CHAPTER4 ELECTROLYSIS PROCESS Monday January 3 2022 Electrolysis









- Slides: 28

CHAPTER-4 ELECTROLYSIS PROCESS

Monday, January 3, 2022 Electrolysis To understand electrolysis

Metal Ores Metals are extracted from metal ores via: Chemical reduction (using carbon) Electrolysis Metals which are higher than carbon in the reactivity series have to be extracted using electrolysis – what are these metals? Potassium Sodium Calcium Magnesium Aluminium *Metals below carbon can be extracted via reduction using carbon as it can only take the oxygen away from the metal oxide if the metal is less reactive than the carbon itself

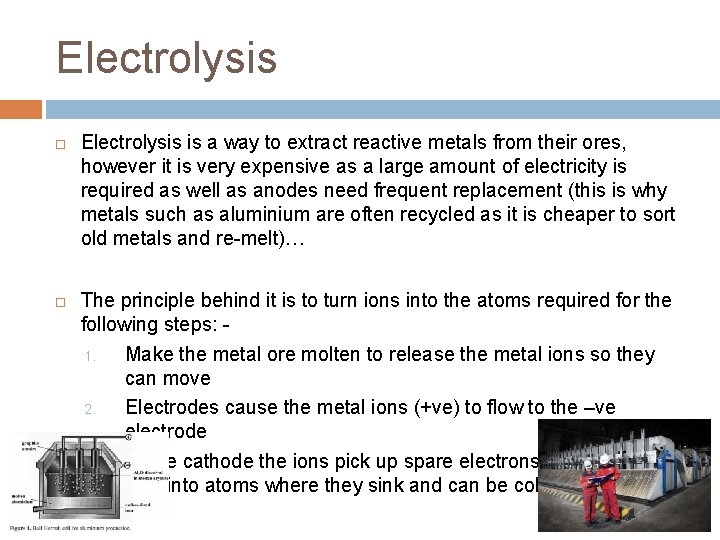
Electrolysis is a way to extract reactive metals from their ores, however it is very expensive as a large amount of electricity is required as well as anodes need frequent replacement (this is why metals such as aluminium are often recycled as it is cheaper to sort old metals and re-melt)… The principle behind it is to turn ions into the atoms required for the following steps: 1. Make the metal ore molten to release the metal ions so they can move 2. Electrodes cause the metal ions (+ve) to flow to the –ve electrode 3. At the cathode the ions pick up spare electrons turning from ions into atoms where they sink and can be collected

Ionic Ionic substances form when a metal reacts with a non-metal – they contain charged particles called ions For example, sodium chloride forms when sodium reacts with chlorine – it contains positively charged sodium ions and negatively charged chloride ions Ionic substances can be broken down by electricity

