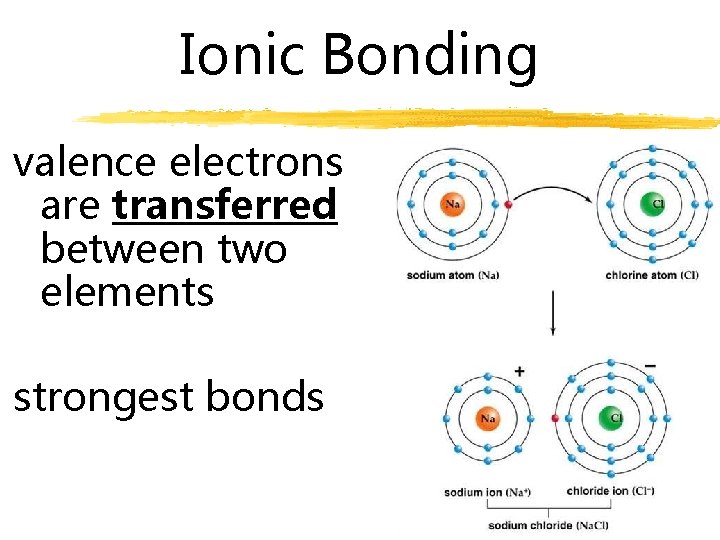
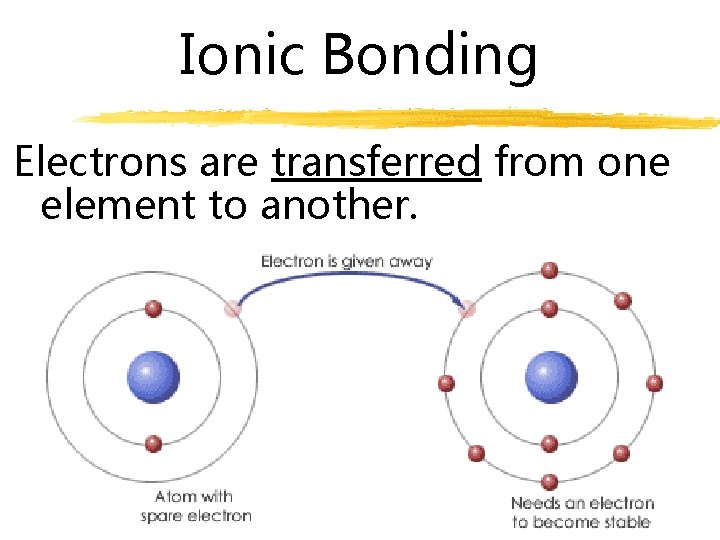
Ionic Bonding valence electrons are transferred between two elements strongest bonds

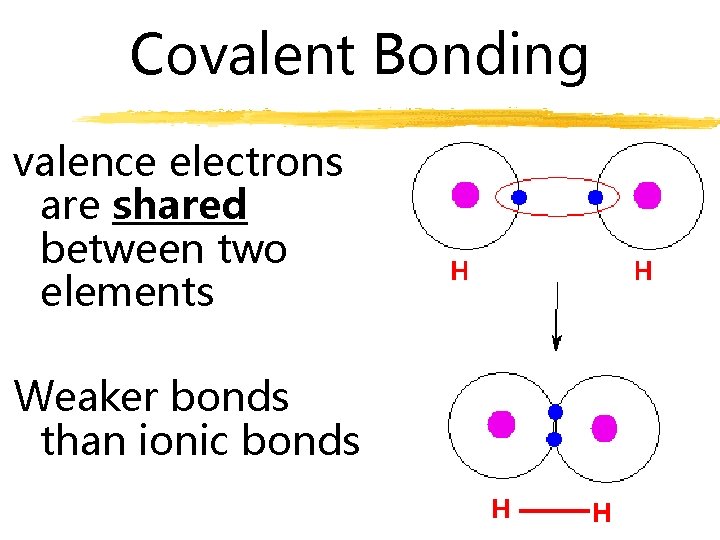
Covalent Bonding valence electrons are shared between two elements Weaker bonds than ionic bonds

Ionic Bonding Electrons are transferred from one element to another.

