CHAPTERS 12 and 13 The Atomic Nucleus Nuclear






















- Slides: 91

CHAPTERS 12 and 13 The Atomic Nucleus / Nuclear Physics n n n Important nomenclature and ideas 12. 1 Discovery of the Neutron 12. 2 Nuclear Properties 12. 3 The Deuteron 12. 4 Nuclear Forces 12. 5 Nuclear Stability 12. 6 Radioactive Decay 12. 7 Alpha, Beta, and Gamma Decays 12. 8 Radioactive Nuclides 13. 4 Fission 13. 5 13. 6 Fission Reactors Fusion It is said that Cockroft and Walton were interested in raising the voltage of their equipment, its reliability, and so on, more and more, as so often happens when you are involved with technical problems, and that eventually Rutherford lost patience and said, “If you don’t put a scintillation screen in and look for alpha particles by the end of the week, I’ll sack the lot of you. ” And they went and found them (the first nuclear transmutations). - Sir Rudolf Peierls in Nuclear Physics in Retrospect 1

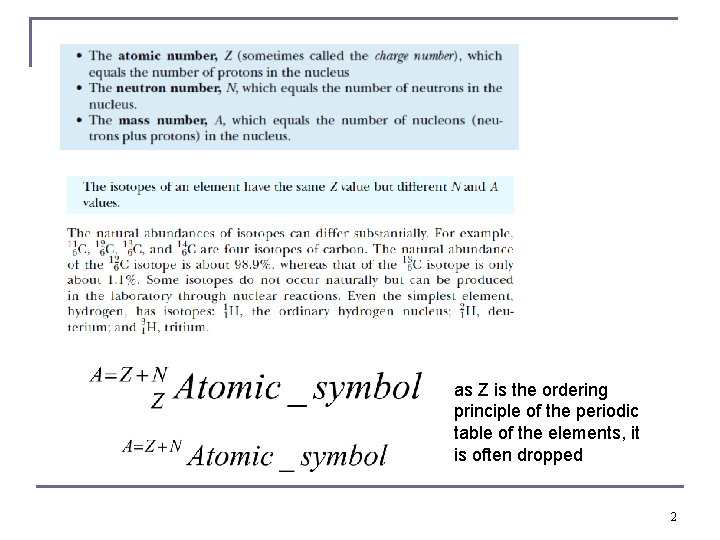
as Z is the ordering principle of the periodic table of the elements, it is often dropped 2

3

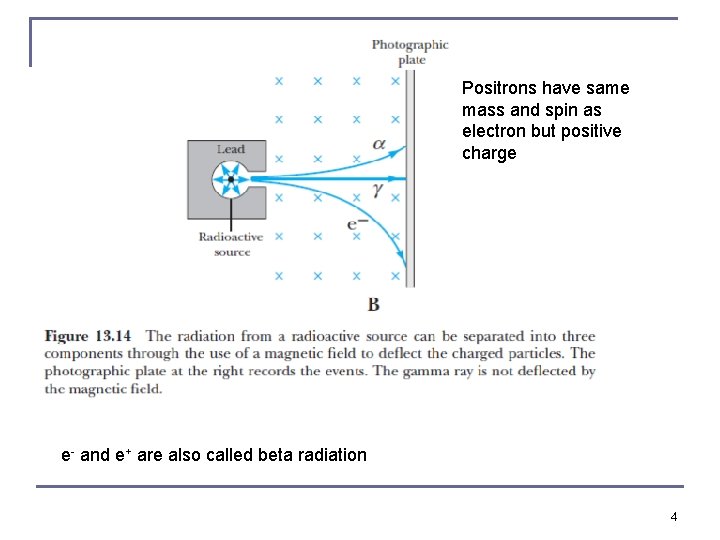
Positrons have same mass and spin as electron but positive charge e- and e+ are also called beta radiation 4

12. 1: Discovery of the Neutron Rutherford proposed the atomic structure with the massive nucleus in 1911. n which particles compose the nucleus was known only in 1932 n Three reasons why electrons cannot exist within the nucleus: 1) Nuclear size The uncertainty principle puts a lower limit on its kinetic energy that is much larger that any kinetic energy observed for an electron emitted from nuclei (its actually the result of β-decay). 2) Nuclear spin If a deuteron nucleus were to consist of protons and electrons, the deuteron must contain 2 protons and 1 electron. A nucleus composed of 3 fermions must result in a half-integral spin. But it has been measured to be 1. So no electrons can possible in the n nucleus (but they apparently come out of certain nuclei) 5

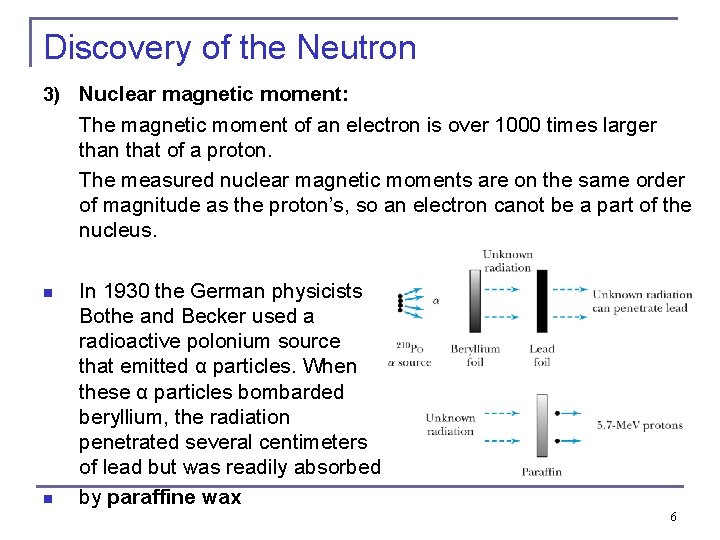
Discovery of the Neutron 3) Nuclear magnetic moment: The magnetic moment of an electron is over 1000 times larger than that of a proton. The measured nuclear magnetic moments are on the same order of magnitude as the proton’s, so an electron canot be a part of the nucleus. n n In 1930 the German physicists Bothe and Becker used a radioactive polonium source that emitted α particles. When these α particles bombarded beryllium, the radiation penetrated several centimeters of lead but was readily absorbed by paraffine wax 6

Discovery of the Neutron n In 1932 Chadwick proposed that the new radiation produced by α + Be consisted of neutrons. His experimental data estimated the neutron’s mass as somewhere between 1. 005 u and 1. 008 u, not far from the modern value of 1. 0087 u. n The electromagnetic radiation (photons) are called gamma rays which have energies on the order of Me. V. n Curie and Joliot performed several measurements to study penetrating high-energy gamma rays. n There also electrons (and positrons) emerging from atoms, beta rays (but they are not constituents of the nucleus themselves) 7

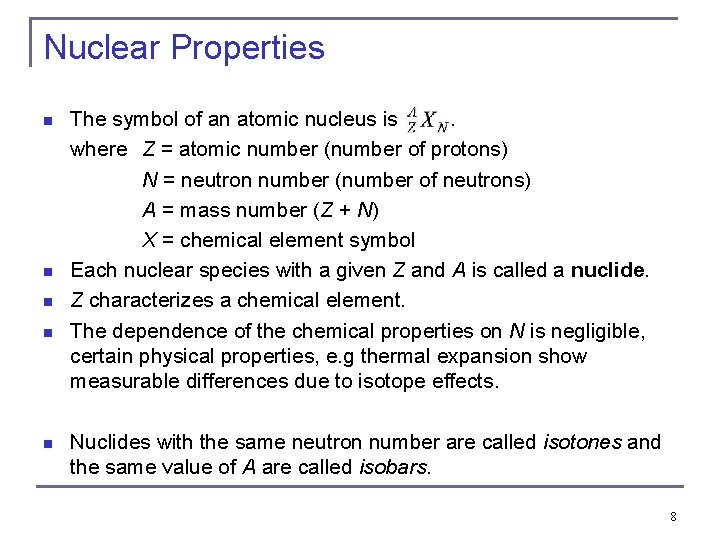
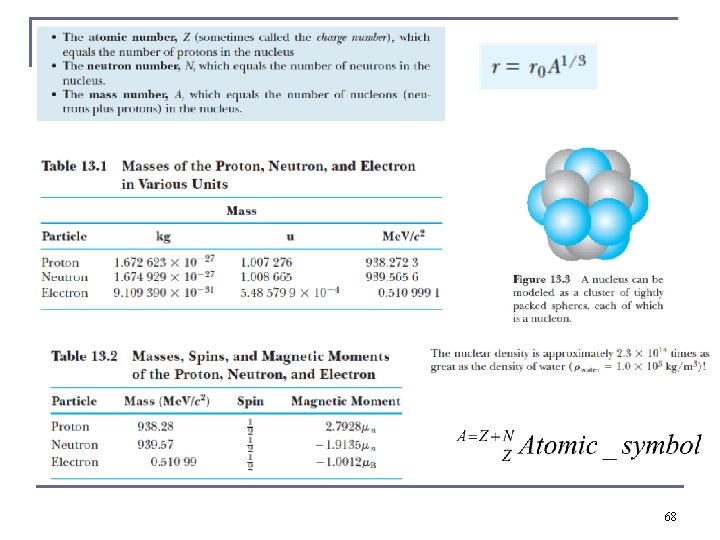
Nuclear Properties n n n The symbol of an atomic nucleus is. where Z = atomic number (number of protons) N = neutron number (number of neutrons) A = mass number (Z + N) X = chemical element symbol Each nuclear species with a given Z and A is called a nuclide. Z characterizes a chemical element. The dependence of the chemical properties on N is negligible, certain physical properties, e. g thermal expansion show measurable differences due to isotope effects. Nuclides with the same neutron number are called isotones and the same value of A are called isobars. 8

12. 2: Nuclear Properties n The nuclear charge is +e times the number (Z) of protons. n Hydrogen’s isotopes: q q n n Deuterium: Heavy hydrogen. Has a neutron as well as a proton in its nucleus. Tritium: Has two neutrons and one proton, is radioactive, about 40 tons on earth. The nuclei of the deuterium and tritium atoms are called deuterons and tritons. Atoms with the same Z, but different mass number A, are called isotopes. 9

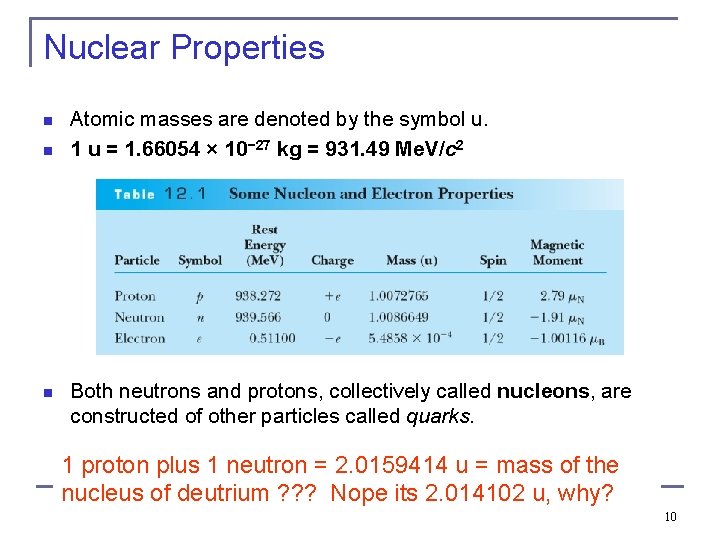
Nuclear Properties n n n Atomic masses are denoted by the symbol u. 1 u = 1. 66054 × 10− 27 kg = 931. 49 Me. V/c 2 Both neutrons and protons, collectively called nucleons, are constructed of other particles called quarks. 1 proton plus 1 neutron = 2. 0159414 u = mass of the nucleus of deutrium ? ? ? Nope its 2. 014102 u, why? 10

Sizes and Shapes of Nuclei n n n Rutherford concluded that the range of the nuclear force must be less than about 10− 14 m. Assume that nuclei are spheres of radius R. Particles (electrons, protons, neutrons, and alphas) scatter when projected close to the nucleus. n The nuclear force is often called the strong force. n There is no simple closed form equation for this force, so we don’t have a simple potential energy function that we could put into the Schrödinger Equation, but quantum mechanics reigns supreme in the nuclear realm as well 11

