CHAPTERS 14 1 and 15 States of matter























- Slides: 29

CHAPTERS 14. 1 and 15 States of matter and Classification of Matter

Classification of Matter ESSENTIAL QUESTIONS: • WHAT IS MATTER? • WHAT ARE THE STATES OF MATTER? • HOW DO WE CLASSIFY MATTER? • WHAT ARE PHYSICAL AND CHEMICAL CHANGES?

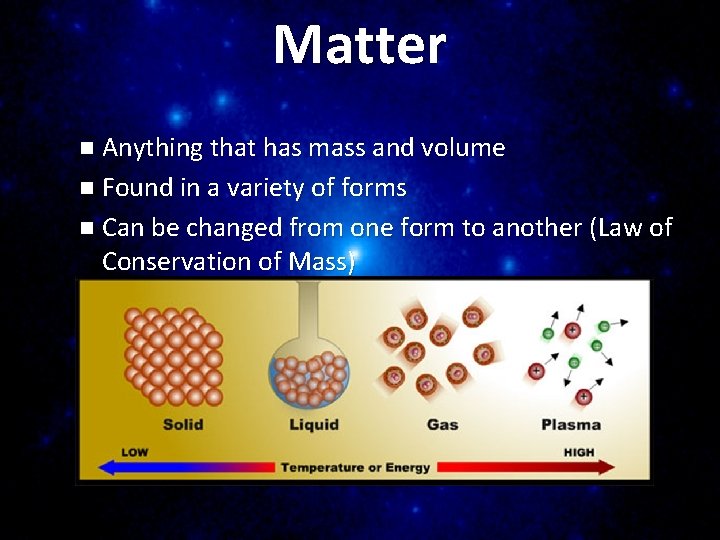
Matter n Anything that has mass and volume n Found in a variety of forms n Can be changed from one form to another (Law of Conservation of Mass)

States of Matter • Kinetic Theory of Matter – states that all particles of matter are in constant motion v The rate at which atoms in a substance move determines its state

• Solid Molecules tightly linked together in a definite shape Vibrate in place Fixed volume and shape

Liquid Molecules NOT as tightly linked as a solid Maintains a fixed volume Able to flow & takes the shape of the container

Gas Molecules have little or no attraction to each other Fill the volume of the container Move very rapidly

• Plasma • • MOST COMMON STATE OF MATTER IN THE UNIVERSE! No definite shape or volume A portion of the particles are IONIZED Acts like a gas but responds to electromagnetic fields

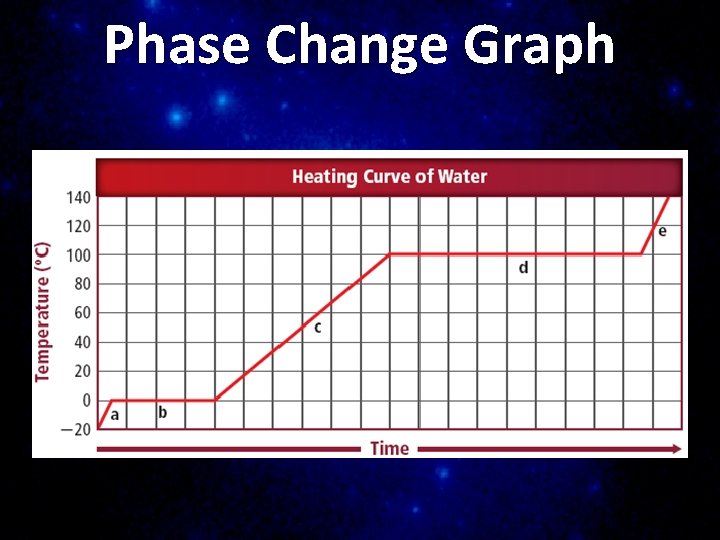
Phase Change A reversible physical change that occurs when a substance changes from one state of matter to another n Temperature does not change during a phase change n Thermal energy must be added or removed to cause a substance to change state

Phase Change Graph

Changing States of Matter

Changing States of Matter Endothermic Reactions – absorb energy – Melting – ie. ice cubes – Vaporization – liquid gas ie. boiling / evaporation / standing water – Sublimation – solid gas without liquid phase ie. dry ice

Changing States of Matter Exothermic Reactions – release energy – Freezing – ie. ice cubes – Condensation – gas liquid ie. morning dew – Deposition – gas solid without liquid phase ie. frost on windows

Changing States of Matter

Composition of Matter • Properties of materials are used to classify them. • The two main categories are – PURE SUBSTANCES and – MIXTURES

PURE SUBSTANCES • A type of matter with a fixed composition. • ELEMENT: a substance that contains ONE TYPE of atom: copper, carbon (graphite/diamond) and oxygen (O 2). .

