CHAPTERS 4 5 The Chemistry of Life All
- Slides: 21

CHAPTERS 4 & 5 The Chemistry of Life

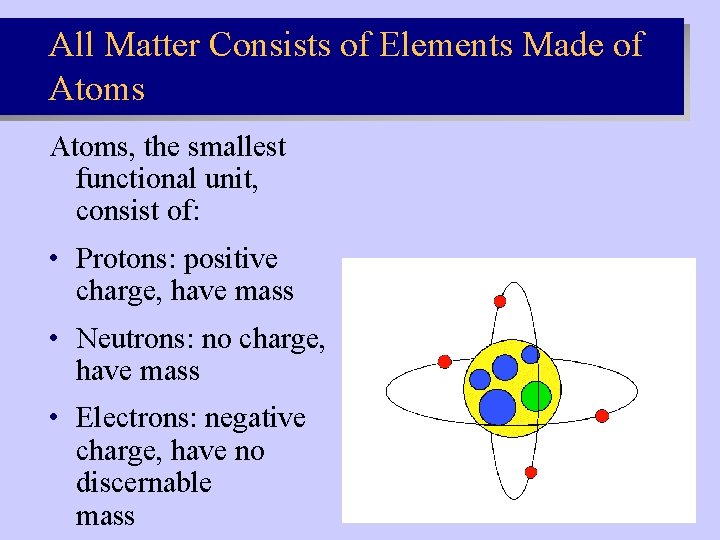
All Matter Consists of Elements Made of Atoms, the smallest functional unit, consist of: • Protons: positive charge, have mass • Neutrons: no charge, have mass • Electrons: negative charge, have no discernable mass Slide 2. 1


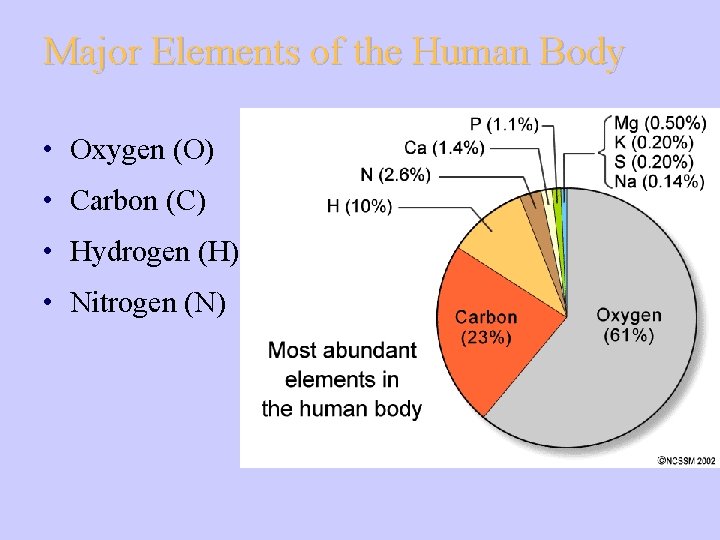
Major Elements of the Human Body • Oxygen (O) • Carbon (C) • Hydrogen (H) • Nitrogen (N)

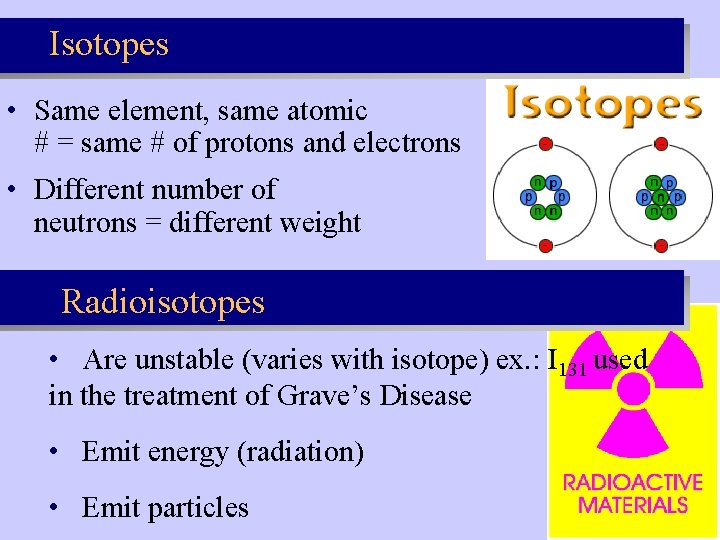
Isotopes • Same element, same atomic # = same # of protons and electrons • Different number of neutrons = different weight Radioisotopes • Are unstable (varies with isotope) ex. : I 131 used in the treatment of Grave’s Disease • Emit energy (radiation) • Emit particles