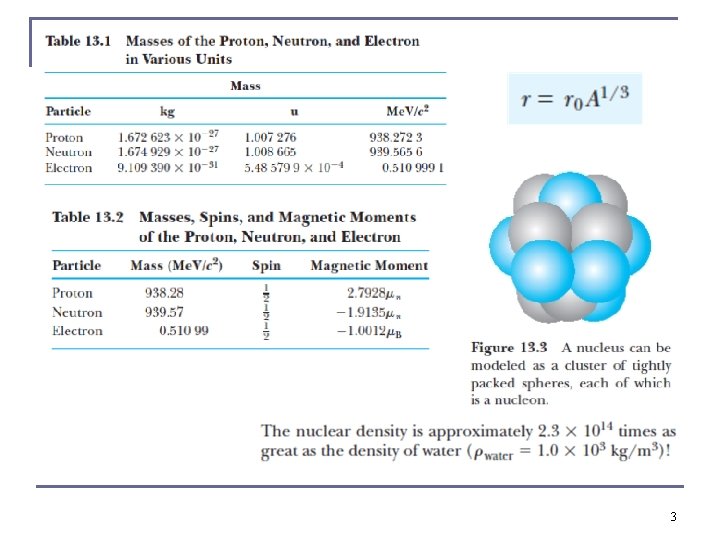
Sizes and Shapes of Nuclei n The nuclear radius may be approximated to be R = r 0 A 1/3 where r 0 ≈ 1. 2 × 10− 15 m. n We use the femtometer with 1 fm = 10− 15 m, or the fermi. 12

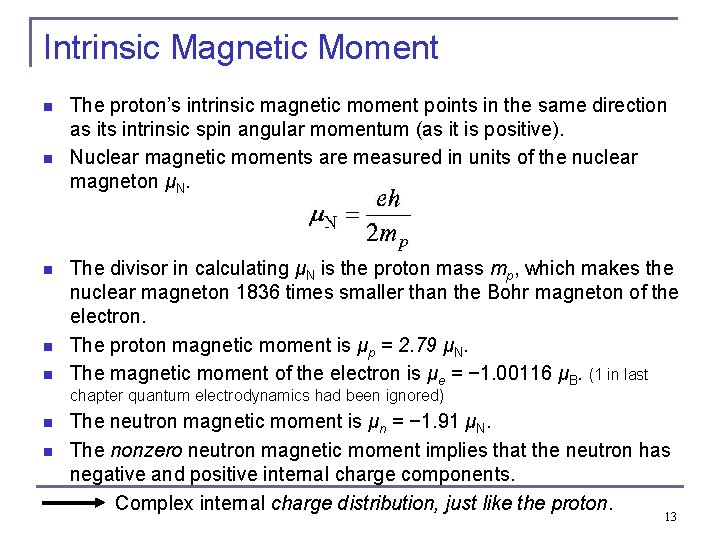
Intrinsic Magnetic Moment n n n The proton’s intrinsic magnetic moment points in the same direction as its intrinsic spin angular momentum (as it is positive). Nuclear magnetic moments are measured in units of the nuclear magneton μN. The divisor in calculating μN is the proton mass mp, which makes the nuclear magneton 1836 times smaller than the Bohr magneton of the electron. The proton magnetic moment is μp = 2. 79 μN. The magnetic moment of the electron is μe = − 1. 00116 μB. (1 in last chapter quantum electrodynamics had been ignored) n n The neutron magnetic moment is μn = − 1. 91 μN. The nonzero neutron magnetic moment implies that the neutron has negative and positive internal charge components. Complex internal charge distribution, just like the proton. 13

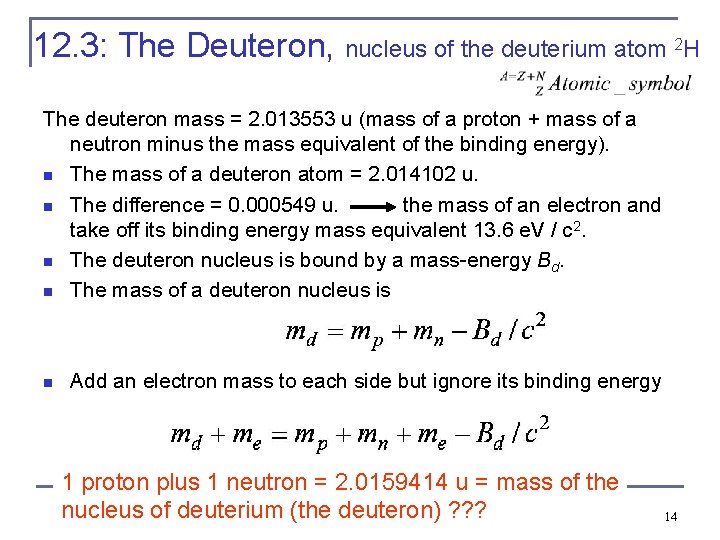
12. 3: The Deuteron, nucleus of the deuterium atom 2 H The deuteron mass = 2. 013553 u (mass of a proton + mass of a neutron minus the mass equivalent of the binding energy). n The mass of a deuteron atom = 2. 014102 u. n The difference = 0. 000549 u. the mass of an electron and take off its binding energy mass equivalent 13. 6 e. V / c 2. n The deuteron nucleus is bound by a mass-energy Bd. n The mass of a deuteron nucleus is n Add an electron mass to each side but ignore its binding energy 1 proton plus 1 neutron = 2. 0159414 u = mass of the nucleus of deuterium (the deuteron) ? ? ? 14

n md + me is the deuterium atom mass M(2 H) and mp + me is the most common atomic hydrogen mass n Because the electron masses cancel in almost all nuclear-mass difference calculations, we use atomic masses rather than nuclear masses. 2 n Convert this to energy using u = 931. 5 Me. V / c 2. n Even for heavier nuclei we effectively neglect the electron binding energies (much larger than 13. 6 e. V) because the nuclear binding energies (e. g. 2 Me. V) are hundreds of thousands times greater. 15

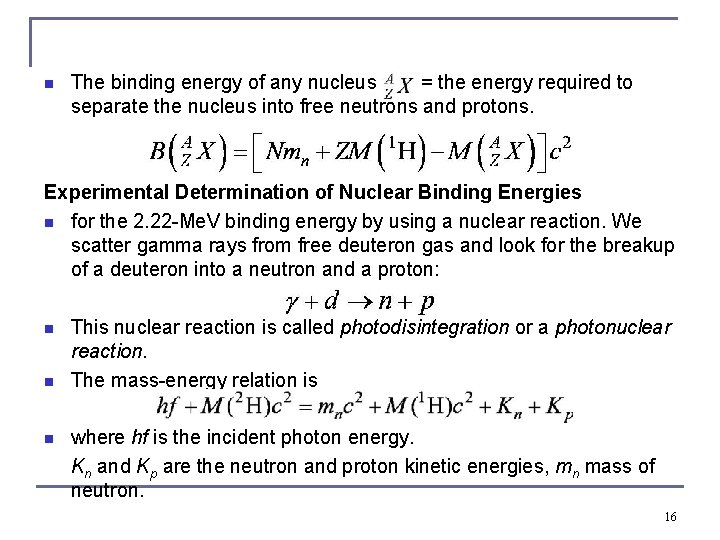
n The binding energy of any nucleus = the energy required to separate the nucleus into free neutrons and protons. Experimental Determination of Nuclear Binding Energies n for the 2. 22 -Me. V binding energy by using a nuclear reaction. We scatter gamma rays from free deuteron gas and look for the breakup of a deuteron into a neutron and a proton: n n n This nuclear reaction is called photodisintegration or a photonuclear reaction. The mass-energy relation is where hf is the incident photon energy. Kn and Kp are the neutron and proton kinetic energies, mn mass of neutron. 16

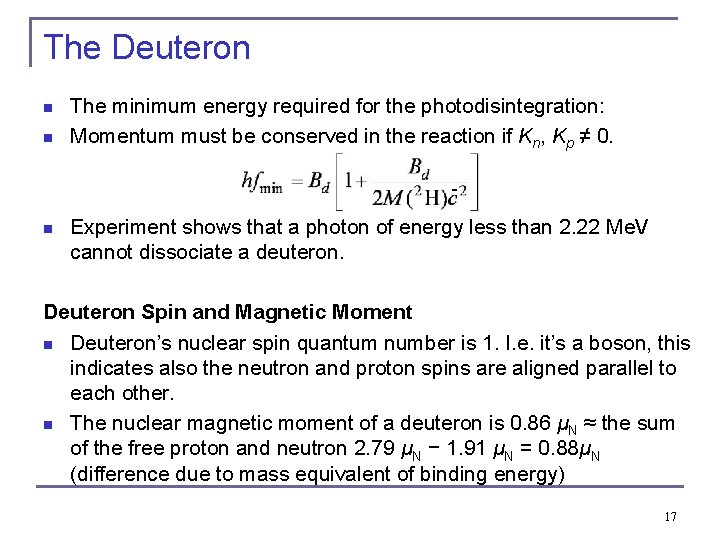
The Deuteron n n The minimum energy required for the photodisintegration: Momentum must be conserved in the reaction if Kn, Kp ≠ 0. - n Experiment shows that a photon of energy less than 2. 22 Me. V cannot dissociate a deuteron. Deuteron Spin and Magnetic Moment n Deuteron’s nuclear spin quantum number is 1. I. e. it’s a boson, this indicates also the neutron and proton spins are aligned parallel to each other. n The nuclear magnetic moment of a deuteron is 0. 86 μN ≈ the sum of the free proton and neutron 2. 79 μN − 1. 91 μN = 0. 88μN (difference due to mass equivalent of binding energy) 17

12. 4: Nuclear Forces n Neutron + proton (np) and proton + proton (pp) elastic collisions. Very high density in the nucleolus, all nuclei are constantly moving about and scatter of reach other Electrostatic hump The nuclear potential energy function for two particles, similar for many particles 18

Nuclear Forces n The inter-nucleon potential has a “hard core” that prevents the nucleons from approaching each other closer than about 0. 4 fm. n The proton has “charge radius” of about 1 fm. Two nucleons within about 2 fm of each other feel an attractive force. n n n The nuclear force (very short range): It falls to zero abruptly with inter-particle separation. The interior nucleons are completely surrounded by other nucleons with which they interact. The nuclear potential between two nucleons is independent of their charge (charge independence of nuclear forces). The major difference between the np and pp potentials is the Coulomb potential shown for r ≥ 3 fm for the pp force. 19

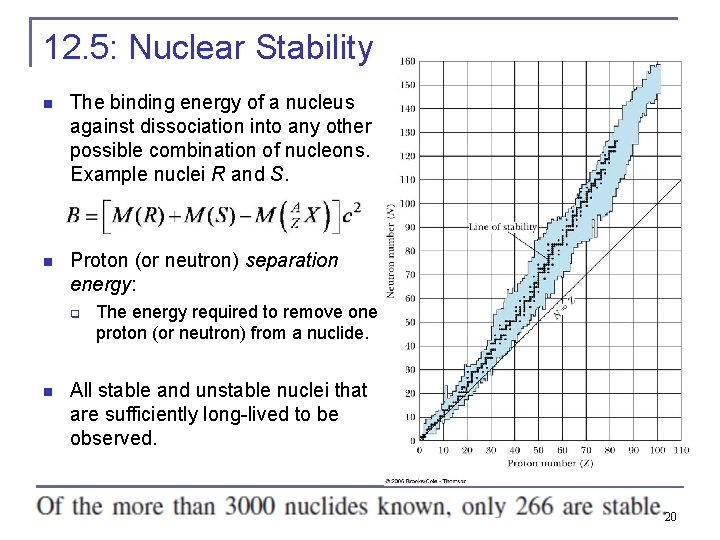
12. 5: Nuclear Stability n The binding energy of a nucleus against dissociation into any other possible combination of nucleons. Example nuclei R and S. n Proton (or neutron) separation energy: q n The energy required to remove one proton (or neutron) from a nuclide. All stable and unstable nuclei that are sufficiently long-lived to be observed. 20

Nuclear Stability n n n The line representing the stable nuclides is the line of stability. It appears that for A ≤ 40, nature prefers the number of protons and neutrons in the nucleus to be about the same Z ≈ N. However, for A ≥ 40, there is a decided preference for N > Z because the nuclear force is independent of whether the particles are nn, np, or pp. As the number of protons increases, the Coulomb force between all the protons becomes stronger until it eventually affects the binding significantly. 21