PURE SUBSTANCES • A type of matter with a fixed composition. • COMPOUND: a substance in which the atoms of two or more elements are combined in a fixed proportion: H 2 O, Ca. CO 3, CO 2, and Na. Cl. • The compound properties are often different from the elements that make them

MIXTURES • A type of matter with NO fixed composition. • HETEROGENEOUS MIXTURES: different materials are distinguished easily: pizza, soup mix, and granite. • Not all are easily recognized

MIXTURES • HOMOGENEOUS MIXTURES: contains two or more gaseous, liquid or solid substances blended evenly throughout: soda, air, sea water, and vinegar. • These are also known as SOLUTIONS: a homogeneous mixture of particles that are CONSTANTLY and UNIFORMLY mixed.

MIXTURES • COLLOID: a mixture with particles that are larger than a solution, but not heavy enough to settle out: milk (water, sugars, fats, proteins), paint (oil, pigment, etc), fog (air, liquids), and smoke in air.

MIXTURES • SUSPENSIONS: heterogeneous mixture containing a liquid in which the particles settle.

Physical Properties of Matter • Physical Properties: Any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. • E. g. viscosity, conductivity, malleability, hardness, melting point, boiling point, and density are examples of physical properties.

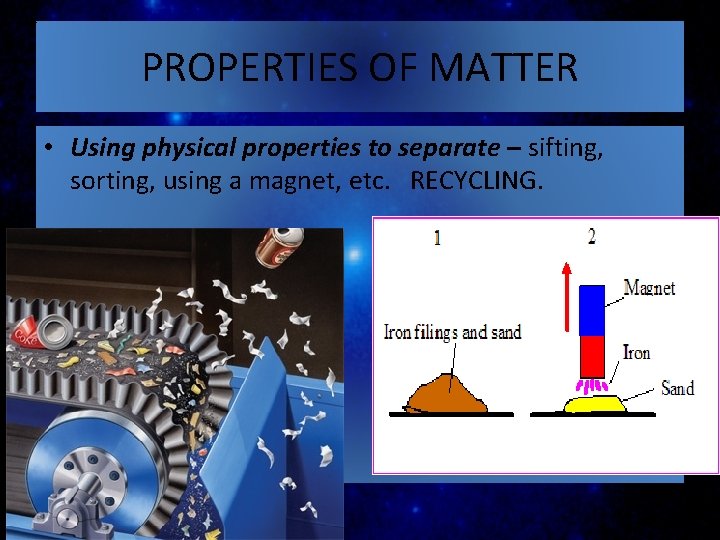
PROPERTIES OF MATTER • Using physical properties to separate – sifting, sorting, using a magnet, etc. RECYCLING.

Section 15. 2: PHYSICAL & CHEMICAL CHANGES OF MATTER • PHYSICAL CHANGE: a change in size, shape, or state of matter. THE IDENTITY REMAINS THE SAME. • These changes may involve energy changes but the properties don’t change. E. g. iron when heated.

PHYSICAL & CHEMICAL CHANGES OF MATTER • PHYSICAL CHANGE: a change in size, shape, or state of matter. THE IDENTITY REMAINS THE SAME. • DISTILLATION – Uses a physical change to separate (evaporation). Used in industry.

Using Properties to Separate Mixtures • Filtration is another separation method. • Filtration is a process that separates materials based on the size of their particles. Some examples: Panning for Gold, archaeologist.