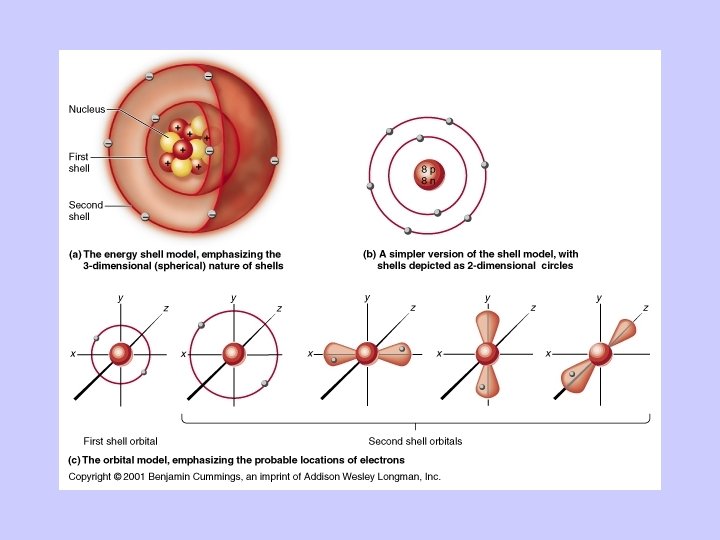
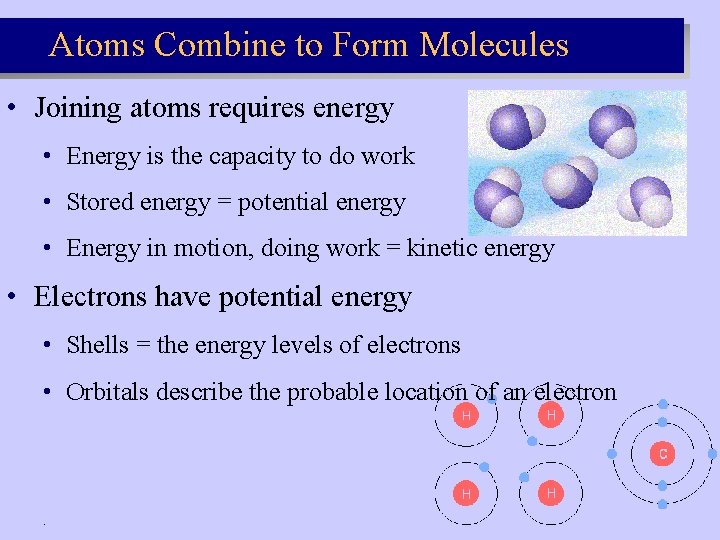
Atoms Combine to Form Molecules • Joining atoms requires energy • Energy is the capacity to do work • Stored energy = potential energy • Energy in motion, doing work = kinetic energy • Electrons have potential energy • Shells = the energy levels of electrons • Orbitals describe the probable location of an electron .