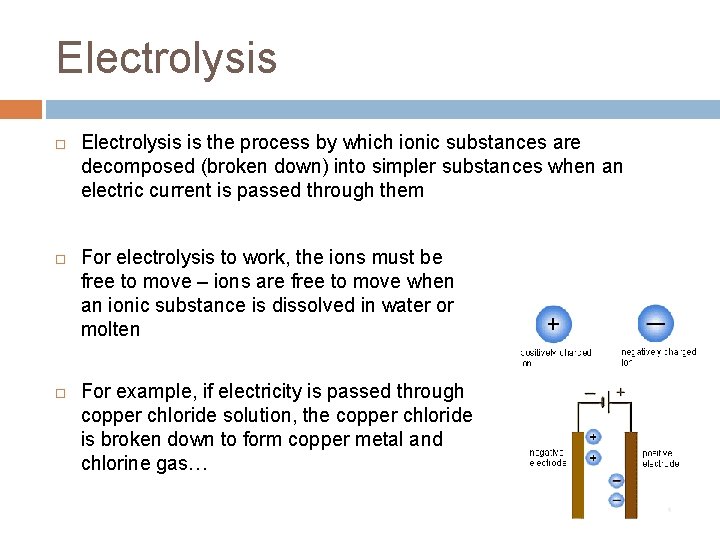
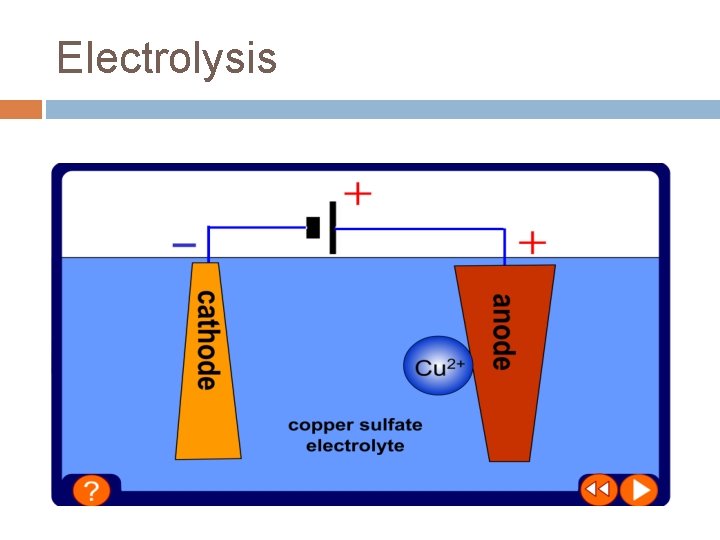
Electrolysis Electrolysis is the process by which ionic substances are decomposed (broken down) into simpler substances when an electric current is passed through them For electrolysis to work, the ions must be free to move – ions are free to move when an ionic substance is dissolved in water or molten For example, if electricity is passed through copper chloride solution, the copper chloride is broken down to form copper metal and chlorine gas…

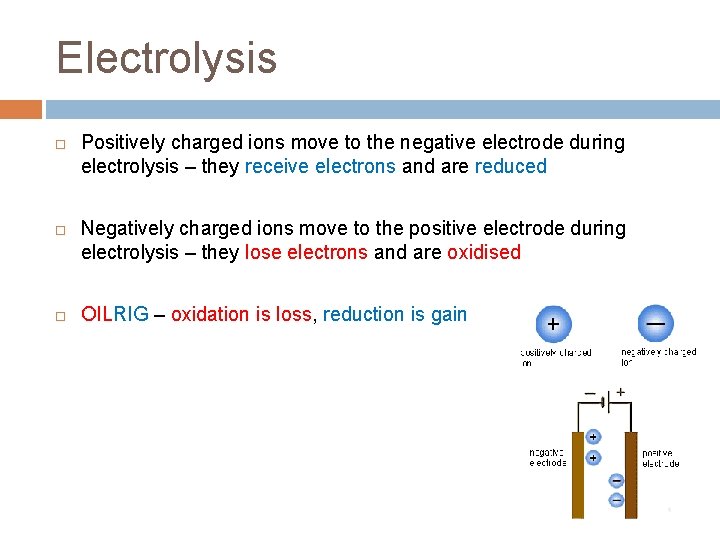
Electrolysis Positively charged ions move to the negative electrode during electrolysis – they receive electrons and are reduced Negatively charged ions move to the positive electrode during electrolysis – they lose electrons and are oxidised OILRIG – oxidation is loss, reduction is gain

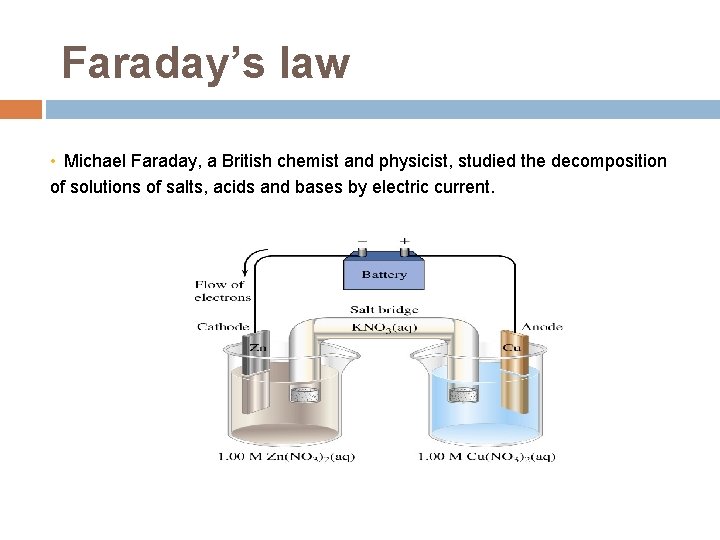
Faraday’s law • Michael Faraday, a British chemist and physicist, studied the decomposition of solutions of salts, acids and bases by electric current.