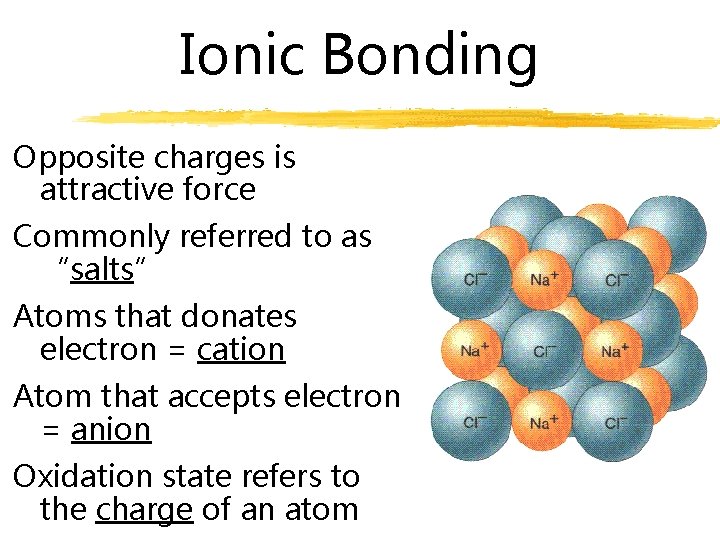
Ionic Bonding Opposite charges is attractive force Commonly referred to as “salts” Atoms that donates electron = cation Atom that accepts electron = anion Oxidation state refers to the charge of an atom

Lewis Dot Formulas Octet Rule: every element wants 8 electrons in its outer shell. a) potassium + chlorine → potassium chloride a) magnesium + fluorine → magnesium fluoride

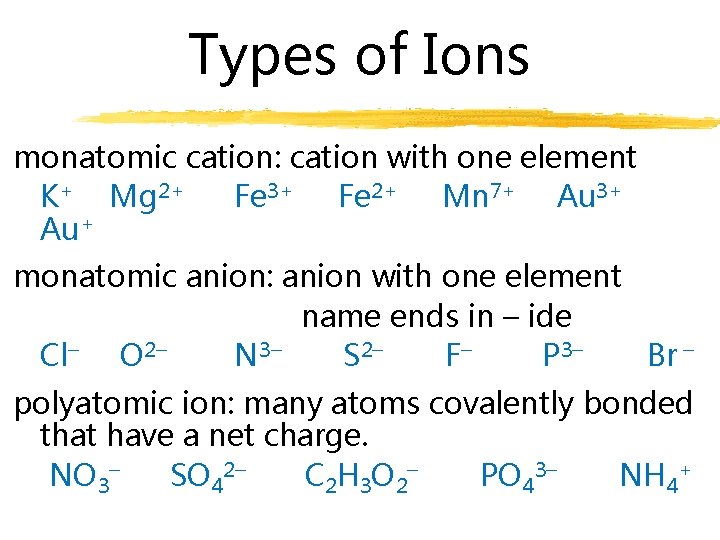
Types of Ions monatomic cation: cation with one element K+ Mg 2+ Fe 3+ Fe 2+ Mn 7+ Au 3+ Au+ monatomic anion: anion with one element name ends in – ide Cl– O 2– N 3– S 2– F– P 3– Br – polyatomic ion: many atoms covalently bonded that have a net charge. NO 3– SO 42– C 2 H 3 O 2– PO 43– NH 4+

Writing Ionic Chemical Formulas 1. 2. 3. 4. 5. 6. Composition number of elements Writing chemical formulas (from the names) Recognize the (+) and (–) ions Write the symbols of the elements with their charge A Roman numeral will tell you what the charge is on the cation if there is more than one possibility 7. Adjust the number of each ion (with subscripts) as needed so the positive charge is equal and opposite the negative charge. 8. If the ions are polyatomic and there is more than one, the ion is enclosed with parentheses with a subscript on the outside.

Writing Ionic Chemical Formulas 1. sodium chloride 2. calcium sulfide 3. calcium sulfate 4. barium phosphate

Naming Ionic Compounds a) Consists of two words: b) Name the cation c) Name the anion d) If the cation has more than one possible charge, a Roman Numeral is used to show the charge. e) All transition metals need roman numerals except: i. Zinc always has a charge of +2 ii. Silver always has a charge of +1