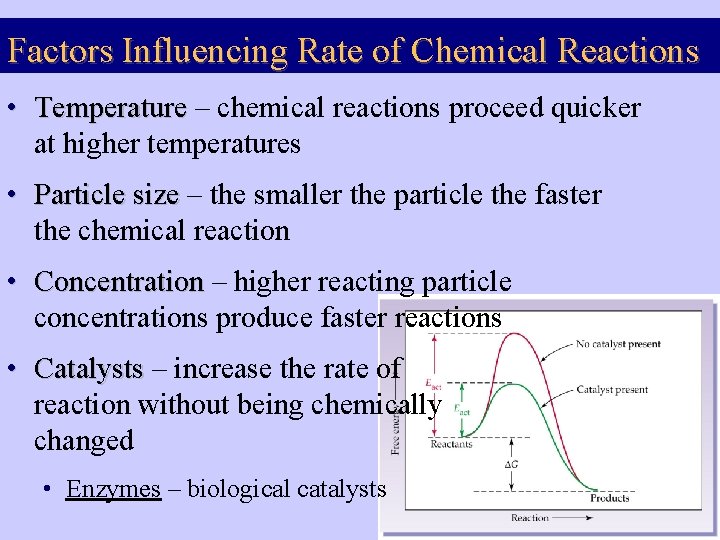
Factors Influencing Rate of Chemical Reactions • Temperature – chemical reactions proceed quicker at higher temperatures • Particle size – the smaller the particle the faster the chemical reaction • Concentration – higher reacting particle concentrations produce faster reactions • Catalysts – increase the rate of reaction without being chemically changed • Enzymes – biological catalysts

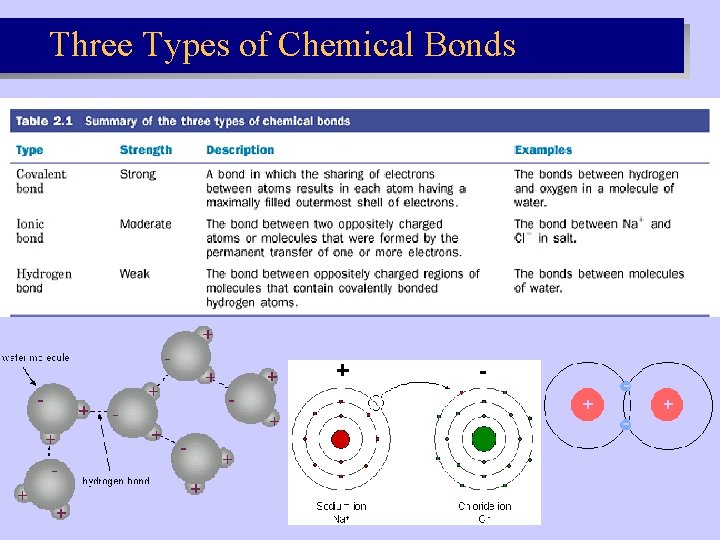
Three Types of Chemical Bonds

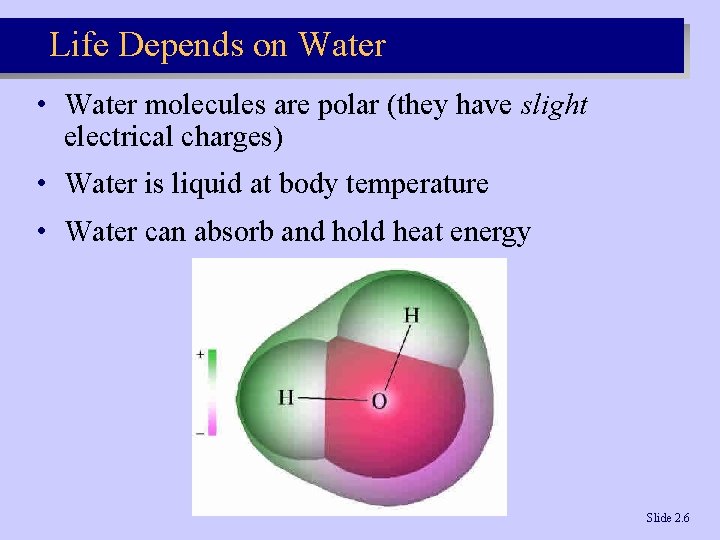
Life Depends on Water • Water molecules are polar (they have slight electrical charges) • Water is liquid at body temperature • Water can absorb and hold heat energy Slide 2. 6