Faraday's law of electrolysis states that the amount of any substance that is deposited or dissolved in electrolysis is directly proportional to the total passed electric charge. It can be expressed as F is Faraday constant. F = Le = 96485. 309 C·mol-1 = 1 Faraday.

Electrolysis

Electrolysis Products Ionic substances in solution break down into elements during electrolysis – different elements are released depending on the particular ionic substance…

Negative Electrode At the negative electrode, positively charged ions gain electrons – this is reduction (ions have been reduced) Metal ions and hydrogen ions are positively charged – whether you get the metal or hydrogen during electrolysis depends on the position of the metal in the reactivity series: The metal will be produced if it is less reactive than hydrogen Hydrogen will be produced if the metal is more reactive than hydrogen E. g. the electrolysis of copper chloride solution produces copper at the negative electrode, but the electrolysis of sodium chloride solution produces

Positive Electrode At the positive electrode, negatively charged ions lose electrons This is oxidation – the ions have been oxidised -ve Ion In Solution Element Given Off At +ve Electrode Chloride, Cl- Chlorine, Cl 2 Bromide, Br- Bromine, Br 2 Iodide, I- Iodine, I 2 Sulfate, SO 4 -2 Oxygen, O 2

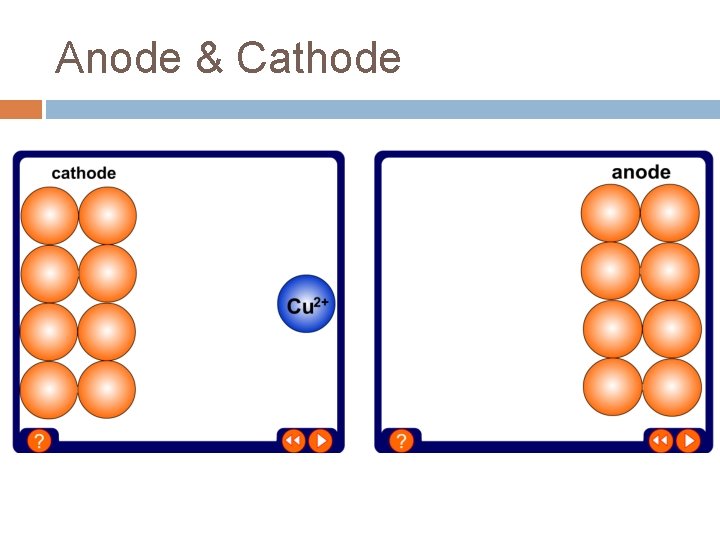
Anode & Cathode

Metallurgy the mineral must first be separated from the surrounding ore material by physical means Extractive metallurgy are the chemical processes that separate a metal from its mineral Refining are the processes that purify the metal for use 15

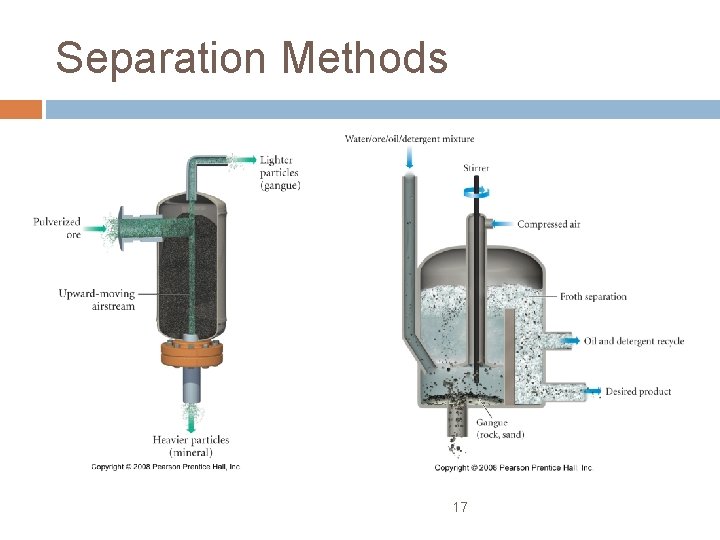
Separation first step is to crush the ore into small particles the mineral is then separated from the gangue by physical means using cyclonic winds to separate by density froth flotation in which the mineral is treated with a wetting agent to make it more attracted to the froth 16

