Chapters 1516 An Introduction to Infrared Spectrometry 1





- Slides: 28

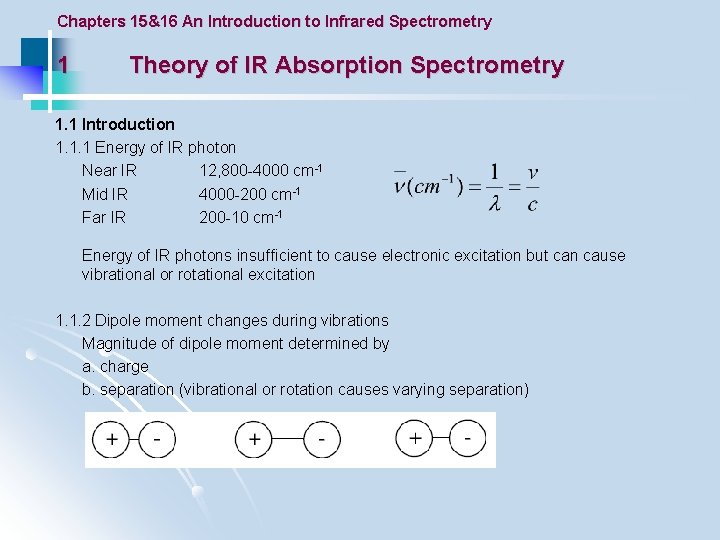
Chapters 15&16 An Introduction to Infrared Spectrometry 1 Theory of IR Absorption Spectrometry 1. 1 Introduction 1. 1. 1 Energy of IR photon Near IR 12, 800 -4000 cm-1 Mid IR 4000 -200 cm-1 Far IR 200 -10 cm-1 Energy of IR photons insufficient to cause electronic excitation but can cause vibrational or rotational excitation 1. 1. 2 Dipole moment changes during vibrations Magnitude of dipole moment determined by a. charge b. separation (vibrational or rotation causes varying separation)

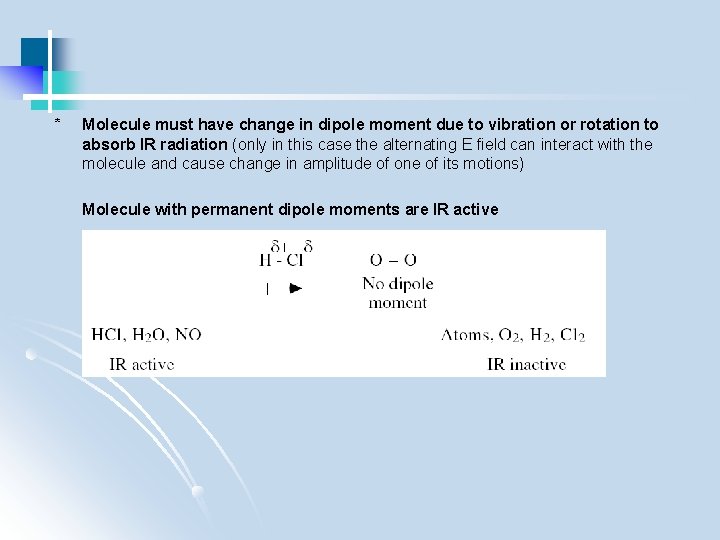
* Molecule must have change in dipole moment due to vibration or rotation to absorb IR radiation (only in this case the alternating E field can interact with the molecule and cause change in amplitude of one of its motions) Molecule with permanent dipole moments are IR active

1. 1. 3 Types of molecular vibrations basic categories: - stretching: change in bond length, symmetric or asymmetric - bending: change in the angle, scissoring, wagging, rocking, twisting/torsion