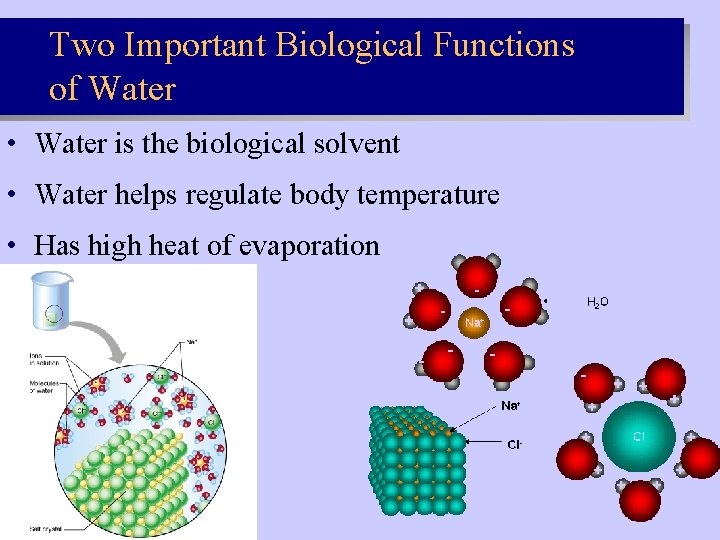
Two Important Biological Functions of Water • Water is the biological solvent • Water helps regulate body temperature • Has high heat of evaporation

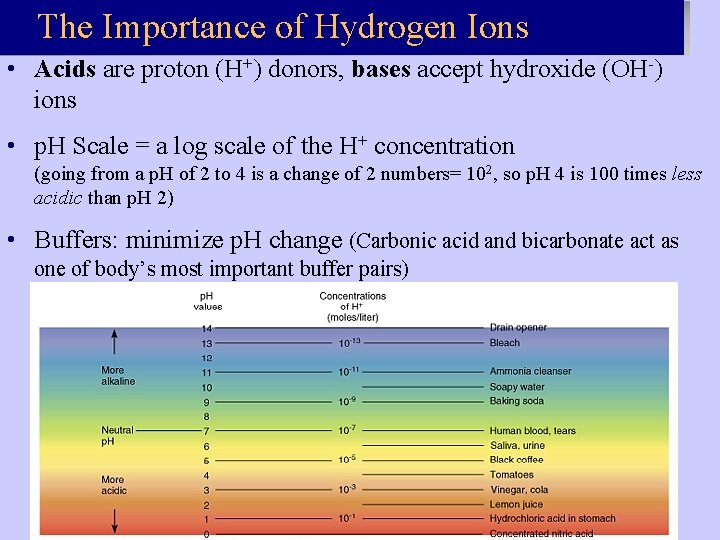
The Importance of Hydrogen Ions • Acids are proton (H+) donors, bases accept hydroxide (OH-) ions • p. H Scale = a log scale of the H+ concentration (going from a p. H of 2 to 4 is a change of 2 numbers= 102, so p. H 4 is 100 times less acidic than p. H 2) • Buffers: minimize p. H change (Carbonic acid and bicarbonate act as one of body’s most important buffer pairs)

The Organic Molecules of Living Organisms Carbon, the building block of living things: • Comprises 18% of body by weight • Forms four covalent bonds • Can form single or double bonds • Can build micro- or macromolecules Carbohydrates Lipids Proteins Nucleic Acids Slide 2. 11

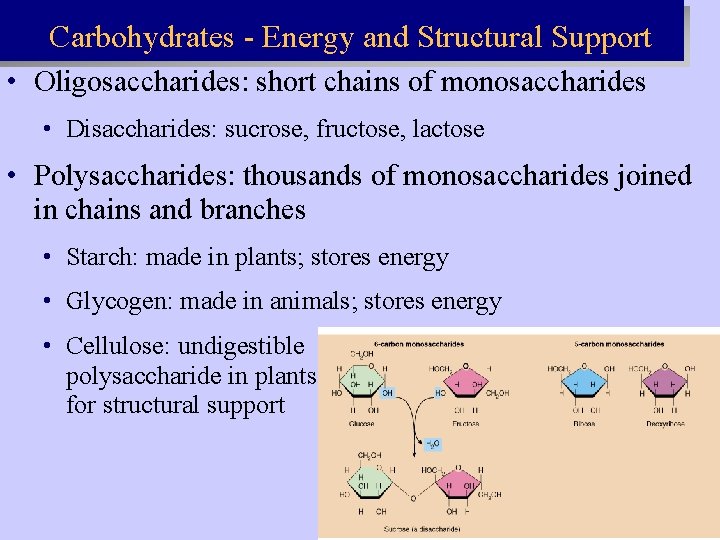
Carbohydrates - Energy and Structural Support • Oligosaccharides: short chains of monosaccharides • Disaccharides: sucrose, fructose, lactose • Polysaccharides: thousands of monosaccharides joined in chains and branches • Starch: made in plants; stores energy • Glycogen: made in animals; stores energy • Cellulose: undigestible polysaccharide in plants for structural support