Separation Methods 17

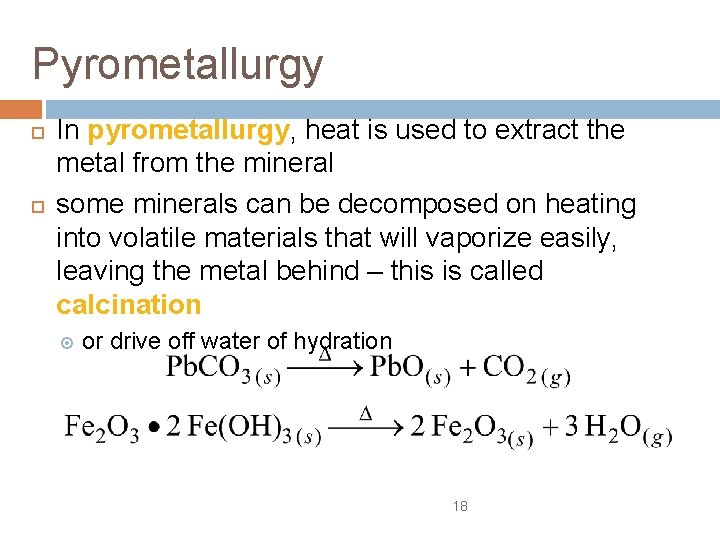
Pyrometallurgy In pyrometallurgy, heat is used to extract the metal from the mineral some minerals can be decomposed on heating into volatile materials that will vaporize easily, leaving the metal behind – this is called calcination or drive off water of hydration 18

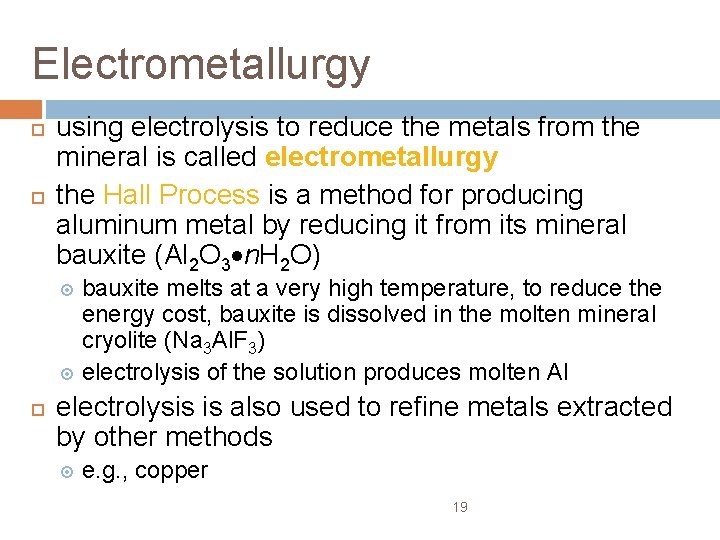
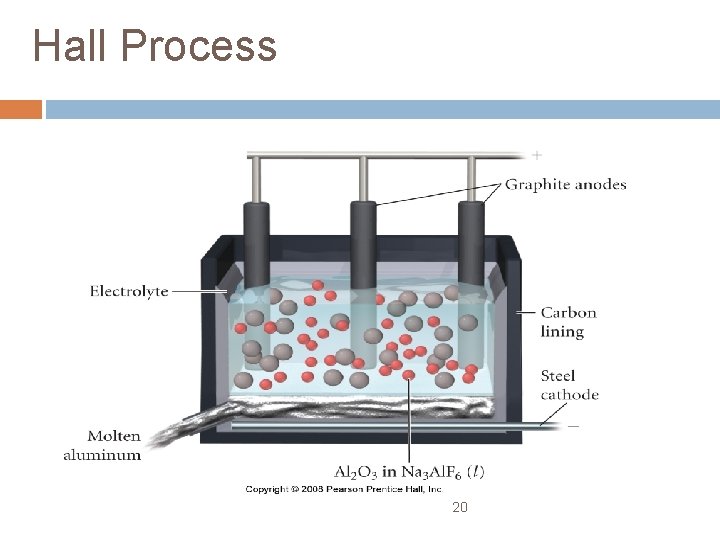
Electrometallurgy using electrolysis to reduce the metals from the mineral is called electrometallurgy the Hall Process is a method for producing aluminum metal by reducing it from its mineral bauxite (Al 2 O 3 n. H 2 O) bauxite melts at a very high temperature, to reduce the energy cost, bauxite is dissolved in the molten mineral cryolite (Na 3 Al. F 3) electrolysis of the solution produces molten Al electrolysis is also used to refine metals extracted by other methods e. g. , copper 19