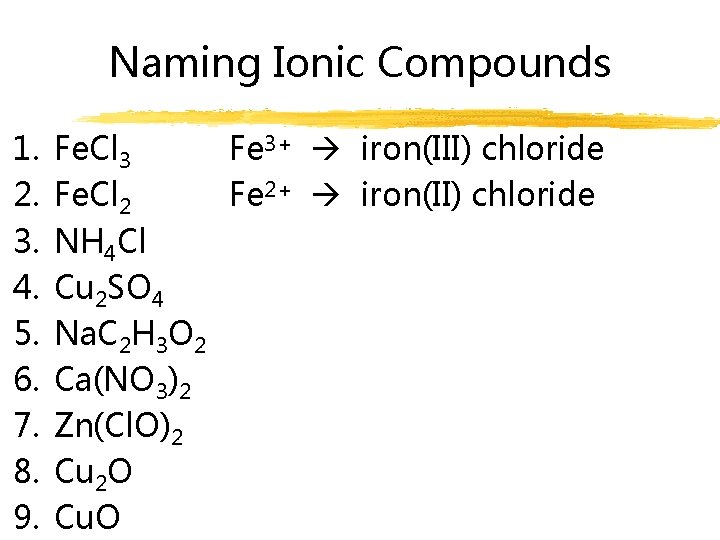
Naming Ionic Compounds 1. 2. 3. 4. 5. 6. 7. 8. 9. Fe. Cl 3 Fe 3+ iron(III) chloride Fe. Cl 2 Fe 2+ iron(II) chloride NH 4 Cl Cu 2 SO 4 Na. C 2 H 3 O 2 Ca(NO 3)2 Zn(Cl. O)2 Cu 2 O Cu. O

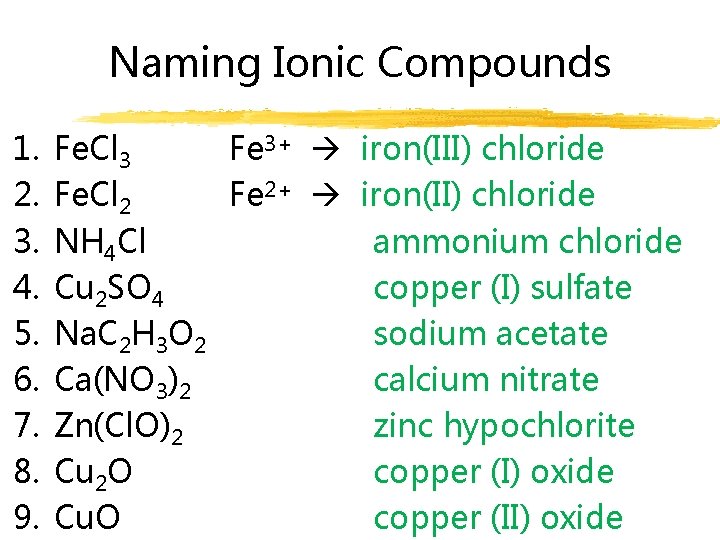
Naming Ionic Compounds 1. 2. 3. 4. 5. 6. 7. 8. 9. Fe. Cl 3 Fe 3+ iron(III) chloride Fe. Cl 2 Fe 2+ iron(II) chloride NH 4 Cl ammonium chloride Cu 2 SO 4 copper (I) sulfate Na. C 2 H 3 O 2 sodium acetate Ca(NO 3)2 calcium nitrate Zn(Cl. O)2 zinc hypochlorite Cu 2 O copper (I) oxide Cu. O copper (II) oxide

Covalent Bonding A. Valence electrons are shared between two elements B. Weaker than ionic bonding

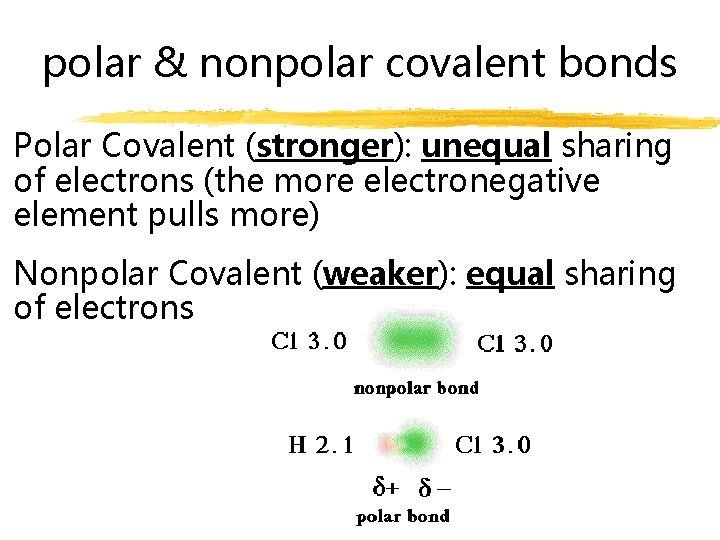
polar & nonpolar covalent bonds Polar Covalent (stronger): unequal sharing of electrons (the more electronegative element pulls more) Nonpolar Covalent (weaker): equal sharing of electrons

Writing Formulas for Covalent Compounds 1. carbon dioxide 2. carbon monoxide 3. dinitrogen monoxide 4. carbon tetrafluoride 5. triphosphorus pentachloride