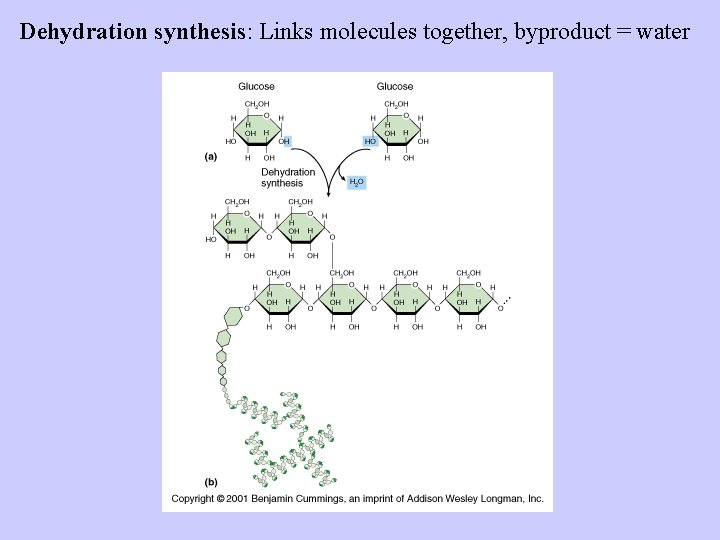
Dehydration synthesis: Links molecules together, byproduct = water

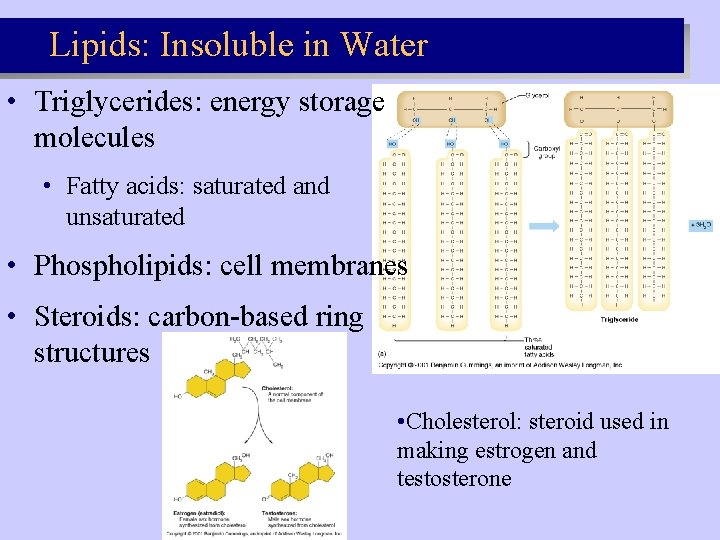
Lipids: Insoluble in Water • Triglycerides: energy storage molecules • Fatty acids: saturated and unsaturated • Phospholipids: cell membranes • Steroids: carbon-based ring structures • Cholesterol: steroid used in making estrogen and testosterone