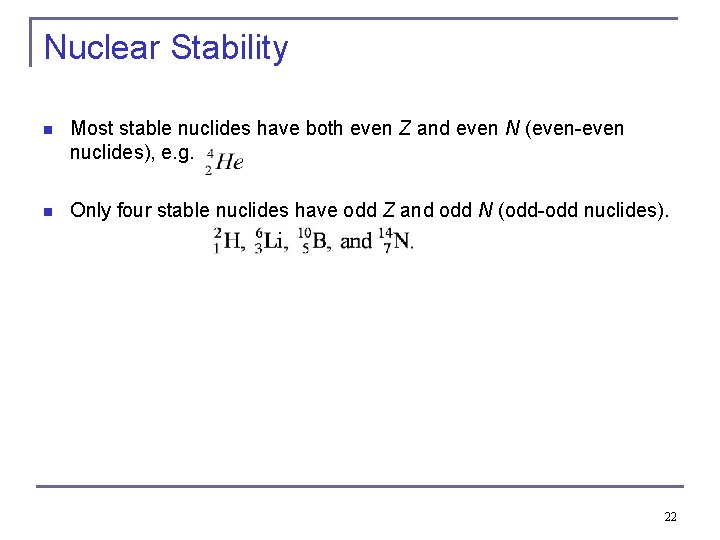
Nuclear Stability n Most stable nuclides have both even Z and even N (even-even nuclides), e. g. n Only four stable nuclides have odd Z and odd N (odd-odd nuclides). 22

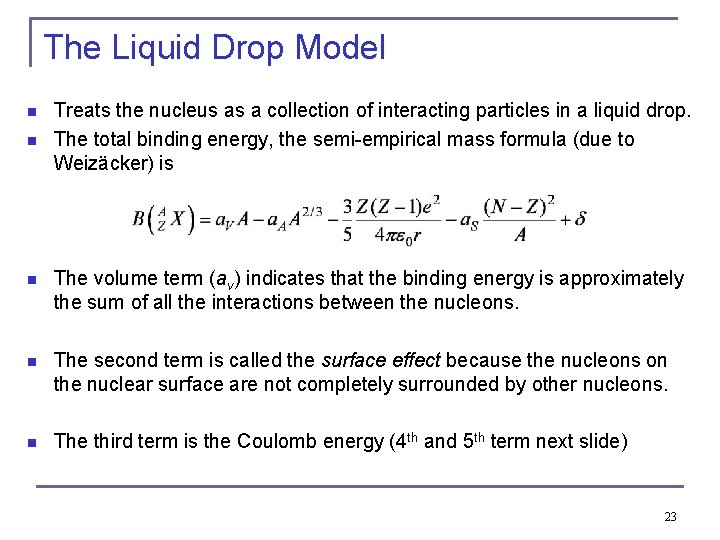
The Liquid Drop Model n n Treats the nucleus as a collection of interacting particles in a liquid drop. The total binding energy, the semi-empirical mass formula (due to Weizäcker) is n The volume term (av) indicates that the binding energy is approximately the sum of all the interactions between the nucleons. n The second term is called the surface effect because the nucleons on the nuclear surface are not completely surrounded by other nucleons. n The third term is the Coulomb energy (4 th and 5 th term next slide) 23

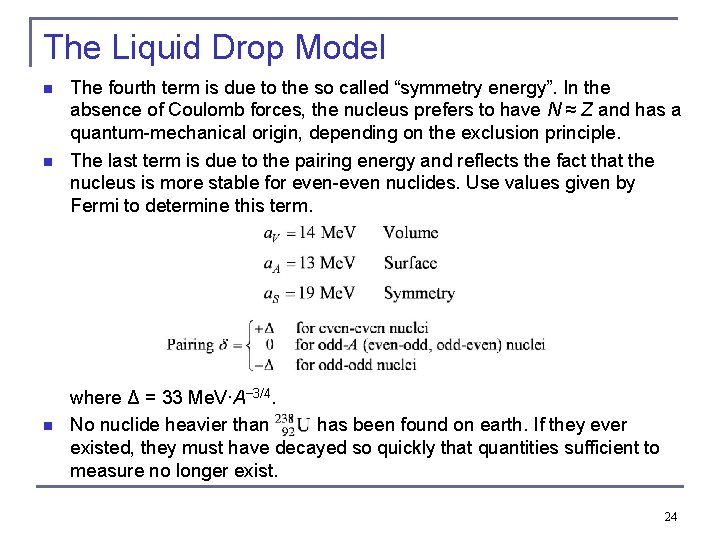
The Liquid Drop Model n n n The fourth term is due to the so called “symmetry energy”. In the absence of Coulomb forces, the nucleus prefers to have N ≈ Z and has a quantum-mechanical origin, depending on the exclusion principle. The last term is due to the pairing energy and reflects the fact that the nucleus is more stable for even-even nuclides. Use values given by Fermi to determine this term. where Δ = 33 Me. V·A− 3/4. No nuclide heavier than has been found on earth. If they ever existed, they must have decayed so quickly that quantities sufficient to measure no longer exist. 24

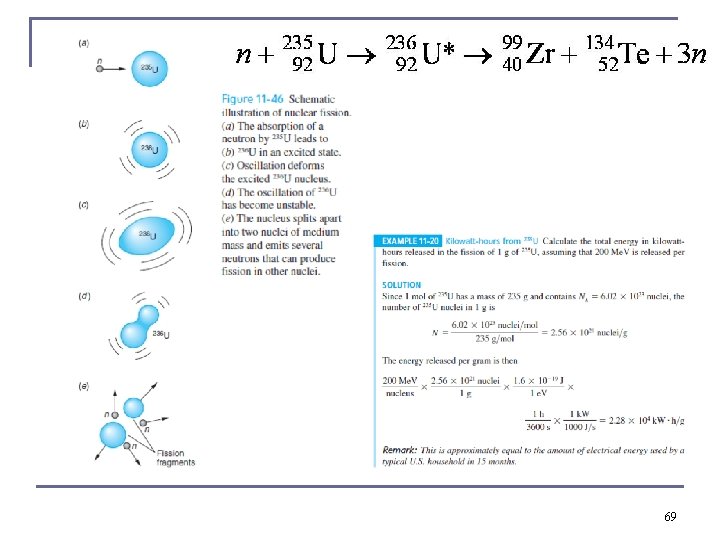
one low energy (room-temperature) neutron being absorbed by 235 U, kaboom, and two to three more medium energy neutrons to make more “kabooms” if more fissionable uranium is around 25

Who is the greatest person that history has forgotten? Marc Morgenstern, lives in The Emerald City of Oz Updated July 6, 2019 · Upvoted by Travis Perry, M. A. History, Wayland Baptist University (2020) and Brayden Swanson, Studied history extensively for six years You’ve probably never even heard of this man, but he’s responsible for saving billions of lives, as well as civilization as we know it: This is Stanislav Yevgrafovich Petrov was a lieutenant colonel of the Soviet Air Defence Forces. On September 26, 1983, three weeks after the Soviet military had shot down Korean Air Lines Flight 007, Petrov was the duty officer at the command center for the Oko nuclear early-warning system when the system reported that a USAF Minuteman missile had been launched from the United States, followed by up to five more. “If notification was received from the Russian early warning systems that inbound missiles had been detected, the Soviet Union's strategy was an immediate and compulsory nuclear counter-attack against the United States (launch on warning), specified in the doctrine of mutual assured destruction, or MAD. ” 26

At the time, nuclear retaliation required that multiple sources confirm an attack before launching retaliatory strikes against the offending nation. Petrov knew that any nuclear strike from the US would be massive, and concluded that the system had triggered a false alarm, that no missiles had been launched from the U. S. , and, disobeying orders from his superiors, stood down the retaliatory launch. “It was subsequently determined that the false alarms were caused by a rare alignment of sunlight on high-altitude clouds and the satellites' Molniya orbits, an error later corrected by cross-referencing a geostationary satellite. ” Petrov’s quick thinking, as well as his refusal to obey orders, prevented what would have most assuredly been the start of World War III, a devastating nuclear holocaust would have ensued, and billions of people might have died, as well as ending civilization as we know it on the Earth. Petrov had, indeed, saved the world. So why do we not hear more about this brave man? The glitches in the Soviets’ early-warning system embarrassed military higher ups, and the entire episode was kept quiet until the incident became known publicly in the 1990 s upon the publication of the memoirs of Colonel General Yuriy Vsyevolodich Votintsev, a retired commander of the Soviet Air Defense's Missile Defense Units and the officer who had been in charge at the time of the incident. 27

“Petrov was neither rewarded nor punished for his actions, but was reassigned to a less sensitive post, took early retirement (although he emphasized that he was not "forced out" of the army, as is sometimes claimed by Western sources), and suffered a nervous breakdown. ” Petrov died on May 19, 2017, of hypostatic pneumonia at 77 years old. 28

October 11, 1986, halfway between Moscow and Washington, D. C. … 29

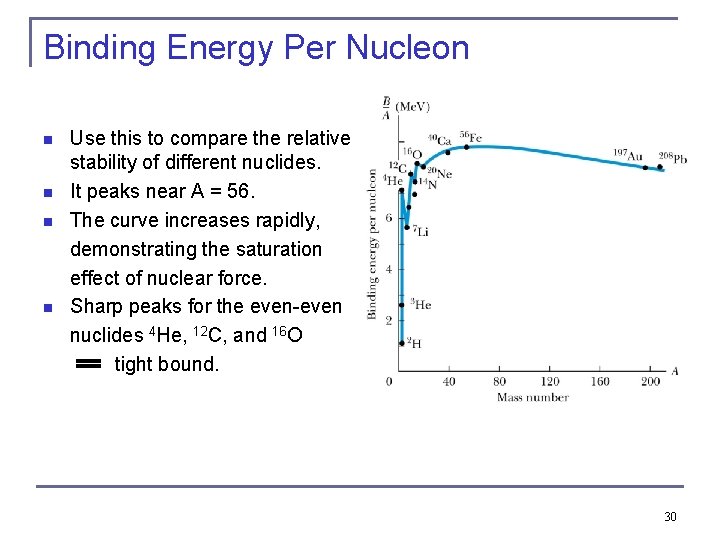
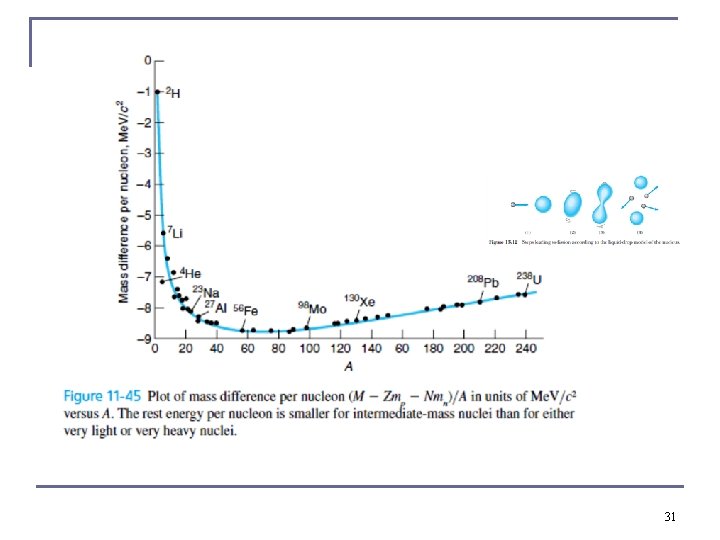
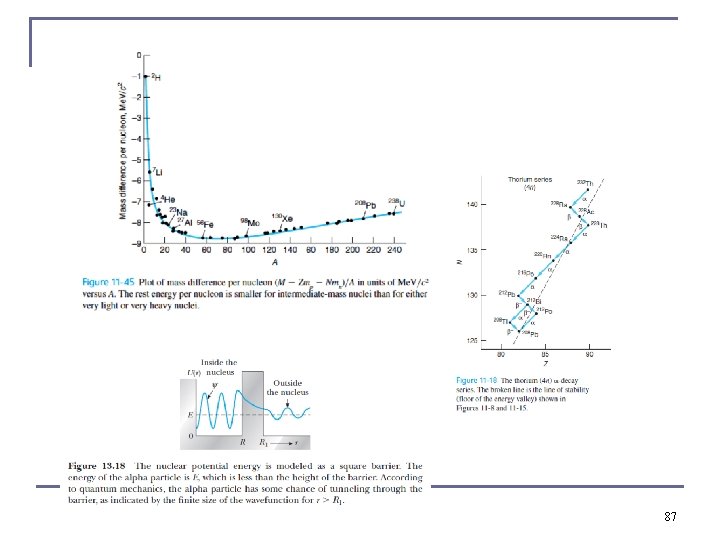
Binding Energy Per Nucleon n n Use this to compare the relative stability of different nuclides. It peaks near A = 56. The curve increases rapidly, demonstrating the saturation effect of nuclear force. Sharp peaks for the even-even nuclides 4 He, 12 C, and 16 O tight bound. 30