Naming Formulas for Covalent Compounds Binary covalent compounds (2 elements) Formulas with two nonmetals Rules: i. First word: 1. prefix indicating the number of atoms for the first element (if there is more than one) 2. name of first element ii. Second word: 1. prefix for the number of atoms of the second element (prefixes on supplement notes sheet) 2. name of second element 3. suffix –ide

Naming Formulas for Covalent Compounds 1. 2. 3. 4. 5. 6. 7. 8. 9. NO NO 2 CBr 4 P 4 O 10 BF 3 Si. I 5 H 2 O S 6 Cl 8 Se 7 O 9

Naming Formulas for Covalent Compounds 1. 2. 3. 4. 5. 6. 7. 8. 9. NO NO 2 CBr 4 P 4 O 10 BF 3 Si. I 5 H 2 O S 6 Cl 8 Se 7 O 9 nitrogen monoxide nitrogen dioxide carbon tetrabromide tetraphosphorus decoxide boron trifluoride silicon pentaiodide dihydrogen monoxide hexasulfur octochloride heptaselenium nonoxide

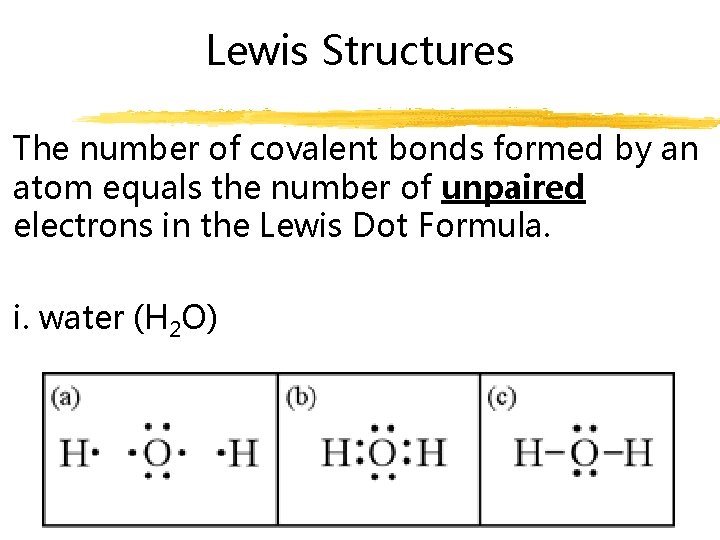
Lewis Structures The number of covalent bonds formed by an atom equals the number of unpaired electrons in the Lewis Dot Formula. i. water (H 2 O)

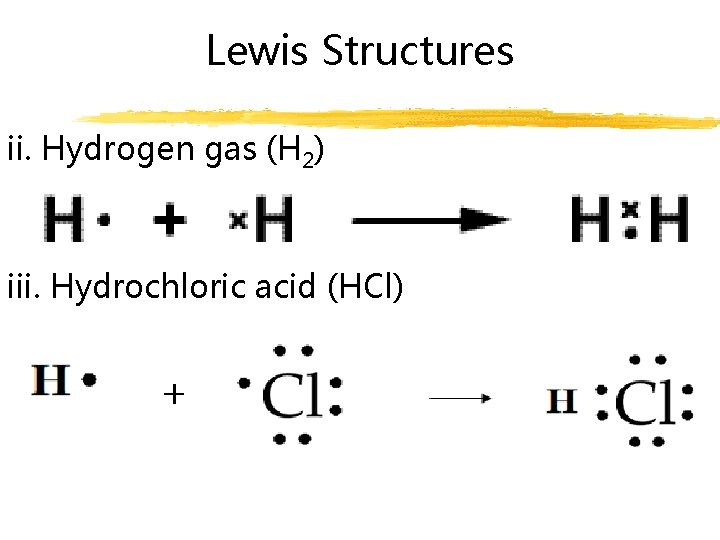
Lewis Structures ii. Hydrogen gas (H 2) iii. Hydrochloric acid (HCl)