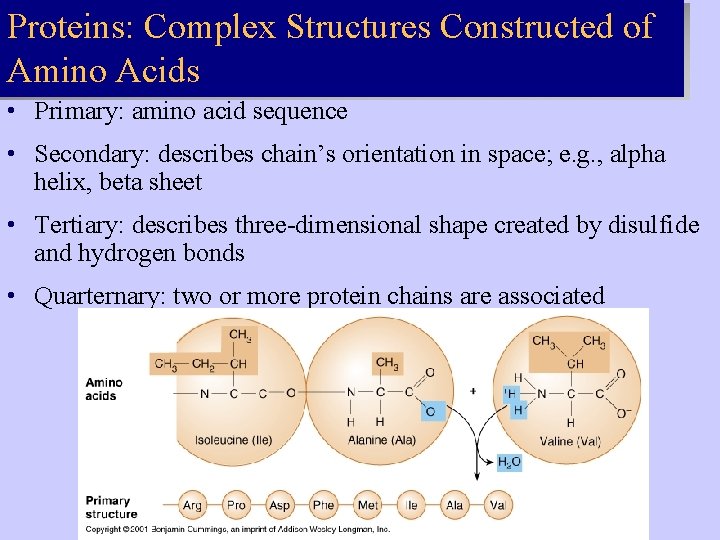
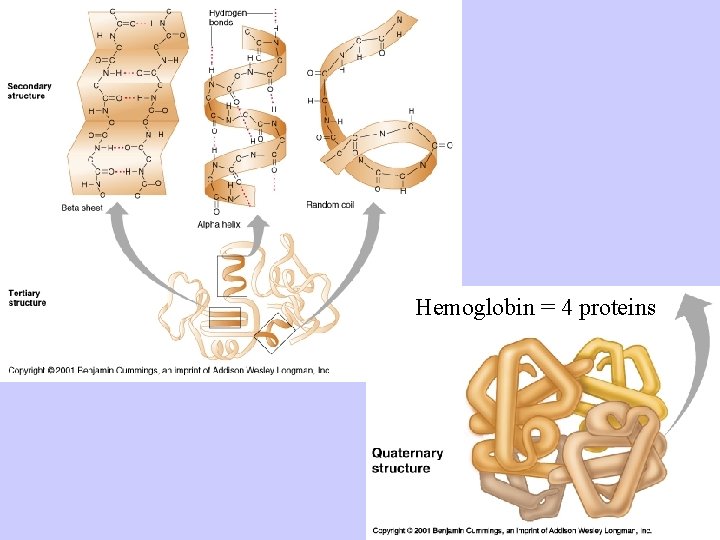
Proteins: Complex Structures Constructed of Amino Acids • Primary: amino acid sequence • Secondary: describes chain’s orientation in space; e. g. , alpha helix, beta sheet • Tertiary: describes three-dimensional shape created by disulfide and hydrogen bonds • Quarternary: two or more protein chains are associated

Hemoglobin = 4 proteins

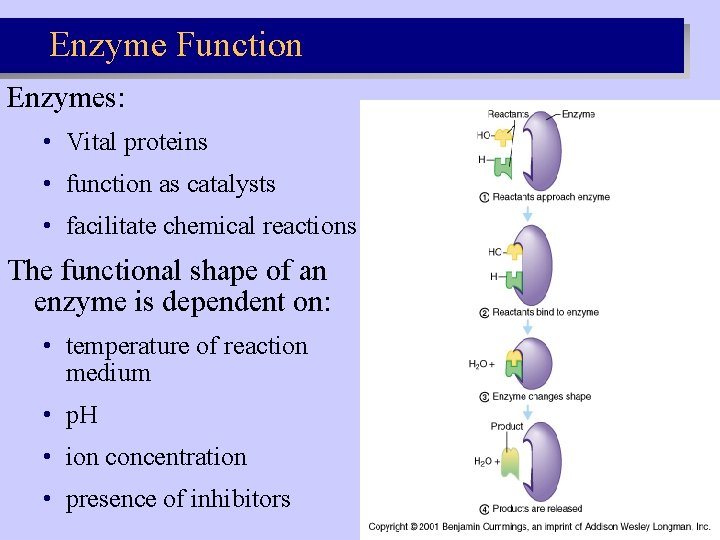
Enzyme Function Enzymes: • Vital proteins • function as catalysts • facilitate chemical reactions The functional shape of an enzyme is dependent on: • temperature of reaction medium • p. H • ion concentration • presence of inhibitors