31

Nuclear Models n Current research focuses on the constituent quarks and physicists have relied on a multitude of models to explain nuclear force behavior. 1) Independent-particle models: The nucleons move nearly independently in a common nuclear potential. The shell model has been the most successful of these. Strong-interaction models: The nucleons are strongly coupled together. The liquid drop model has been successful in explaining nuclear masses as well as nuclear fission. 2) 32

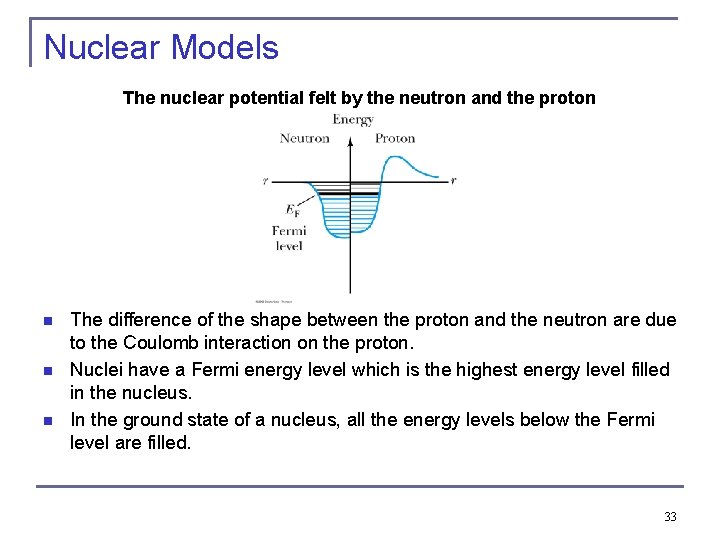
Nuclear Models The nuclear potential felt by the neutron and the proton n The difference of the shape between the proton and the neutron are due to the Coulomb interaction on the proton. Nuclei have a Fermi energy level which is the highest energy level filled in the nucleus. In the ground state of a nucleus, all the energy levels below the Fermi level are filled. 33

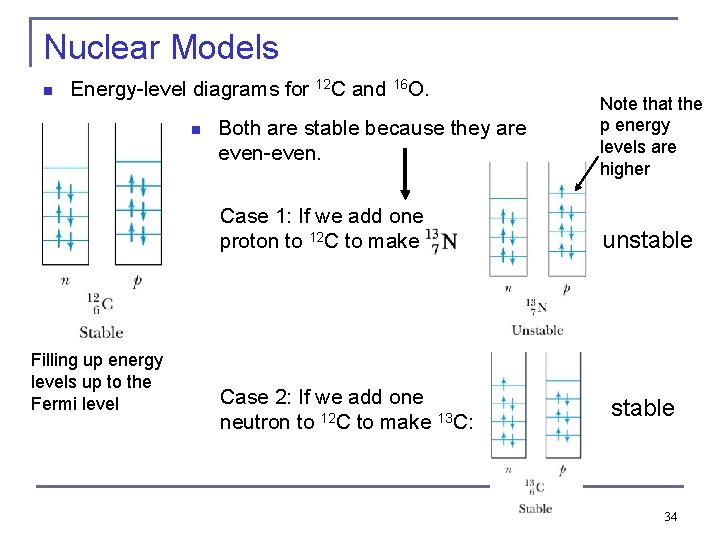
Nuclear Models n Energy-level diagrams for 12 C and 16 O. n Both are stable because they are even-even. Case 1: If we add one proton to 12 C to make Filling up energy levels up to the Fermi level Case 2: If we add one neutron to 12 C to make 13 C: Note that the p energy levels are higher unstable 34

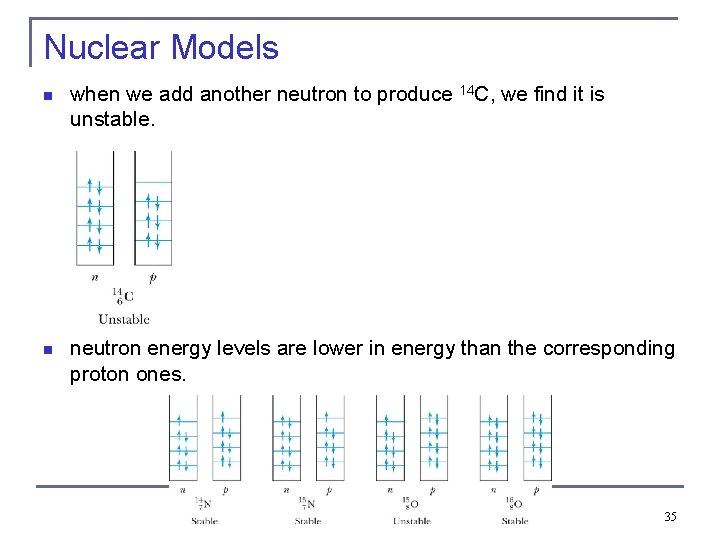
Nuclear Models n when we add another neutron to produce 14 C, we find it is unstable. n neutron energy levels are lower in energy than the corresponding proton ones. 35

12. 6: Radioactive Decay n An empirical law that is fulfilled only statistically n Marie Curie and her husband Pierre discovered polonium and radium in 1898. q q n The simplest decay form is that of a gamma ray, which represents the nucleus changing from an excited state to lower energy state. Other modes of decay include emission of α particles, β (– and +) particles, protons, neutrons, and fission. The decays per unit time (activity). where d. N / dt is negative because total number N decreases with time. 36

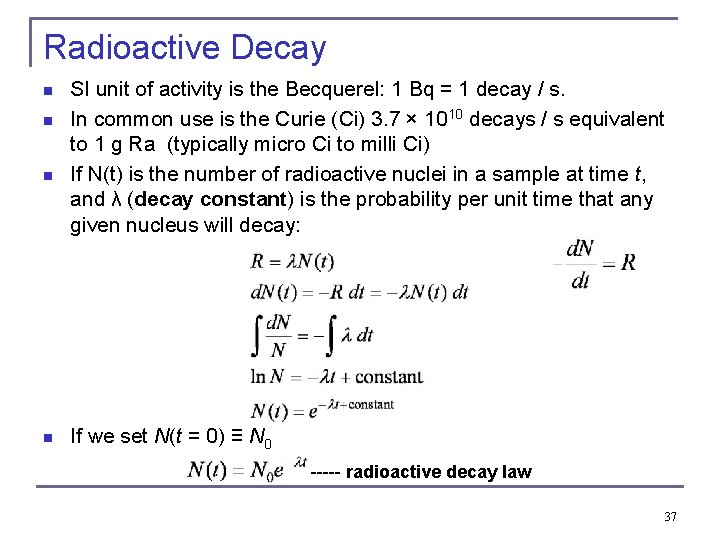
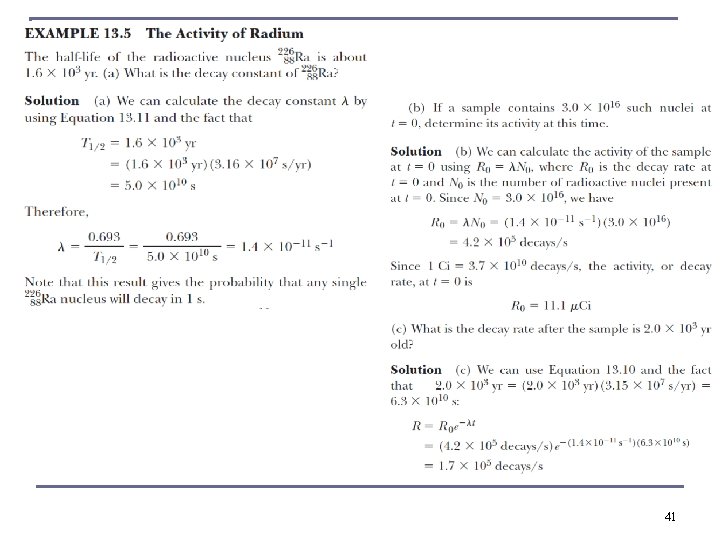
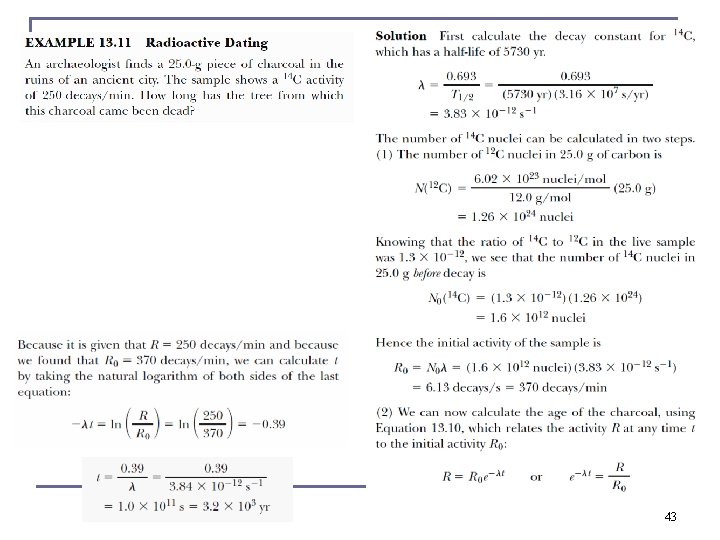
Radioactive Decay n n SI unit of activity is the Becquerel: 1 Bq = 1 decay / s. In common use is the Curie (Ci) 3. 7 × 1010 decays / s equivalent to 1 g Ra (typically micro Ci to milli Ci) If N(t) is the number of radioactive nuclei in a sample at time t, and λ (decay constant) is the probability per unit time that any given nucleus will decay: If we set N(t = 0) ≡ N 0 ----- radioactive decay law 37

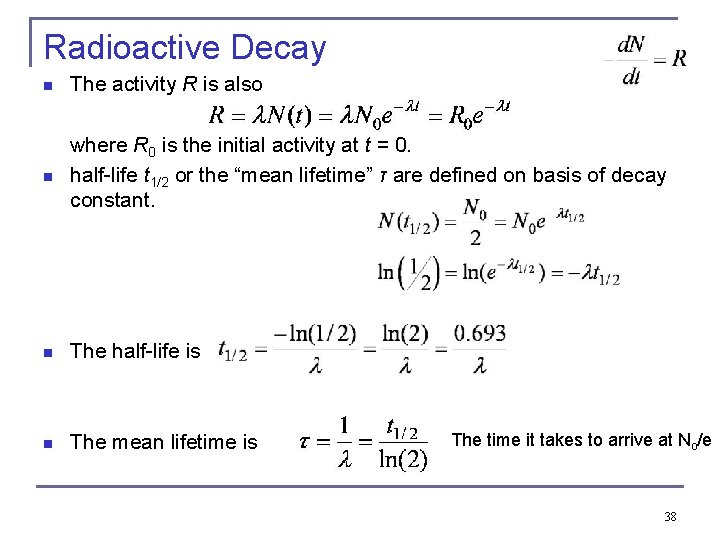
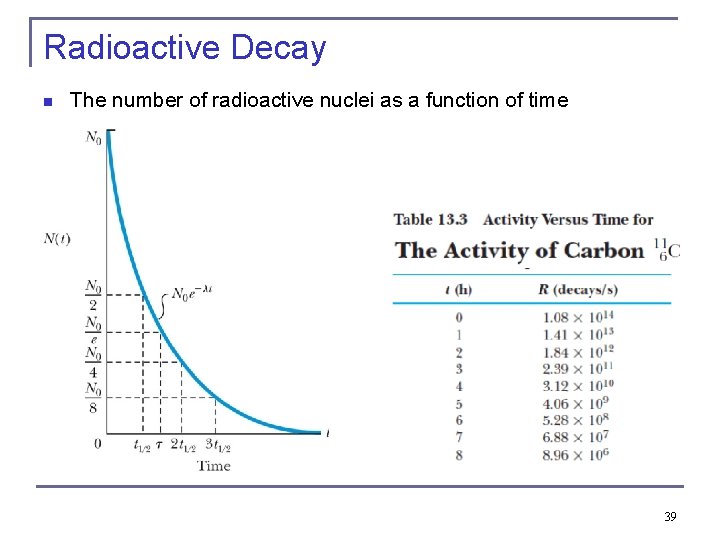
Radioactive Decay n The activity R is also n where R 0 is the initial activity at t = 0. half-life t 1/2 or the “mean lifetime” τ are defined on basis of decay constant. n The half-life is n The mean lifetime is The time it takes to arrive at No/e 38