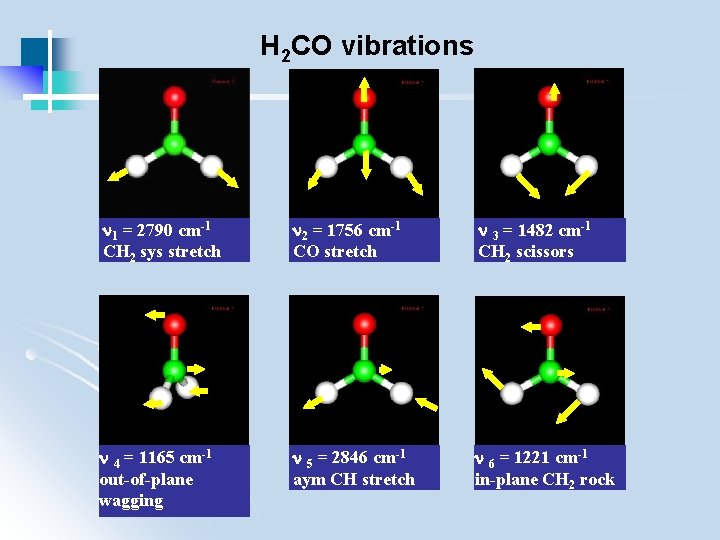
H 2 CO vibrations 1 = 2790 cm-1 CH 2 sys stretch 2 = 1756 cm-1 CO stretch 3 = 1482 cm-1 CH 2 scissors 4 = 1165 cm-1 out-of-plane wagging 5 = 2846 cm-1 aym CH stretch 6 = 1221 cm-1 in-plane CH 2 rock

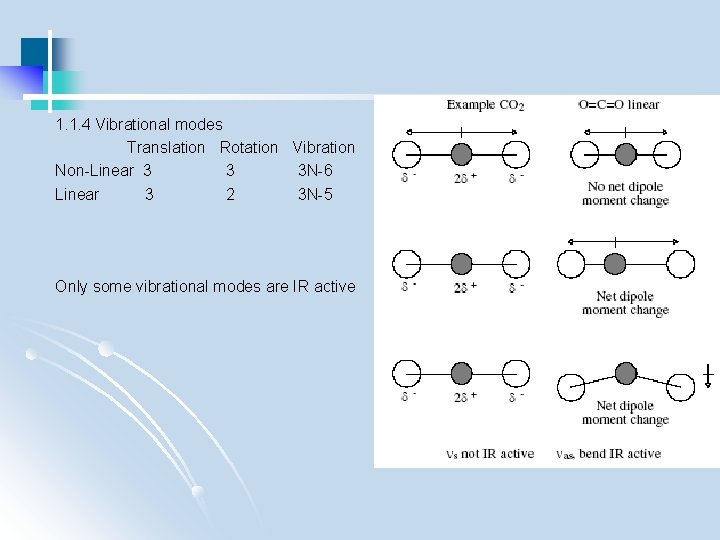
1. 1. 4 Vibrational modes Translation Rotation Vibration Non-Linear 3 3 3 N-6 Linear 3 2 3 N-5 Only some vibrational modes are IR active

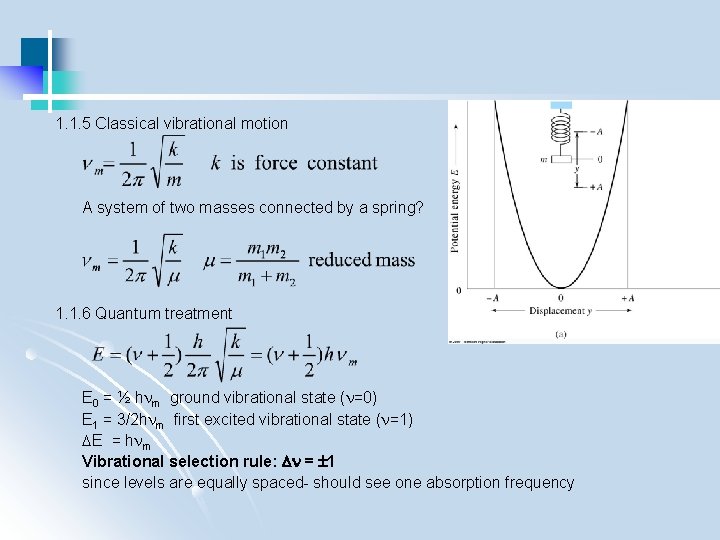
1. 1. 5 Classical vibrational motion A system of two masses connected by a spring? 1. 1. 6 Quantum treatment E 0 = ½ h m ground vibrational state ( =0) E 1 = 3/2 h m first excited vibrational state ( =1) E = h m Vibrational selection rule: = 1 since levels are equally spaced- should see one absorption frequency