Hall Process 20

Copper is a good conductor of electricity, and is used extensively to make electrical wiring and components The extraction of copper from copper ore is done by reduction with carbon, however, the copper produced is not pure enough for use as a conductor, so it is purified using electrolysis

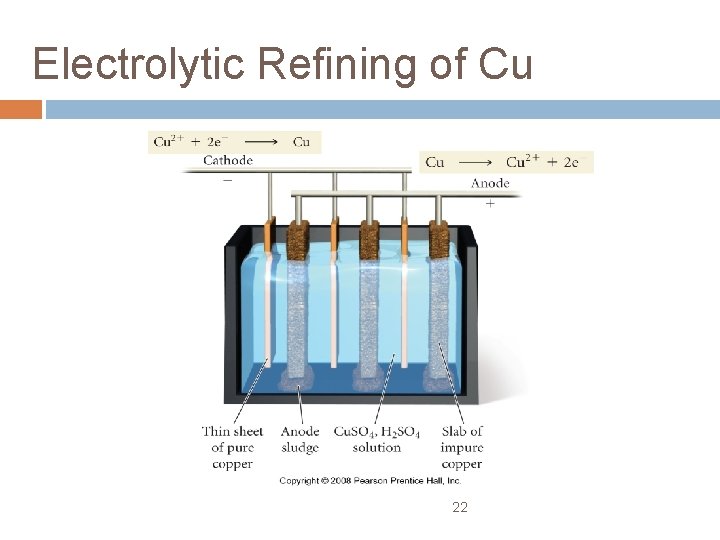
Electrolytic Refining of Cu 22

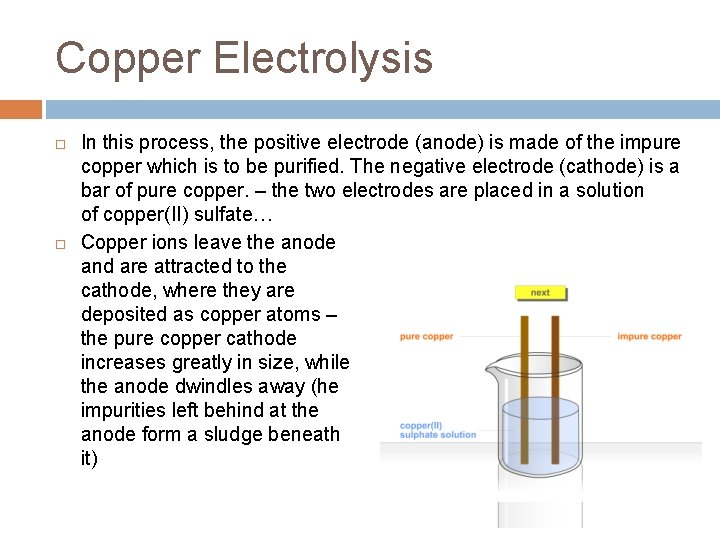
Copper Electrolysis In this process, the positive electrode (anode) is made of the impure copper which is to be purified. The negative electrode (cathode) is a bar of pure copper. – the two electrodes are placed in a solution of copper(II) sulfate… Copper ions leave the anode and are attracted to the cathode, where they are deposited as copper atoms – the pure copper cathode increases greatly in size, while the anode dwindles away (he impurities left behind at the anode form a sludge beneath it)