Radioactive Decay n The number of radioactive nuclei as a function of time 39

40

41

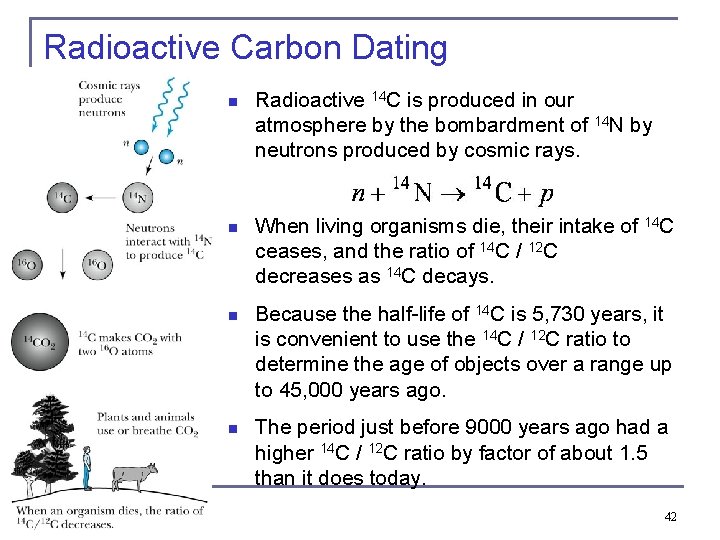
Radioactive Carbon Dating n Radioactive 14 C is produced in our atmosphere by the bombardment of 14 N by neutrons produced by cosmic rays. n When living organisms die, their intake of 14 C ceases, and the ratio of 14 C / 12 C decreases as 14 C decays. n Because the half-life of 14 C is 5, 730 years, it is convenient to use the 14 C / 12 C ratio to determine the age of objects over a range up to 45, 000 years ago. n The period just before 9000 years ago had a higher 14 C / 12 C ratio by factor of about 1. 5 than it does today. 42

43

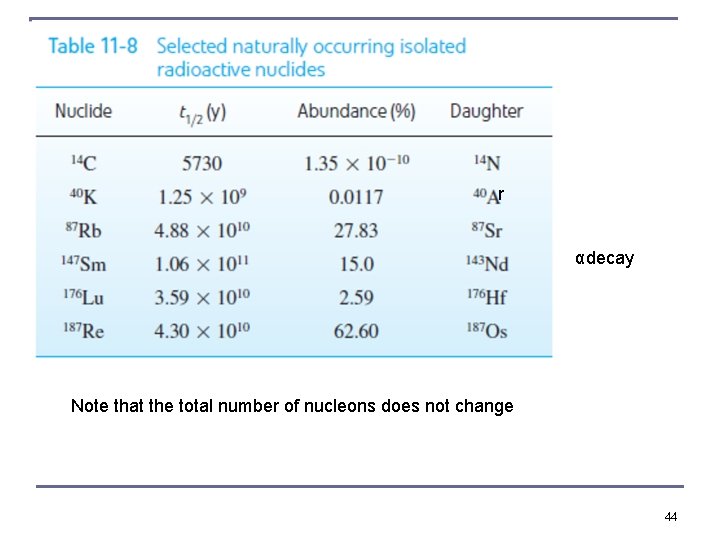
r αdecay Note that the total number of nucleons does not change 44

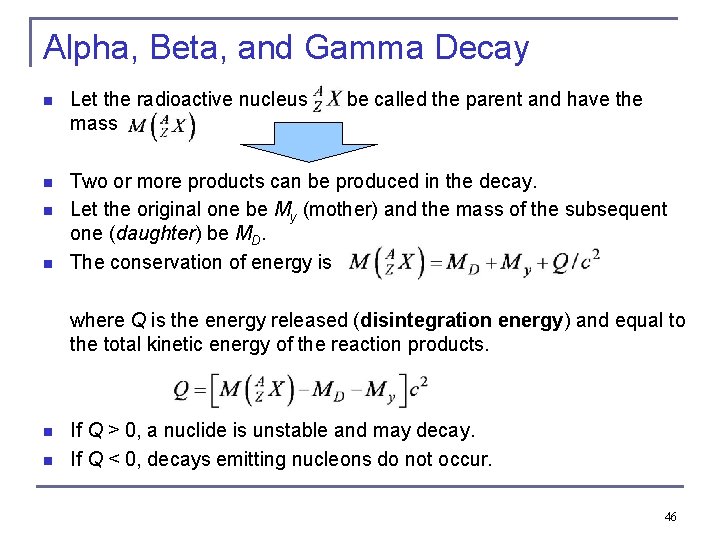
12. 7: Alpha, Beta, and Gamma Decay When a nucleus decays, all the conservation laws must be observed: n Mass-energy n Linear momentum n Angular momentum n Electric charge n Conservation of nucleons q The total number of nucleons (A, the mass number) must be conserved in a typical (relatively low energy) nuclear reaction or decay. 45

Alpha, Beta, and Gamma Decay n Let the radioactive nucleus mass n Two or more products can be produced in the decay. Let the original one be My (mother) and the mass of the subsequent one (daughter) be MD. The conservation of energy is n n be called the parent and have the where Q is the energy released (disintegration energy) and equal to the total kinetic energy of the reaction products. n n If Q > 0, a nuclide is unstable and may decay. If Q < 0, decays emitting nucleons do not occur. 46

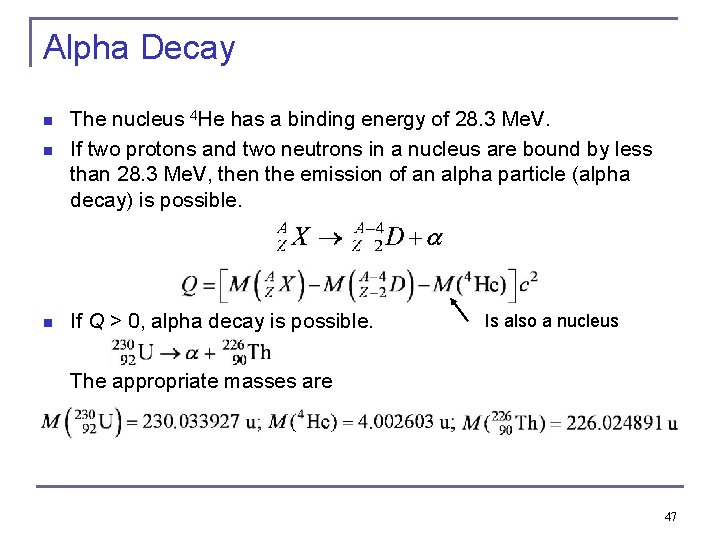
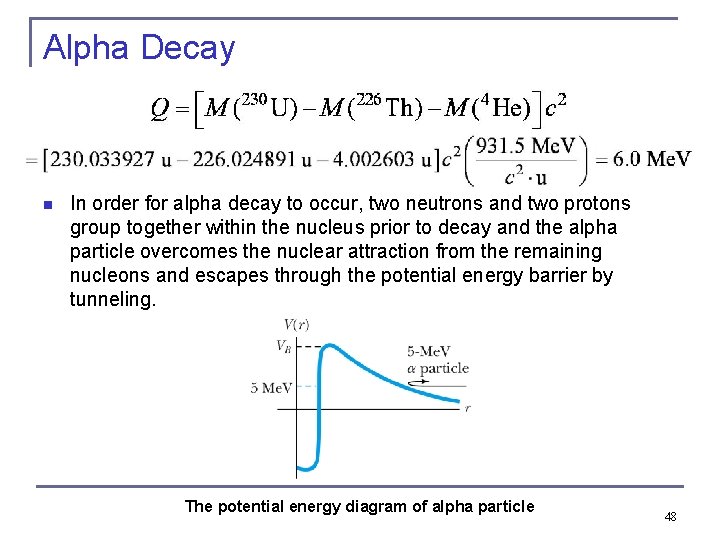
Alpha Decay n n n The nucleus 4 He has a binding energy of 28. 3 Me. V. If two protons and two neutrons in a nucleus are bound by less than 28. 3 Me. V, then the emission of an alpha particle (alpha decay) is possible. If Q > 0, alpha decay is possible. Is also a nucleus The appropriate masses are 47

Alpha Decay n In order for alpha decay to occur, two neutrons and two protons group together within the nucleus prior to decay and the alpha particle overcomes the nuclear attraction from the remaining nucleons and escapes through the potential energy barrier by tunneling. The potential energy diagram of alpha particle 48

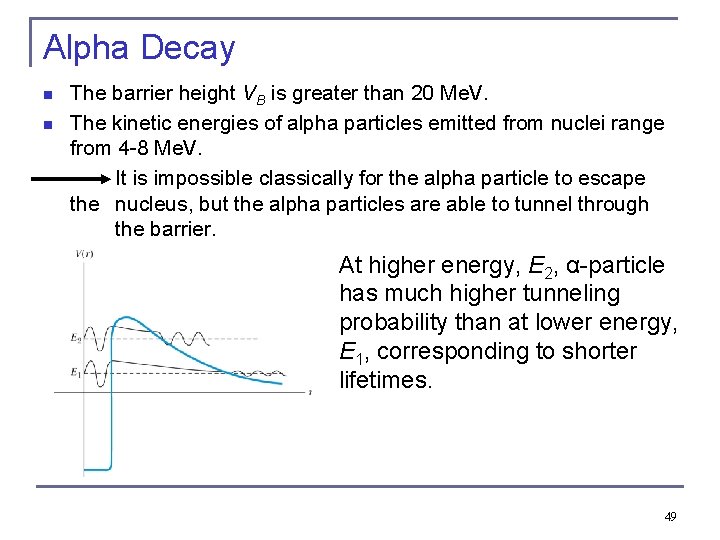
Alpha Decay n n The barrier height VB is greater than 20 Me. V. The kinetic energies of alpha particles emitted from nuclei range from 4 -8 Me. V. It is impossible classically for the alpha particle to escape the nucleus, but the alpha particles are able to tunnel through the barrier. At higher energy, E 2, α-particle has much higher tunneling probability than at lower energy, E 1, corresponding to shorter lifetimes. 49