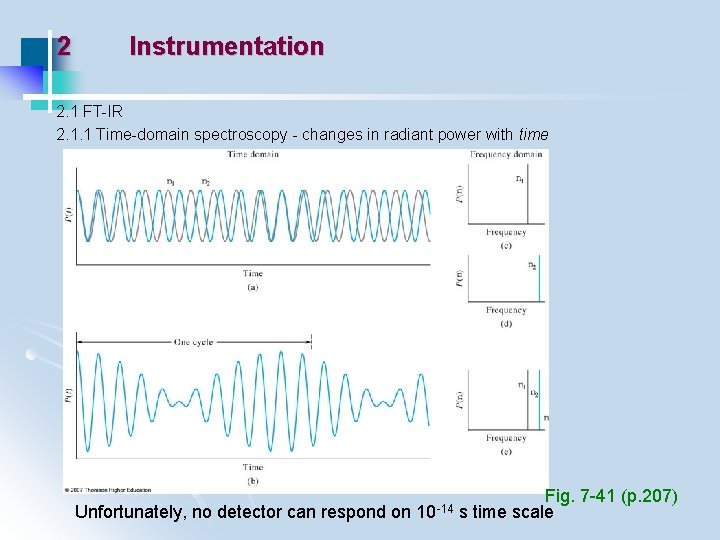
2 Instrumentation 2. 1 FT-IR 2. 1. 1 Time-domain spectroscopy - changes in radiant power with time Unfortunately, no detector can respond on 10 -14 Fig. 7 -41 (p. 207) s time scale

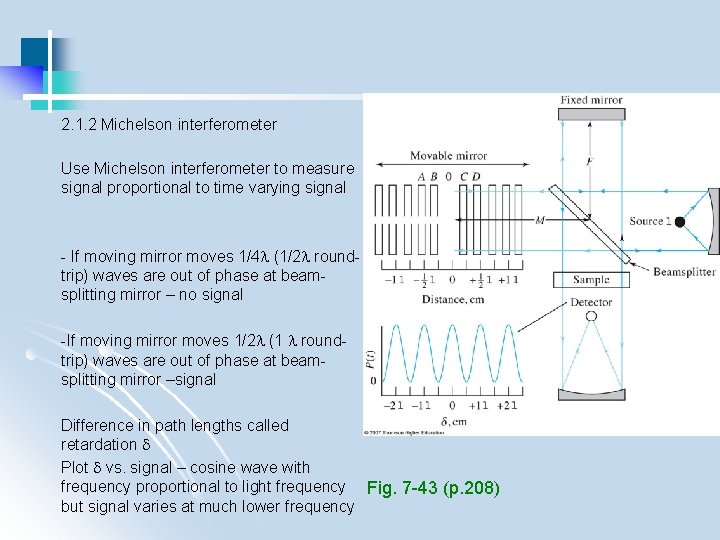
2. 1. 2 Michelson interferometer Use Michelson interferometer to measure signal proportional to time varying signal - If moving mirror moves 1/4 (1/2 roundtrip) waves are out of phase at beamsplitting mirror – no signal -If moving mirror moves 1/2 (1 roundtrip) waves are out of phase at beamsplitting mirror –signal Difference in path lengths called retardation Plot vs. signal – cosine wave with frequency proportional to light frequency Fig. 7 -43 (p. 208) but signal varies at much lower frequency

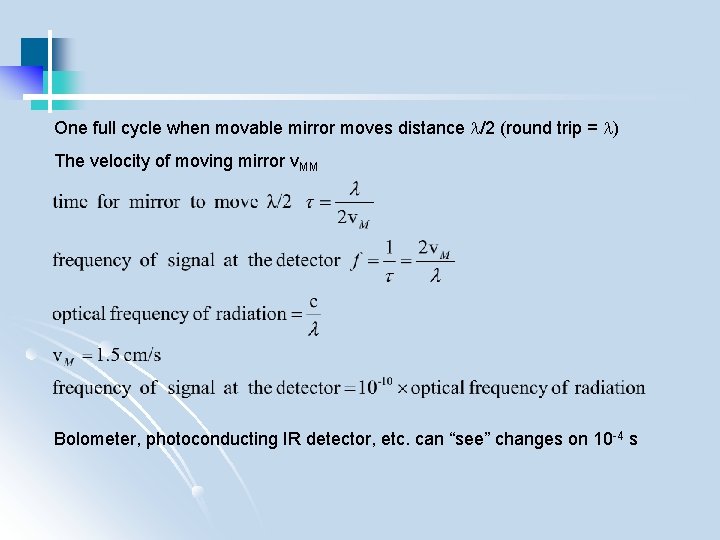
One full cycle when movable mirror moves distance /2 (round trip = ) The velocity of moving mirror v. MM Bolometer, photoconducting IR detector, etc. can “see” changes on 10 -4 s