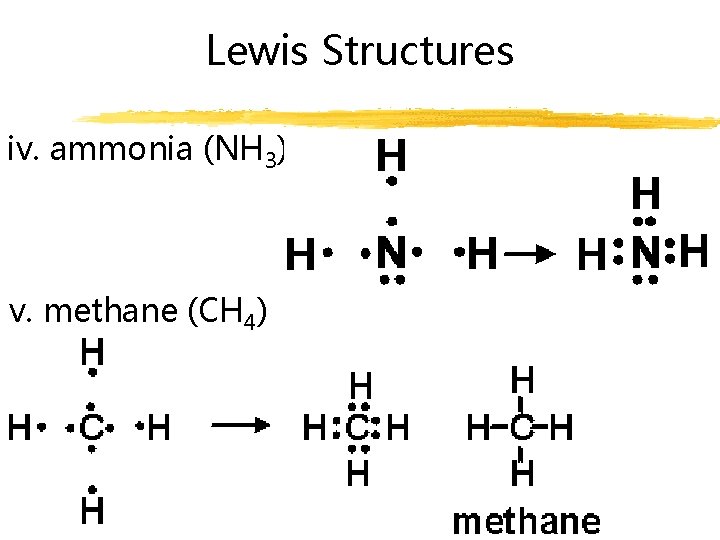
Lewis Structures iv. ammonia (NH 3) v. methane (CH 4)

Multiple Bonds i. double bonds: two pairs of electrons shared O 2 ii. triple bonds: three pairs of electrons shared N 2

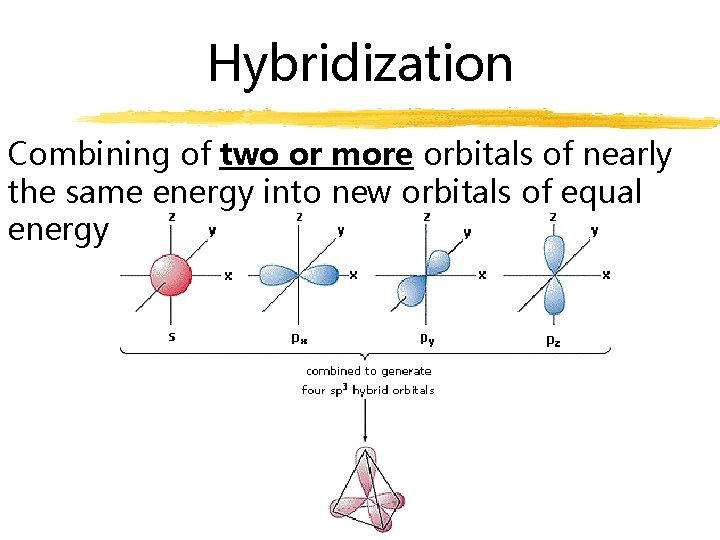
Hybridization Combining of two or more orbitals of nearly the same energy into new orbitals of equal energy

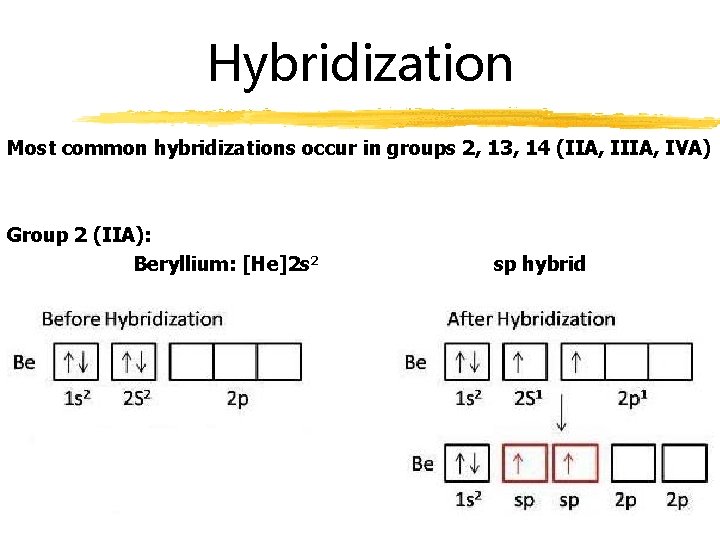
Hybridization Most common hybridizations occur in groups 2, 13, 14 (IIA, IVA) Group 2 (IIA): Beryllium: [He]2 s 2 sp hybrid

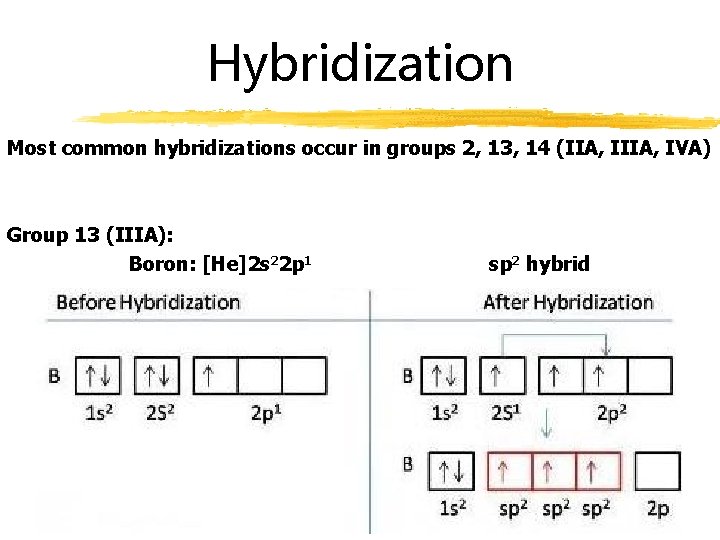
Hybridization Most common hybridizations occur in groups 2, 13, 14 (IIA, IVA) Group 13 (IIIA): Boron: [He]2 s 22 p 1 sp 2 hybrid