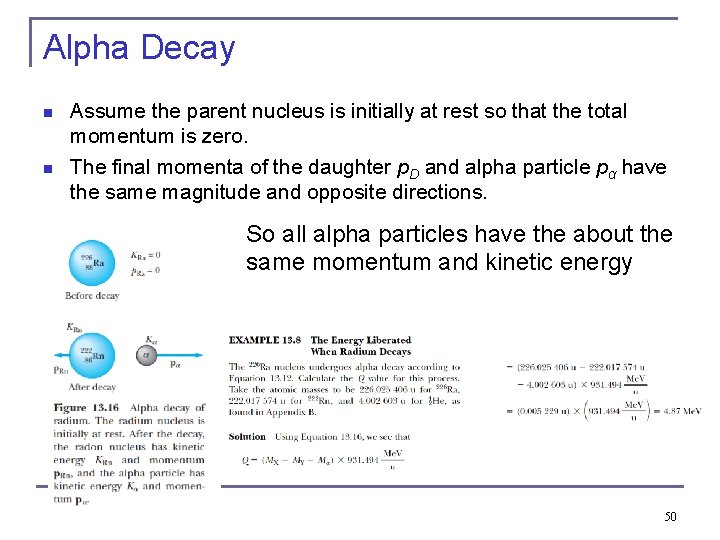
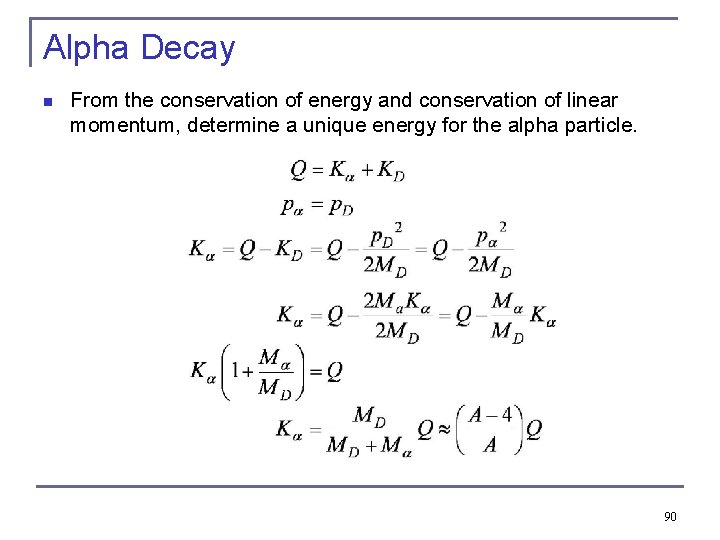
Alpha Decay n n Assume the parent nucleus is initially at rest so that the total momentum is zero. The final momenta of the daughter p. D and alpha particle pα have the same magnitude and opposite directions. So all alpha particles have the about the same momentum and kinetic energy 50

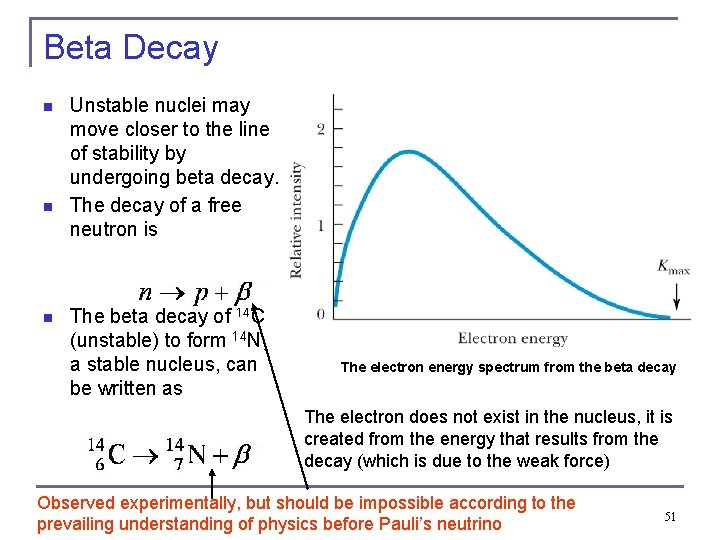
Beta Decay n n n Unstable nuclei may move closer to the line of stability by undergoing beta decay. The decay of a free neutron is The beta decay of 14 C (unstable) to form 14 N, a stable nucleus, can be written as The electron energy spectrum from the beta decay The electron does not exist in the nucleus, it is created from the energy that results from the decay (which is due to the weak force) Observed experimentally, but should be impossible according to the prevailing understanding of physics before Pauli’s neutrino 51

Beta Decay n There was a problem in neutron decay, the spin ½ neutron cannot decay to two spin ½ particles, a proton and an electron. 14 C has spin 0, 14 N has spin 1, and the electron has spin ½. we cannot combine spin ½ & 1 to obtain a spin 0. n Wolfgang Pauli proposed a new particle, the neutrino, that must be produced in beta decay. It has spin quantum number ½, charge 0, and carries away the energy that is apparently missing in the fig. in the previous slide Momentum seems not to be conserved either, so Pauli predicts a particle that must exist and carry away just the right amount of energy so that the conservation principle of total energy is not conserved, that way, momentum will also be conserved and everything is Oki-Doki again n That particle will actually be much later experimentally observed 52

Beta Decay n An occasional electron is detected with the kinetic energy close to the required Kmax (to conserve energy), but in most cases the electron’s kinetic energy is less than Kmax. the neutrino has very little mass, and most of its energy is kinetic. n Neutrinos have no charge and do not interact electromagnetically. They are not affected by the strong force of the nucleus. They are due to the weak interaction (result from the weak force). The unification of the electromagnetic and weak force is the electroweak force. n n n 53

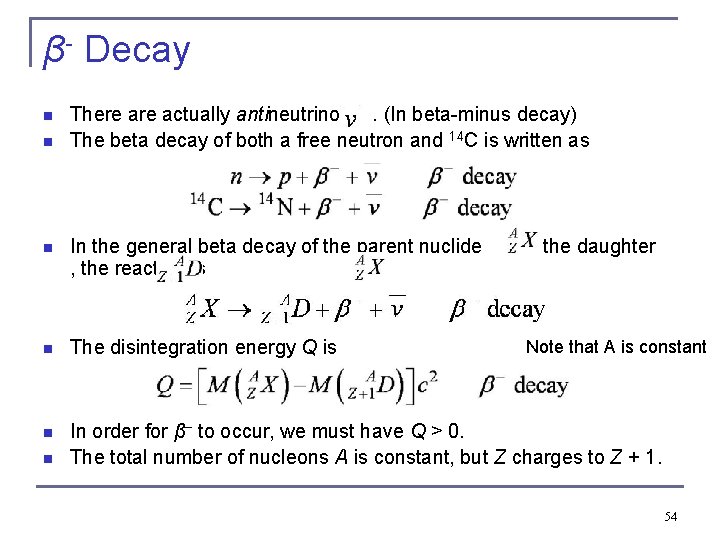
β- Decay n n There actually antineutrinos. (In beta-minus decay) The beta decay of both a free neutron and 14 C is written as n In the general beta decay of the parent nuclide , the reaction is to the daughter n The disintegration energy Q is Note that A is constant n In order for β− to occur, we must have Q > 0. The total number of nucleons A is constant, but Z charges to Z + 1. n 54

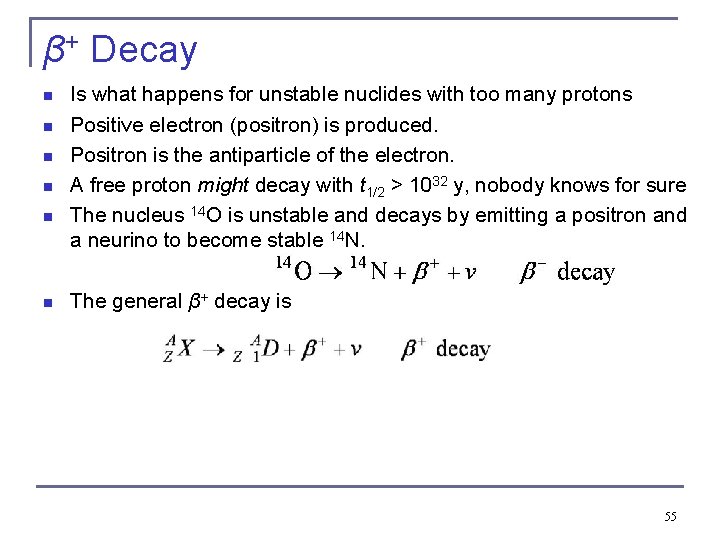
β+ Decay n n n Is what happens for unstable nuclides with too many protons Positive electron (positron) is produced. Positron is the antiparticle of the electron. A free proton might decay with t 1/2 > 1032 y, nobody knows for sure The nucleus 14 O is unstable and decays by emitting a positron and a neurino to become stable 14 N. The general β+ decay is 55

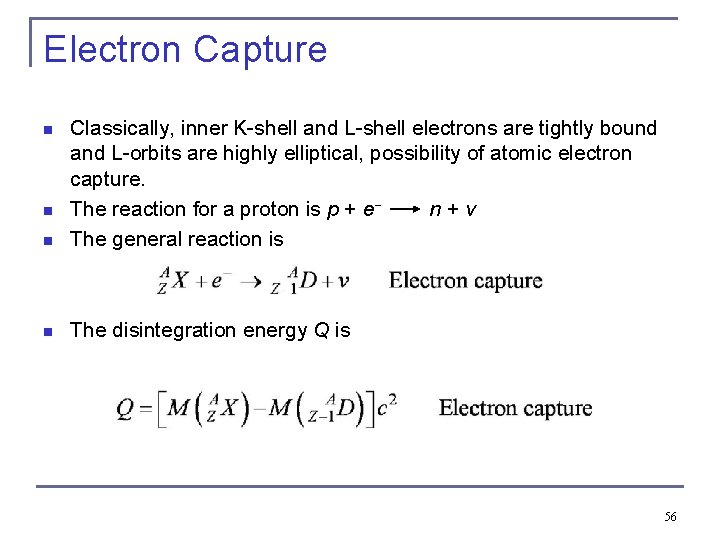
Electron Capture n Classically, inner K-shell and L-shell electrons are tightly bound and L-orbits are highly elliptical, possibility of atomic electron capture. The reaction for a proton is p + e− n+v The general reaction is n The disintegration energy Q is n n 56

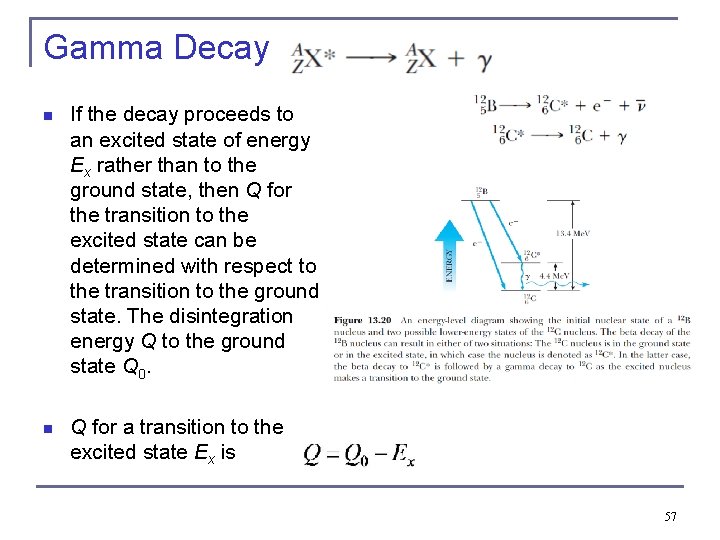
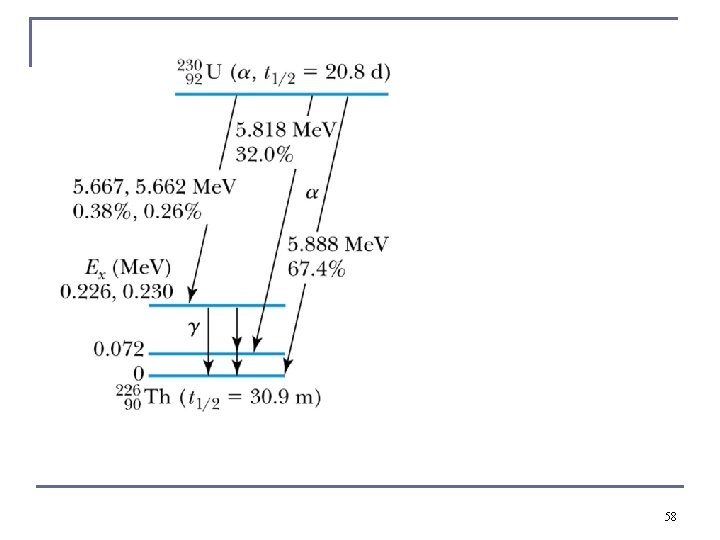
Gamma Decay n If the decay proceeds to an excited state of energy Ex rather than to the ground state, then Q for the transition to the excited state can be determined with respect to the transition to the ground state. The disintegration energy Q to the ground state Q 0. n Q for a transition to the excited state Ex is 57