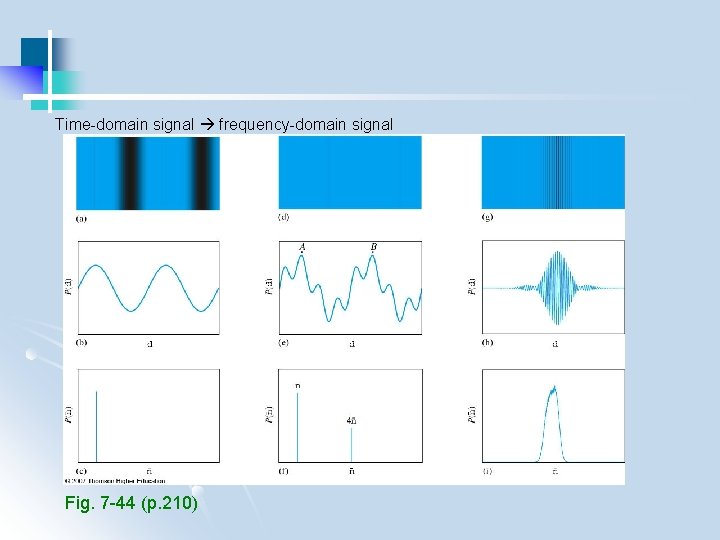
Time-domain signal frequency-domain signal Fig. 7 -44 (p. 210)

2. 1. 3 Resolution Two closely spaced lines only separated if one complete “beat” is recorded. As lines get closer together, must increase Mirror motion is 1/2 , resolution is governed by distance moving mirror travels. 2. 1. 4 Advantages of FT-IR (reading assignment, will be in exam)

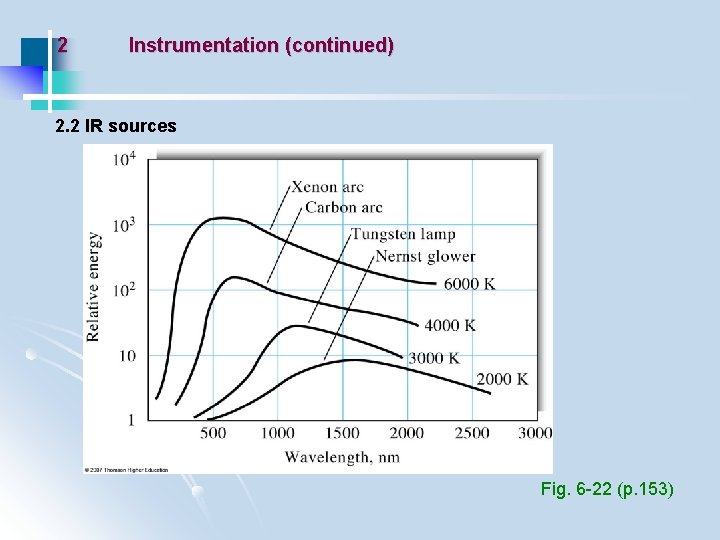
2 Instrumentation (continued) 2. 2 IR sources Fig. 6 -22 (p. 153)

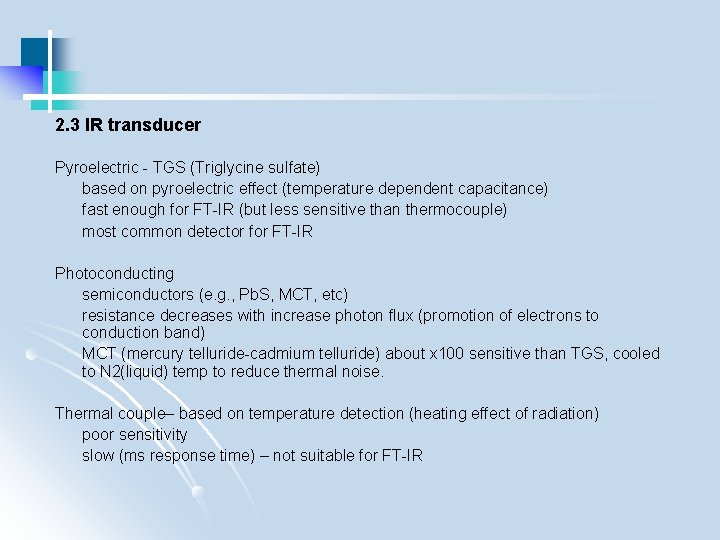
2. 3 IR transducer Pyroelectric - TGS (Triglycine sulfate) based on pyroelectric effect (temperature dependent capacitance) fast enough for FT-IR (but less sensitive than thermocouple) most common detector for FT-IR Photoconducting semiconductors (e. g. , Pb. S, MCT, etc) resistance decreases with increase photon flux (promotion of electrons to conduction band) MCT (mercury telluride-cadmium telluride) about x 100 sensitive than TGS, cooled to N 2(liquid) temp to reduce thermal noise. Thermal couple– based on temperature detection (heating effect of radiation) poor sensitivity slow (ms response time) – not suitable for FT-IR