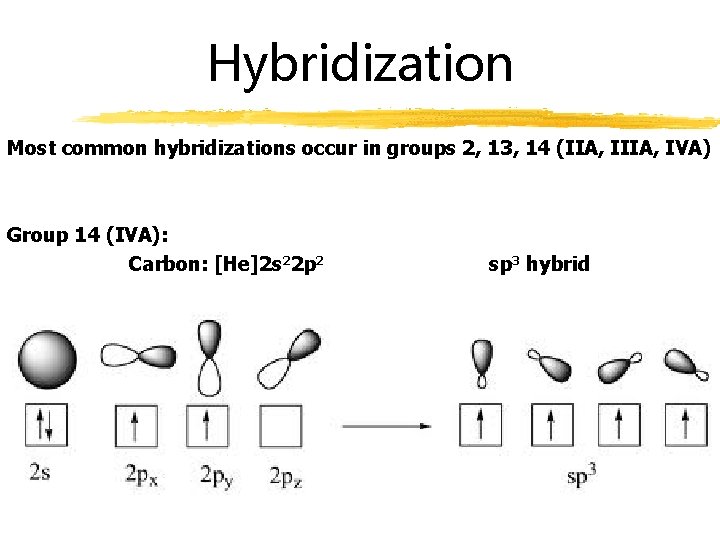
Hybridization Most common hybridizations occur in groups 2, 13, 14 (IIA, IVA) Group 14 (IVA): Carbon: [He]2 s 22 p 2 sp 3 hybrid

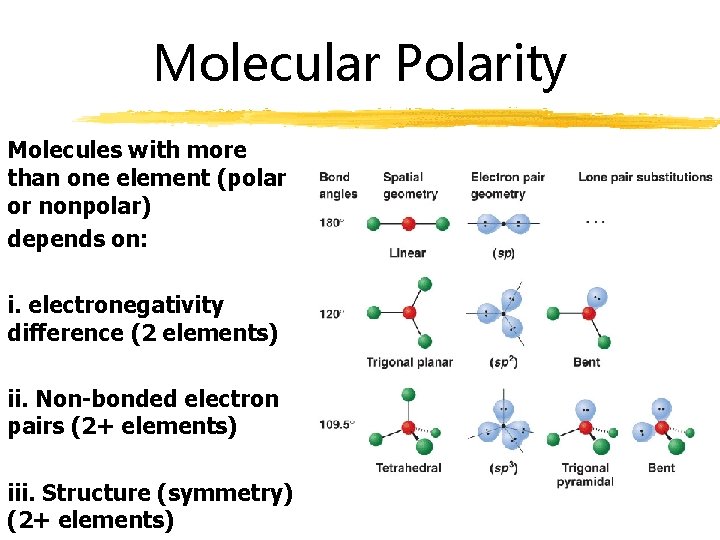
Molecular Polarity Molecules with more than one element (polar or nonpolar) depends on: i. electronegativity difference (2 elements) ii. Non-bonded electron pairs (2+ elements) iii. Structure (symmetry) (2+ elements)

“Inter-chemical” forces A. Inter = between B. Whole salts or molecules attract and bond with one another

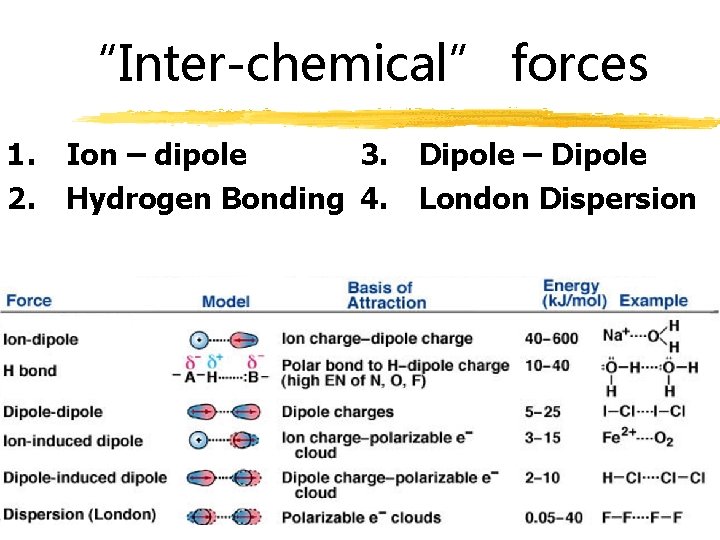
“Inter-chemical” forces 1. Ion – dipole 3. Dipole – Dipole 2. Hydrogen Bonding 4. London Dispersion