58

Gamma Decay n n n The excitation energies tend to be much larger, many ke. V or even Me. V. The possibilities for the nucleus to rid itself of this extra energy is to emit a very high energy photon (gamma ray). The gamma-ray energy hf is given by the difference of the higher energy state E> and lower one E<. n The decay of an excited state of AX* (where * is an excited state) to its ground state is n A transition between two nuclear excited states E> and E< is 59

Gamma Decay n The gamma rays are normally emitted soon after a nucleus is put into an excited state. n Sometimes selection rules prohibit a certain transition, and the excited state may live for a long time. These states are called isomers or isomeric states and are denoted by a small m for metastable. n n Example: one state of at 0. 271 Me. V excitation energy does not gamma decay because of a large spin difference transition. 60

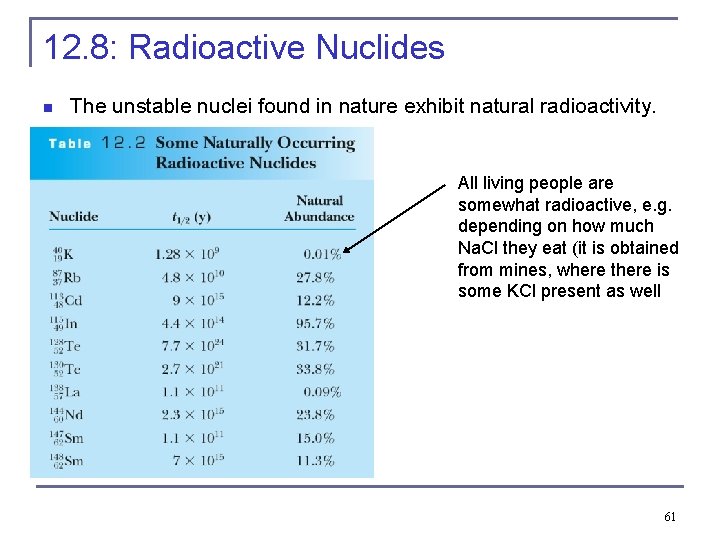
12. 8: Radioactive Nuclides n The unstable nuclei found in nature exhibit natural radioactivity. All living people are somewhat radioactive, e. g. depending on how much Na. Cl they eat (it is obtained from mines, where there is some KCl present as well 61

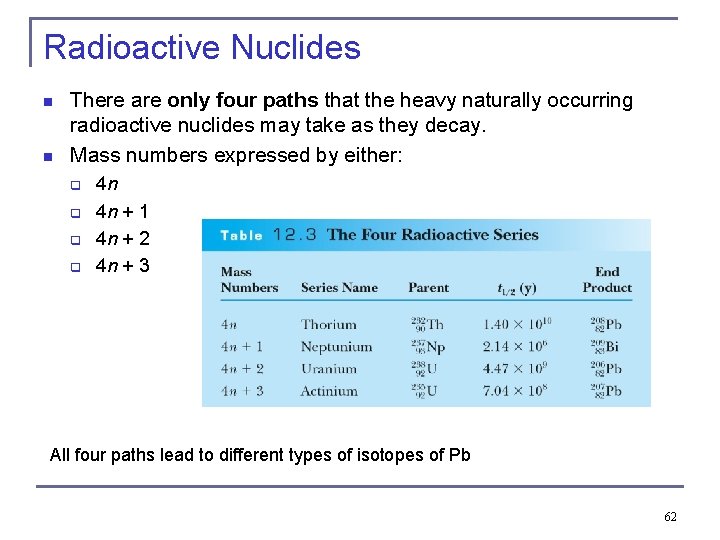
Radioactive Nuclides n n There are only four paths that the heavy naturally occurring radioactive nuclides may take as they decay. Mass numbers expressed by either: q q 4 n 4 n + 1 4 n + 2 4 n + 3 All four paths lead to different types of isotopes of Pb 62

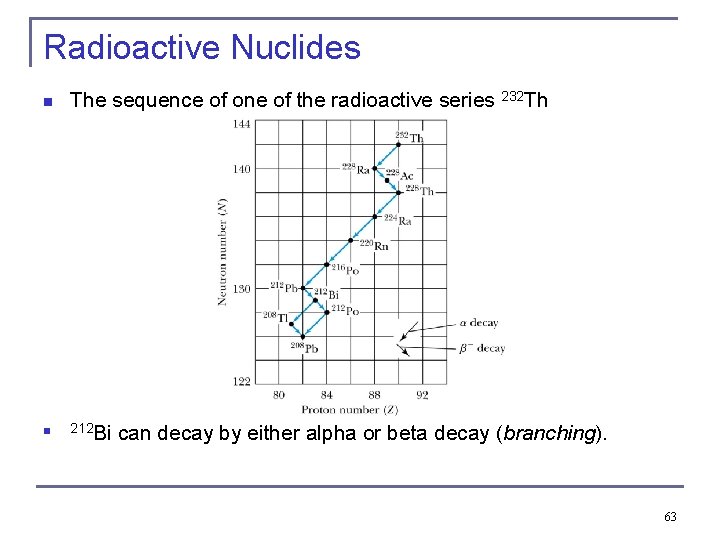
Radioactive Nuclides n The sequence of one of the radioactive series 232 Th n 212 Bi can decay by either alpha or beta decay (branching). 63

Time Dating Using Lead Isotopes n A plot of the abundance ratio of 206 Pb / 204 Pb versus 207 Pb / 204 Pb can be a sensitive indicator of the age of lead ores. Such techniques have been used to show that meteorites and the earth, believed to be left over from the formation of the solar system, are 4. 55 billion years old. 64

65

66

67

68

69

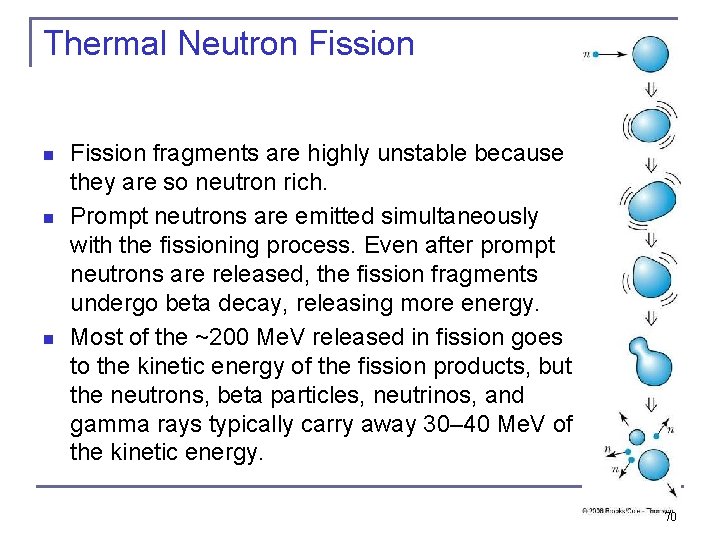
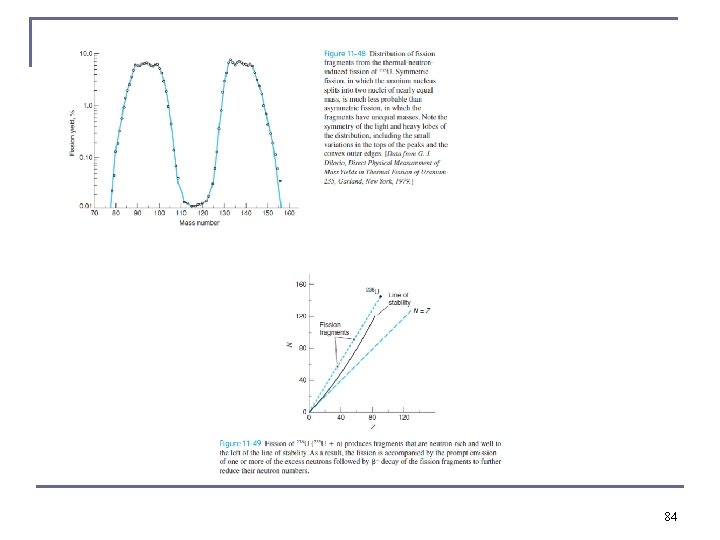
Thermal Neutron Fission fragments are highly unstable because they are so neutron rich. Prompt neutrons are emitted simultaneously with the fissioning process. Even after prompt neutrons are released, the fission fragments undergo beta decay, releasing more energy. Most of the ~200 Me. V released in fission goes to the kinetic energy of the fission products, but the neutrons, beta particles, neutrinos, and gamma rays typically carry away 30– 40 Me. V of the kinetic energy. 70

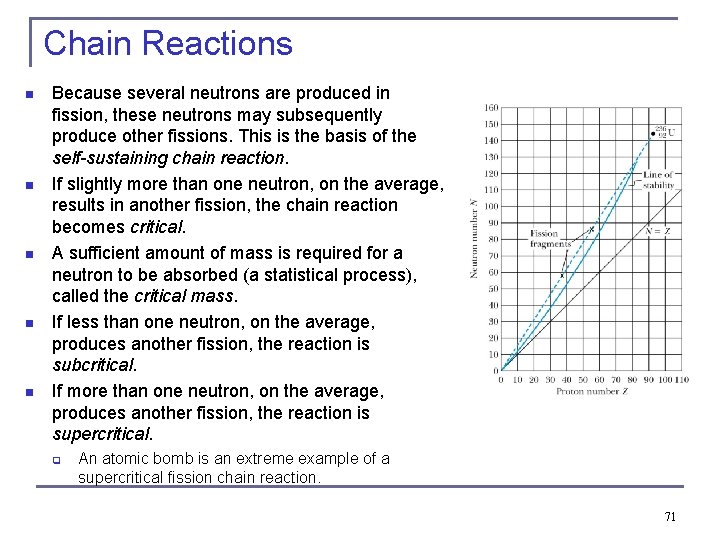
Chain Reactions n n n Because several neutrons are produced in fission, these neutrons may subsequently produce other fissions. This is the basis of the self-sustaining chain reaction. If slightly more than one neutron, on the average, results in another fission, the chain reaction becomes critical. A sufficient amount of mass is required for a neutron to be absorbed (a statistical process), called the critical mass. If less than one neutron, on the average, produces another fission, the reaction is subcritical. If more than one neutron, on the average, produces another fission, the reaction is supercritical. q An atomic bomb is an extreme example of a supercritical fission chain reaction. 71

Chain Reactions n A critical fission reaction can be controlled by absorbing neutrons. A self-sustaining controlled fission process requires that not all the neutrons are prompt. Some of the neutrons are delayed by several seconds and are emitted by daughter nuclides. These delayed neutrons allow the control of the nuclear reactor. n Control rods regulate the absorption of neutrons to sustain a controlled reaction. 72

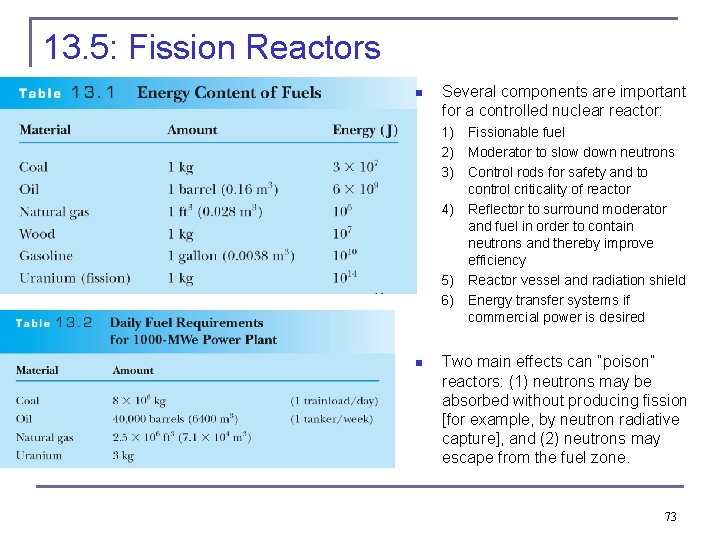
13. 5: Fission Reactors n Several components are important for a controlled nuclear reactor: 1) 2) 3) 4) 5) 6) n Fissionable fuel Moderator to slow down neutrons Control rods for safety and to control criticality of reactor Reflector to surround moderator and fuel in order to contain neutrons and thereby improve efficiency Reactor vessel and radiation shield Energy transfer systems if commercial power is desired Two main effects can “poison” reactors: (1) neutrons may be absorbed without producing fission [for example, by neutron radiative capture], and (2) neutrons may escape from the fuel zone. 73