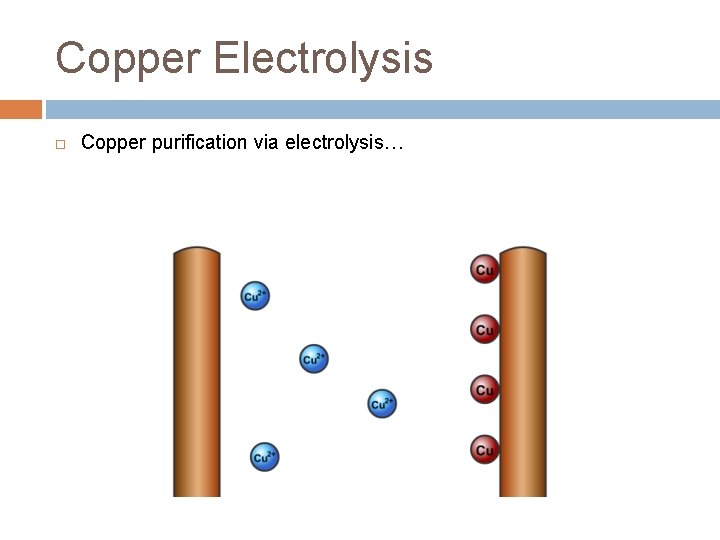
Copper Electrolysis Copper purification via electrolysis…

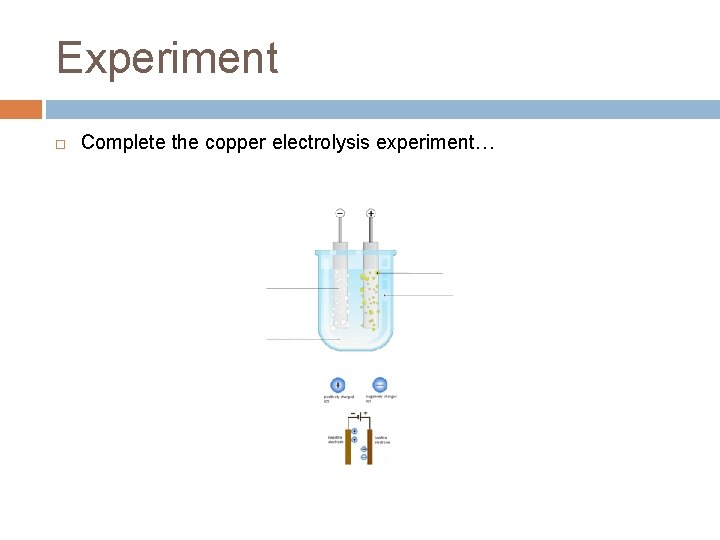
Experiment Complete the copper electrolysis experiment…

Half Equations A half-equation shows you what happens at one of the electrodes during electrolysis – electrons are shown as e. A half-equation is balanced by adding, or taking away, a number of electrons equal to the total number of charges on the ions in the equation… At the negative electrode – positive ions gain electrons at the negative electrode, so are reduced: Cu 2+ + 2 e- → Cu At the positive electrode – negative ions or neutral atoms lose electrons at the positive electrode and are oxidised: Cu → Cu 2++ 2 e-

Half Equations A half-equation shows you what happens at one of the electrodes during electrolysis – electrons are shown as e. A half-equation is balanced by adding, or taking away, a number of electrons equal to the total number of charges on the ions in the equation… At the negative electrode – positive ions gain electrons at the negative electrode, so are reduced: Al 3+ + 3 e- → Al At the positive electrode – negative ions or neutral atoms lose electrons at the positive electrode and are oxidised: 2 O 2 - → O 2 + 4 e-

THANK U