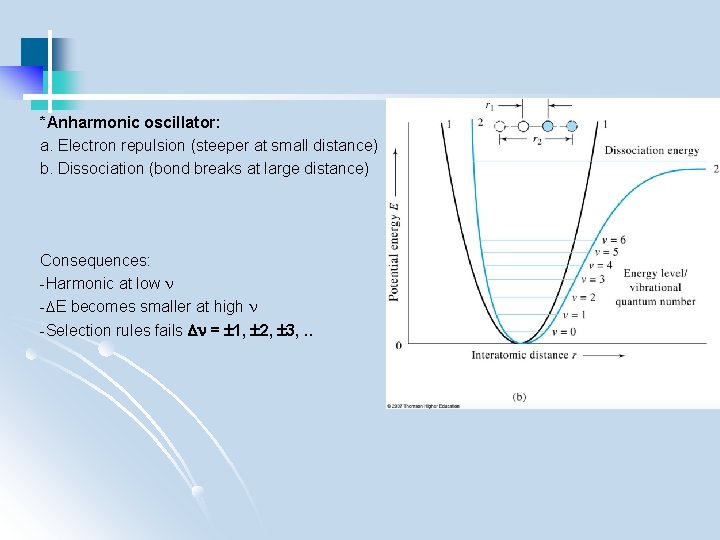
*Anharmonic oscillator: a. Electron repulsion (steeper at small distance) b. Dissociation (bond breaks at large distance) Consequences: -Harmonic at low - E becomes smaller at high -Selection rules fails = 1, 2, 3, . .

3 Absorption Spectrometry 3. 1 Sample handling • • • IR (especially FT-IR) is very widely used for qualitative quantitative Analysis of gases liquids solids Most time-consuming part is sample handling

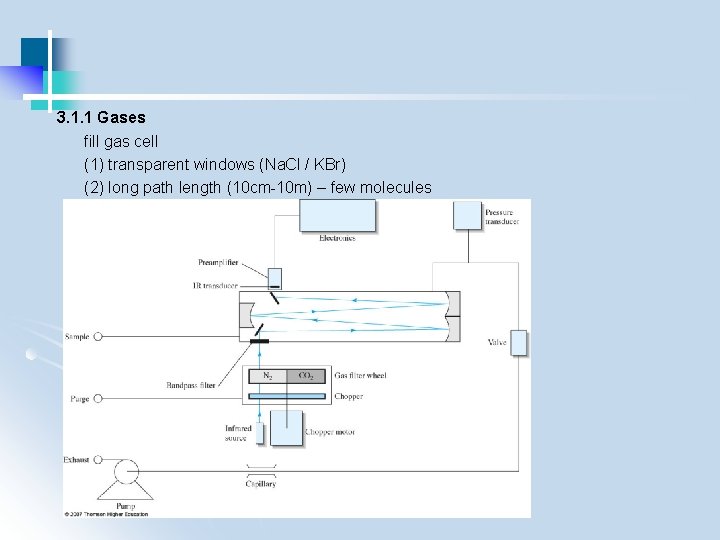
3. 1. 1 Gases fill gas cell (1) transparent windows (Na. Cl / KBr) (2) long path length (10 cm-10 m) – few molecules