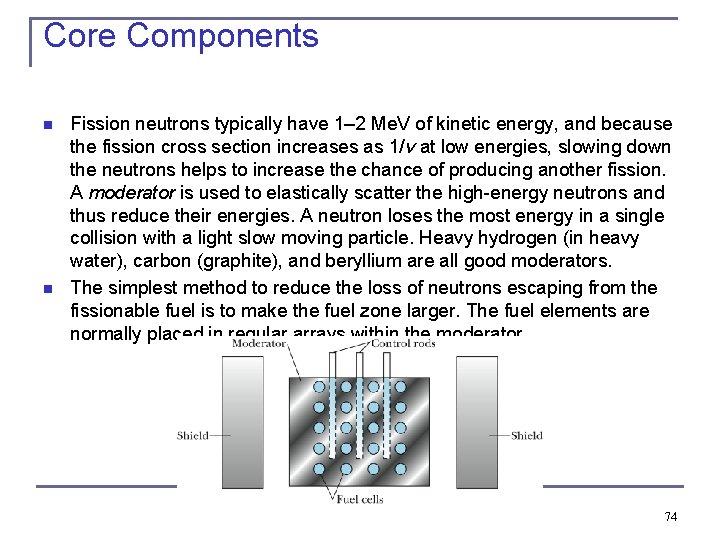
Core Components n n Fission neutrons typically have 1– 2 Me. V of kinetic energy, and because the fission cross section increases as 1/v at low energies, slowing down the neutrons helps to increase the chance of producing another fission. A moderator is used to elastically scatter the high-energy neutrons and thus reduce their energies. A neutron loses the most energy in a single collision with a light slow moving particle. Heavy hydrogen (in heavy water), carbon (graphite), and beryllium are all good moderators. The simplest method to reduce the loss of neutrons escaping from the fissionable fuel is to make the fuel zone larger. The fuel elements are normally placed in regular arrays within the moderator. 74

Core Components n n The delayed neutrons produced in fission allow the mechanical movement of the rods to control the fission reaction. A “fail-safe” system automatically drops the control rods into the reactor in an emergency shutdown. If the fuel and moderator are surrounded by a material with a very low neutron capture cross section, there is a reasonable chance that after one or even many scatterings, the neutron will be backscattered or “reflected” back into the fuel area. Water is often used both as moderator and reflector. 75

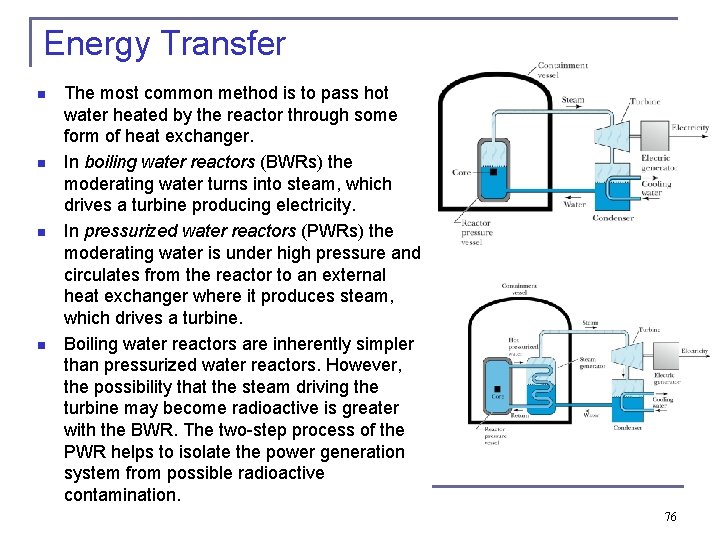
Energy Transfer n n The most common method is to pass hot water heated by the reactor through some form of heat exchanger. In boiling water reactors (BWRs) the moderating water turns into steam, which drives a turbine producing electricity. In pressurized water reactors (PWRs) the moderating water is under high pressure and circulates from the reactor to an external heat exchanger where it produces steam, which drives a turbine. Boiling water reactors are inherently simpler than pressurized water reactors. However, the possibility that the steam driving the turbine may become radioactive is greater with the BWR. The two-step process of the PWR helps to isolate the power generation system from possible radioactive contamination. 76

Types of Reactors n n n Power reactors produce commercial electricity. Research reactors are operated to produce high neutron fluxes for neutron-scattering experiments. Heat production reactors supply heat in some cold countries. Some reactors are designed to produce radioisotopes. Several training reactors are located on college campuses. 77

Nuclear Reactor Problems n n n The danger of a serious accident in which radioactive elements are released into the atmosphere or groundwater is of great concern to the general public. Thermal pollution both in the atmosphere and in lakes and rivers used for cooling may be a significant ecological problem. A more serious problem is the safe disposal of the radioactive wastes produced in the fissioning process, because some fission fragments have a half-life of thousands of years. Two widely publicized accidents at nuclear reactor facilities—one at Three Mile Island in Pennsylvania in 1979, the other at Chernobyl in Ukraine in 1986—have significantly dampened the general public’s support for nuclear reactors. Large expansion of nuclear power can succeed only if four critical problems are overcome: lower costs, improved safety, better nuclear waste management, and lower proliferation risk. 78

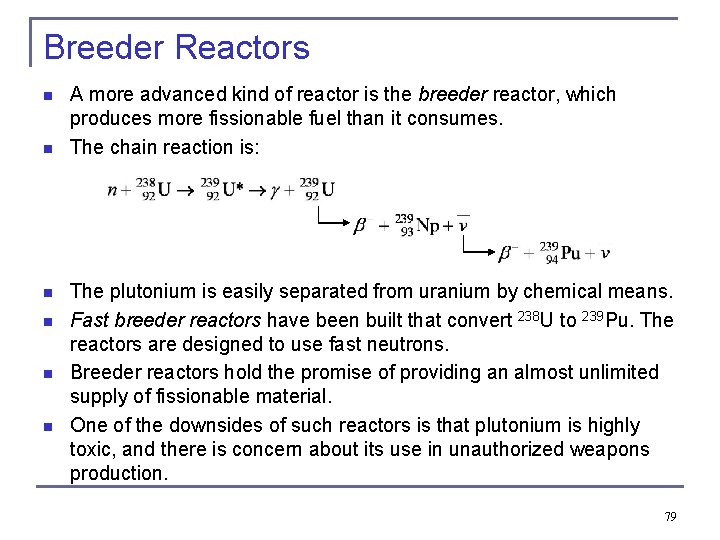
Breeder Reactors n n n A more advanced kind of reactor is the breeder reactor, which produces more fissionable fuel than it consumes. The chain reaction is: The plutonium is easily separated from uranium by chemical means. Fast breeder reactors have been built that convert 238 U to 239 Pu. The reactors are designed to use fast neutrons. Breeder reactors hold the promise of providing an almost unlimited supply of fissionable material. One of the downsides of such reactors is that plutonium is highly toxic, and there is concern about its use in unauthorized weapons production. 79

13. 6: Fusion n If two light nuclei fuse together, they also form a nucleus with a larger binding energy per nucleon and energy is released. This reaction is called nuclear fusion. n The most energy is released if two isotopes of hydrogen fuse together in the reaction. 80

The European Fusion project, 1991 81

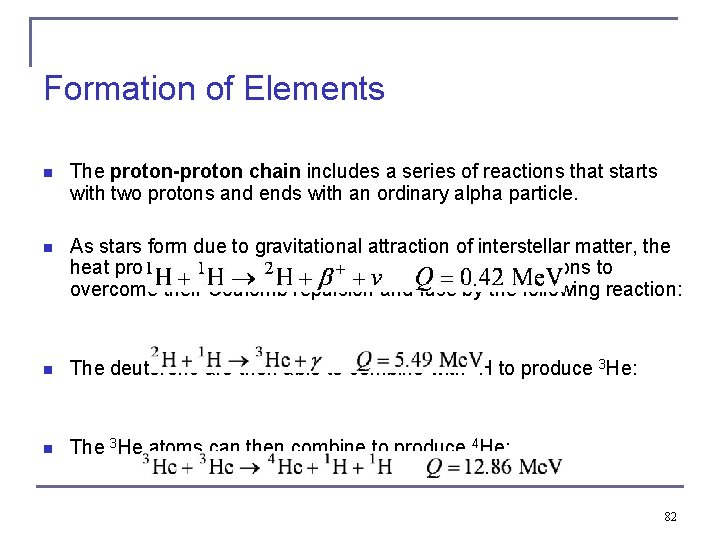
Formation of Elements n The proton-proton chain includes a series of reactions that starts with two protons and ends with an ordinary alpha particle. n As stars form due to gravitational attraction of interstellar matter, the heat produced by the attraction is enough to cause protons to overcome their Coulomb repulsion and fuse by the following reaction: n The deuterons are then able to combine with 1 H to produce 3 He: n The 3 He atoms can then combine to produce 4 He: 82

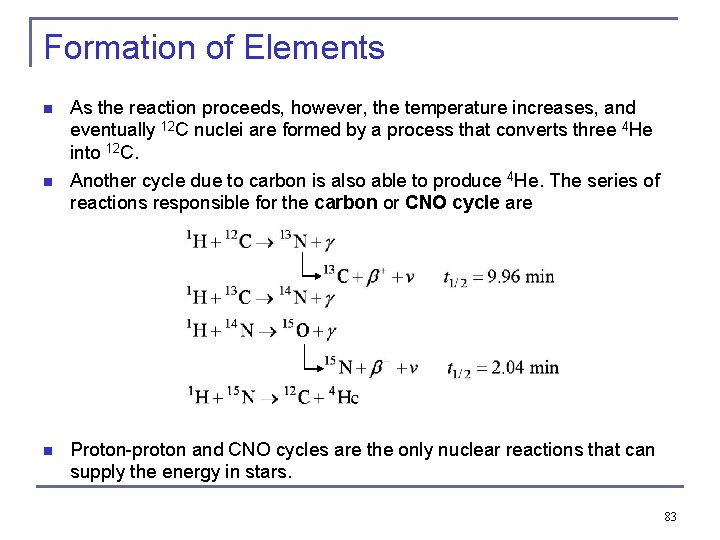
Formation of Elements n n n As the reaction proceeds, however, the temperature increases, and eventually 12 C nuclei are formed by a process that converts three 4 He into 12 C. Another cycle due to carbon is also able to produce 4 He. The series of reactions responsible for the carbon or CNO cycle are Proton-proton and CNO cycles are the only nuclear reactions that can supply the energy in stars. 83

84

85

86

87

88

89

Alpha Decay n From the conservation of energy and conservation of linear momentum, determine a unique energy for the alpha particle. 90

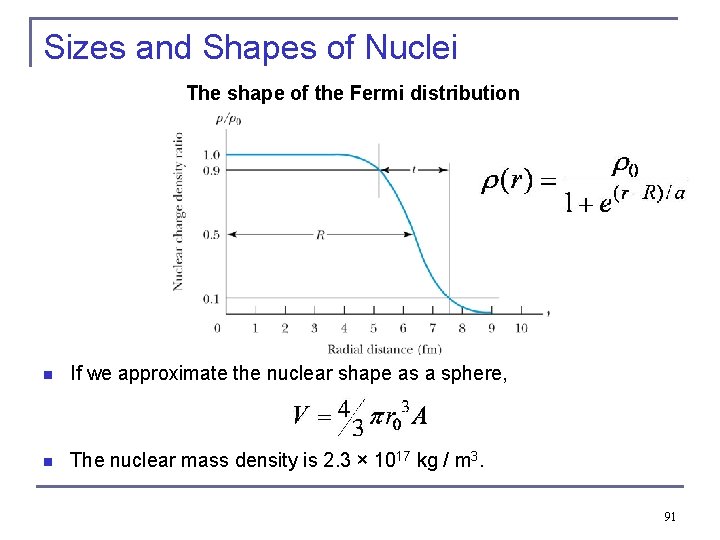
Sizes and Shapes of Nuclei The shape of the Fermi distribution n If we approximate the nuclear shape as a sphere, n The nuclear mass density is 2. 3 × 1017 kg / m 3. 91