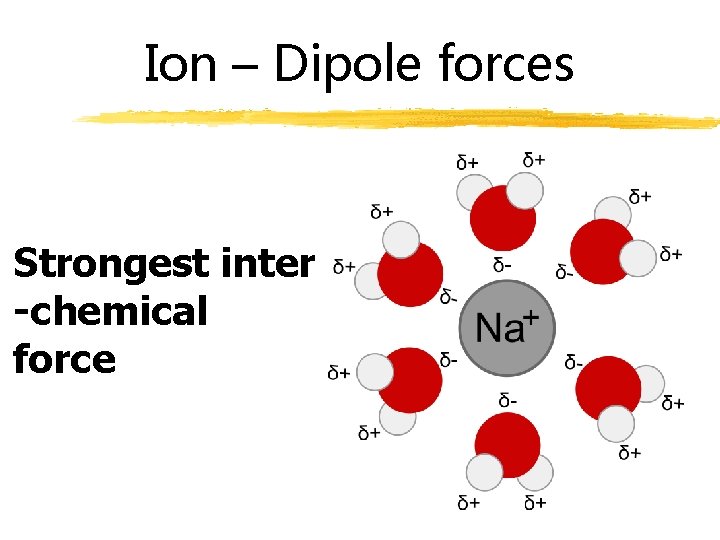
Ion – Dipole forces Strongest inter -chemical force

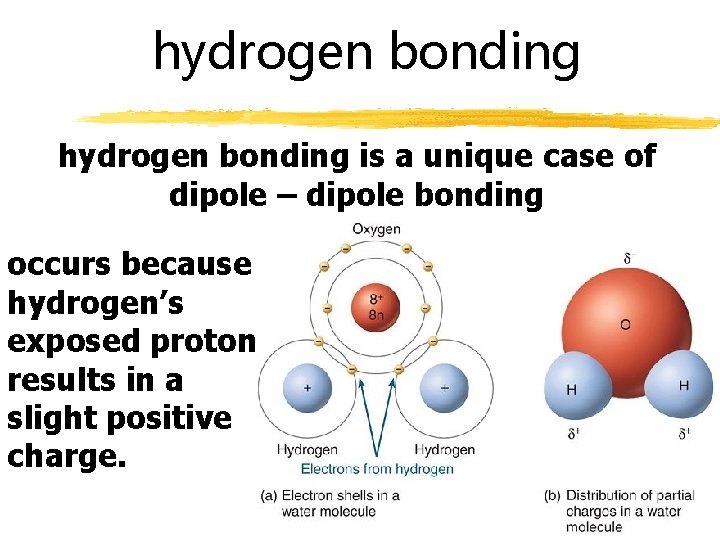
hydrogen bonding is a unique case of dipole – dipole bonding occurs because hydrogen’s exposed proton results in a slight positive charge.

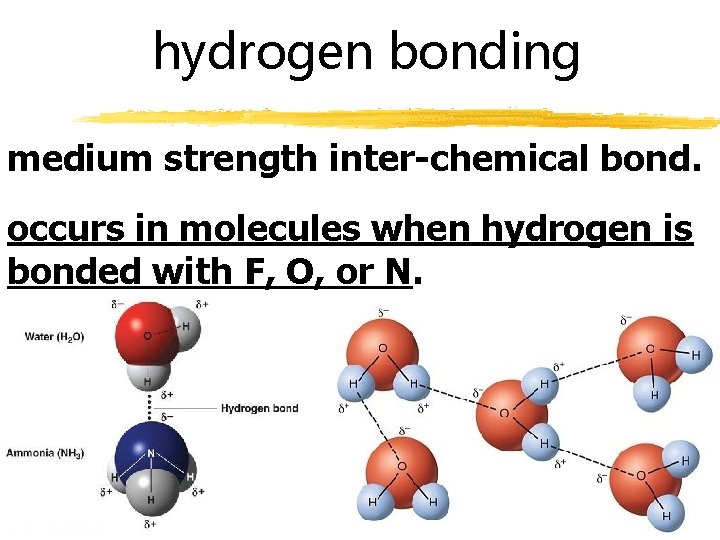
hydrogen bonding medium strength inter-chemical bond. occurs in molecules when hydrogen is bonded with F, O, or N.