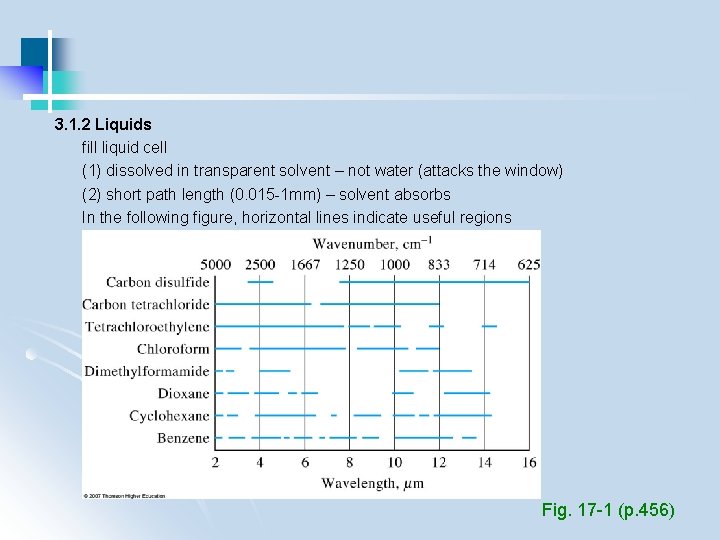
3. 1. 2 Liquids fill liquid cell (1) dissolved in transparent solvent – not water (attacks the window) (2) short path length (0. 015 -1 mm) – solvent absorbs In the following figure, horizontal lines indicate useful regions Fig. 17 -1 (p. 456)

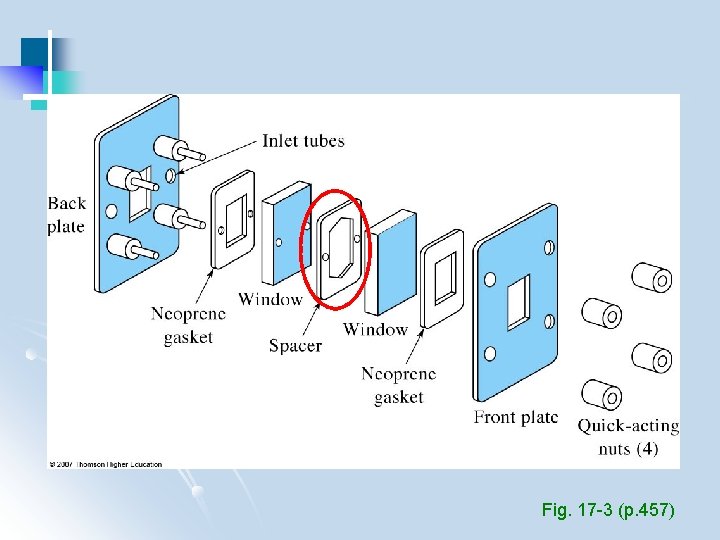
Fig. 17 -3 (p. 457)

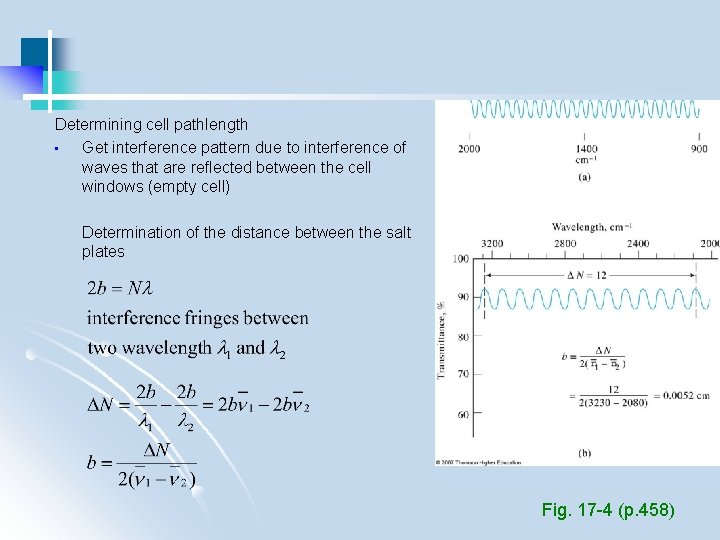
Determining cell pathlength • Get interference pattern due to interference of waves that are reflected between the cell windows (empty cell) Determination of the distance between the salt plates Fig. 17 -4 (p. 458)

3. 1. 3 Solids (1) make semi-transparent pellet with KBr (2) grind and mix with mineral oil to form mull. One drop (film) between Na. Cl plates.