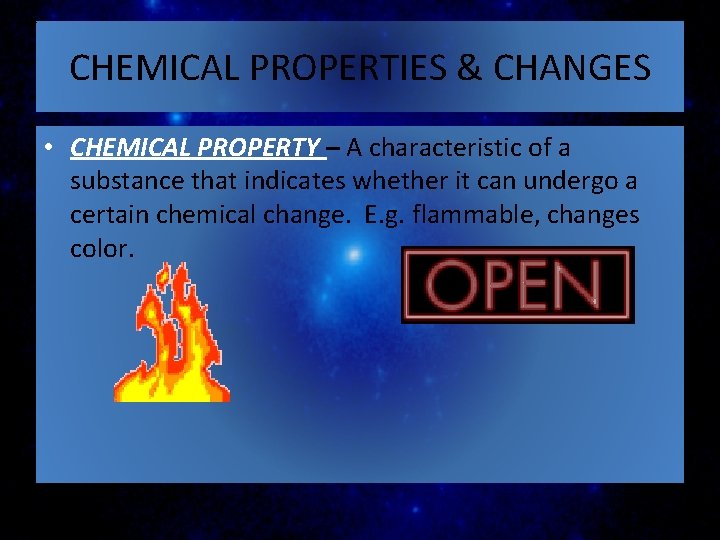
CHEMICAL PROPERTIES & CHANGES • CHEMICAL PROPERTY – A characteristic of a substance that indicates whether it can undergo a certain chemical change. E. g. flammable, changes color.

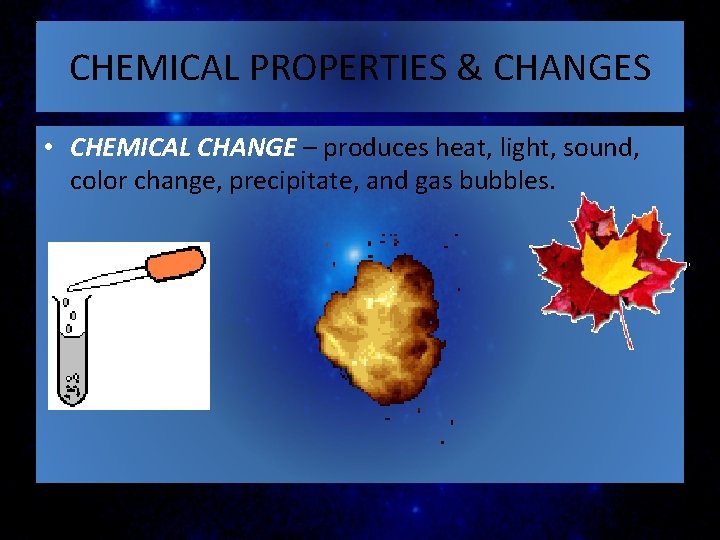
CHEMICAL PROPERTIES & CHANGES • CHEMICAL CHANGE – produces heat, light, sound, color change, precipitate, and gas bubbles.

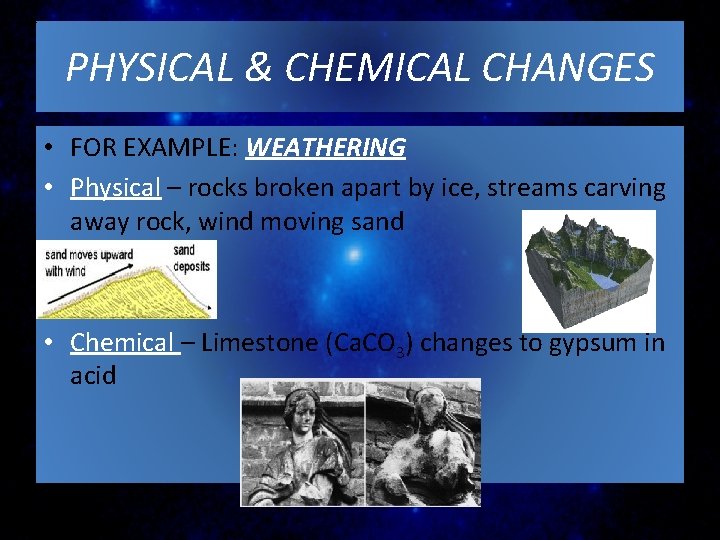
PHYSICAL & CHEMICAL CHANGES • FOR EXAMPLE: WEATHERING • Physical – rocks broken apart by ice, streams carving away rock, wind moving sand • Chemical – Limestone (Ca. CO 3) changes to gypsum in acid