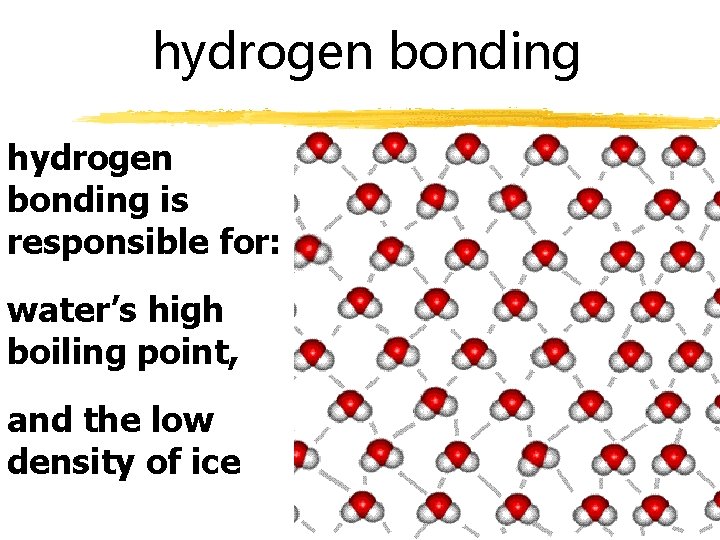
hydrogen bonding is responsible for: water’s high boiling point, and the low density of ice

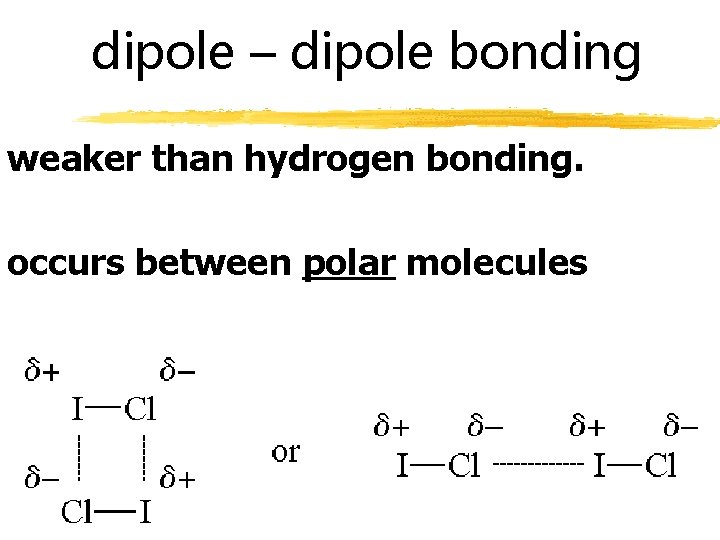
dipole – dipole bonding weaker than hydrogen bonding. occurs between polar molecules

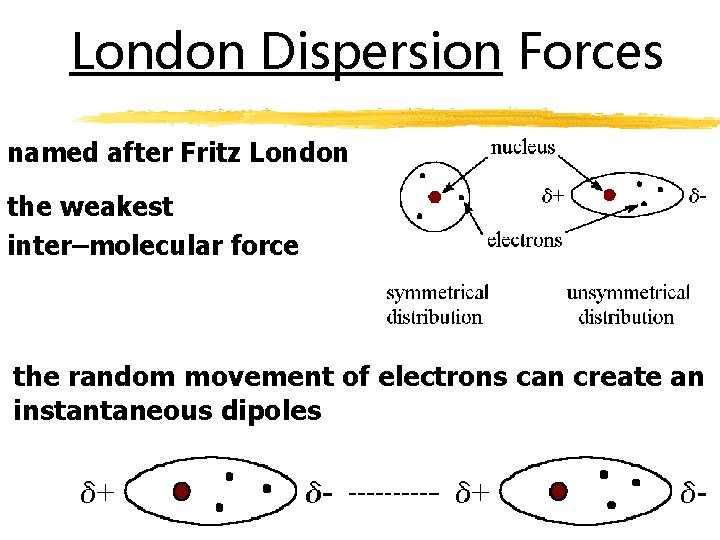
London Dispersion Forces named after Fritz London the weakest inter–molecular force the random movement of electrons can create an instantaneous dipoles