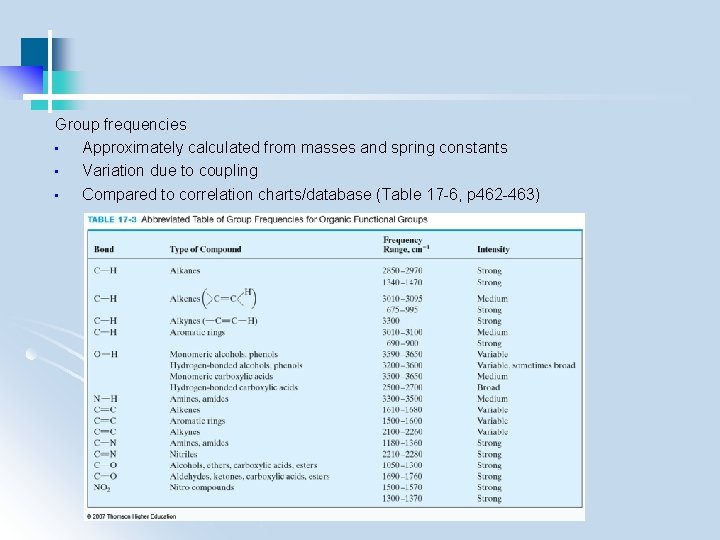
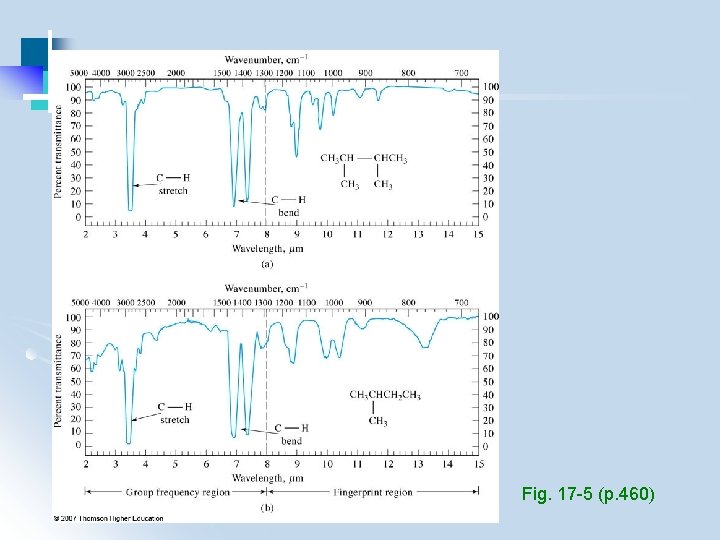
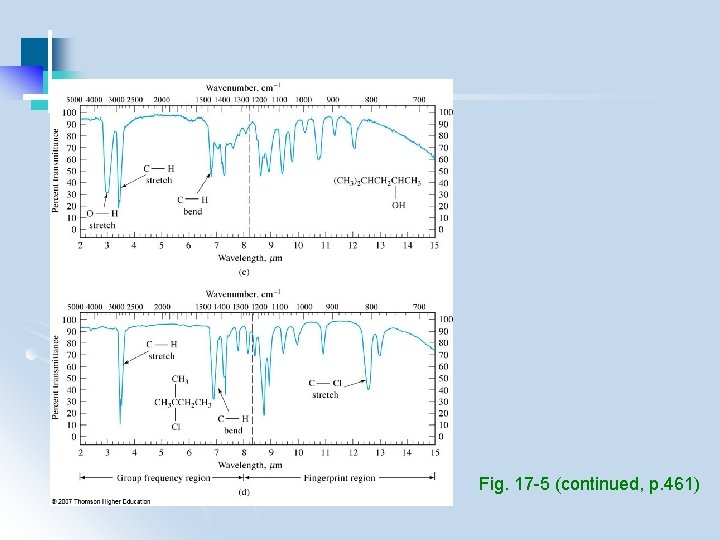
3. 2 Qualitative Analysis (1) Identify functional groups (group frequency region, 3600 -1250 cm-1) (2) Compare with standard spectra containing these functional groups (fingerprint region, 1200 – 600 cm-1) - use computerized spectral search engines - use IR assignments in conjunction with other info (e. g. , chemical, physical, spectroscopic)

Group frequencies • Approximately calculated from masses and spring constants • Variation due to coupling • Compared to correlation charts/database (Table 17 -6, p 462 -463)

Fig. 17 -5 (p. 460)

Fig. 17 -5 (continued, p. 461)

3. 3 Quantitative Analysis IR more difficult than UV-Vis because (1) narrow bands (variation in ) (2) complex spectra (3) weak incident beam (4) low transducer sensitivity (5) solvent absorption IR mostly used for rapid qualitative but not quantitative analysis