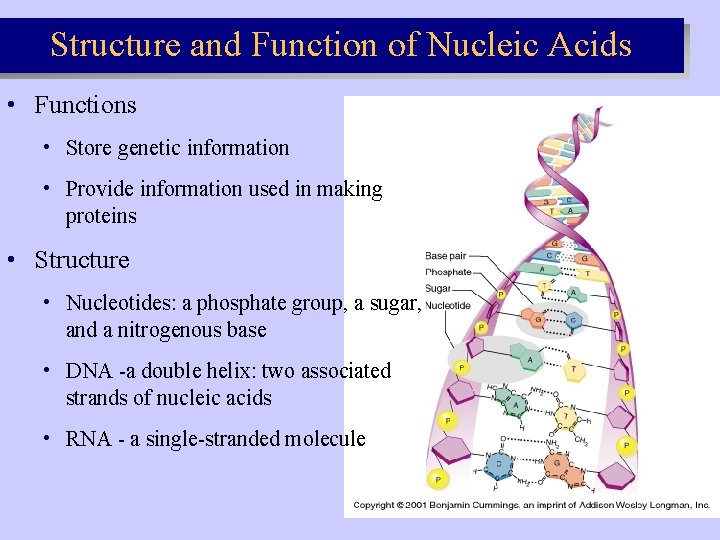
Structure and Function of Nucleic Acids • Functions • Store genetic information • Provide information used in making proteins • Structure • Nucleotides: a phosphate group, a sugar, and a nitrogenous base • DNA -a double helix: two associated strands of nucleic acids • RNA - a single-stranded molecule

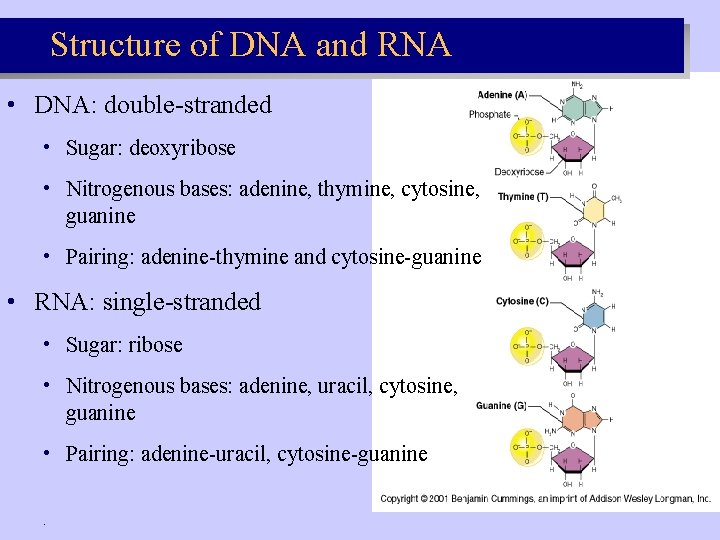
Structure of DNA and RNA • DNA: double-stranded • Sugar: deoxyribose • Nitrogenous bases: adenine, thymine, cytosine, guanine • Pairing: adenine-thymine and cytosine-guanine • RNA: single-stranded • Sugar: ribose • Nitrogenous bases: adenine, uracil, cytosine, guanine • Pairing: adenine-uracil, cytosine-guanine.

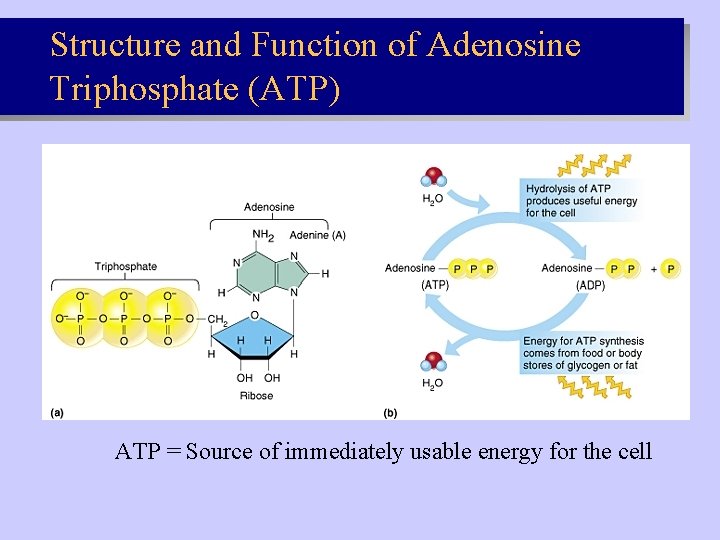
Structure and Function of Adenosine Triphosphate (ATP) ATP = Source of immediately usable energy for the cell