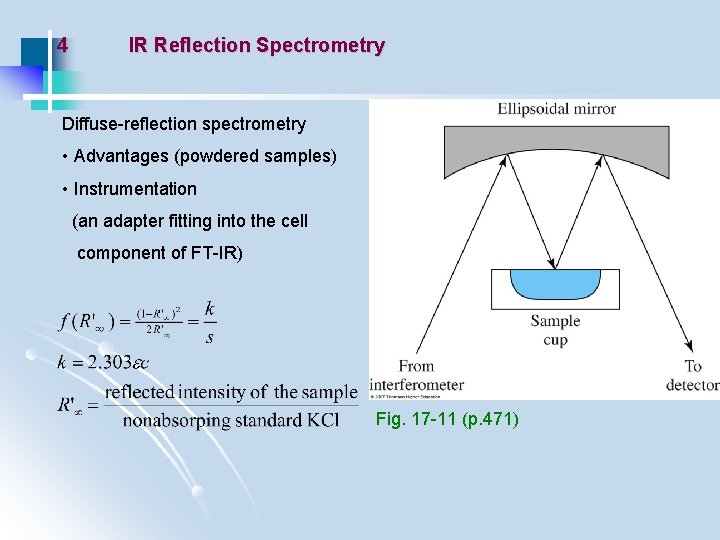
4 IR Reflection Spectrometry Diffuse-reflection spectrometry • Advantages (powdered samples) • Instrumentation (an adapter fitting into the cell component of FT-IR) Fig. 17 -11 (p. 471)

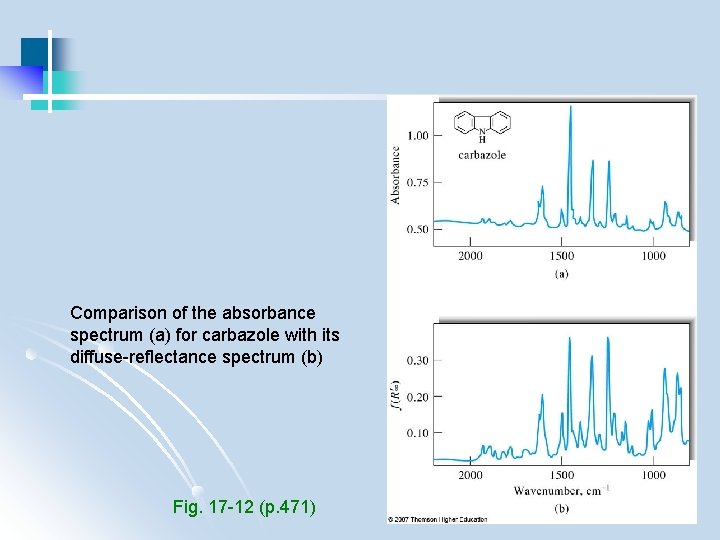
Comparison of the absorbance spectrum (a) for carbazole with its diffuse-reflectance spectrum (b) Fig. 17 -12 (p. 471)

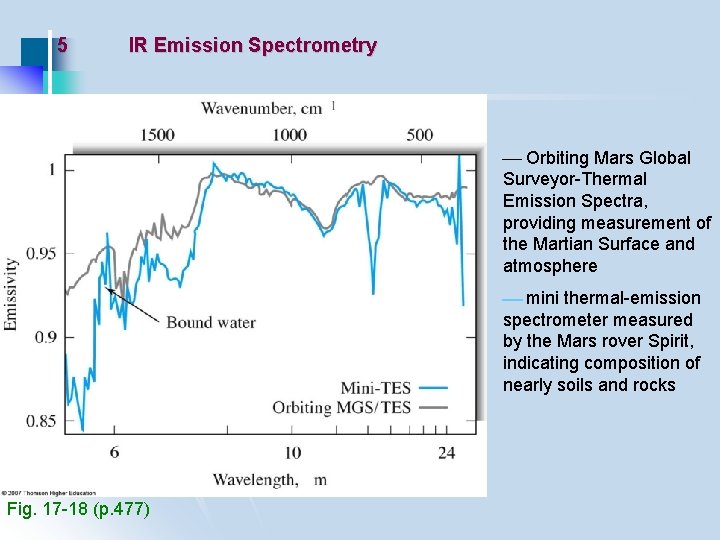
5 IR Emission Spectrometry Orbiting Mars Global Surveyor-Thermal Emission Spectra, providing measurement of the Martian Surface and atmosphere mini thermal-emission spectrometer measured by the Mars rover Spirit, indicating composition of nearly soils and rocks Fig. 17 -18 (p. 477)