Chapter3 PART II stoichiometry Limiting Reactants Chemical calculations


- Slides: 10

Chapter-3 PART –II stoichiometry Limiting Reactants Chemical calculations ¢ Theoretical yield ¢ Percentage yield ¢ 1

Limiting Reactant A limiting reactant in a chemical reaction is the substance that • Is used up first. • Stops the reaction. • Limits the amount of product that can form. 2

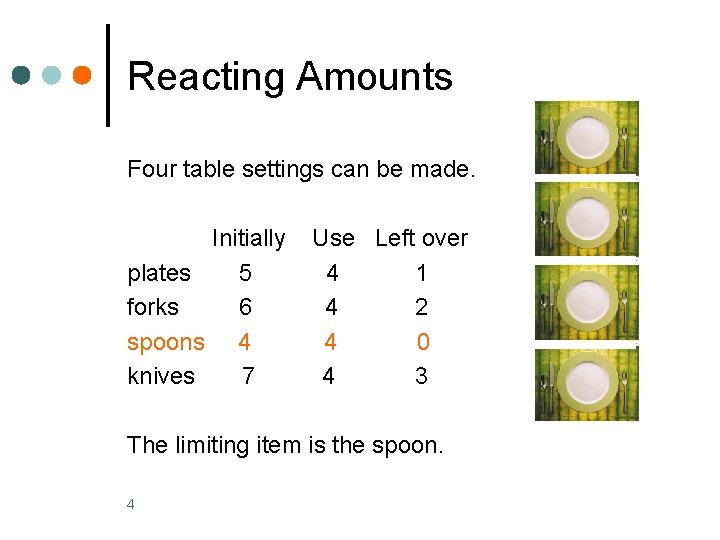
Reacting Amounts In a table setting, there is 1 plate, 1 fork, 1 knife, and 1 spoon. How many table settings are possible from 5 plates, 6 forks, 4 spoons, and 7 knives? What is the limiting item? 3 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

Reacting Amounts Four table settings can be made. Initially plates 5 forks 6 spoons 4 knives 7 Use Left over 4 1 4 2 4 0 4 3 The limiting item is the spoon. 4

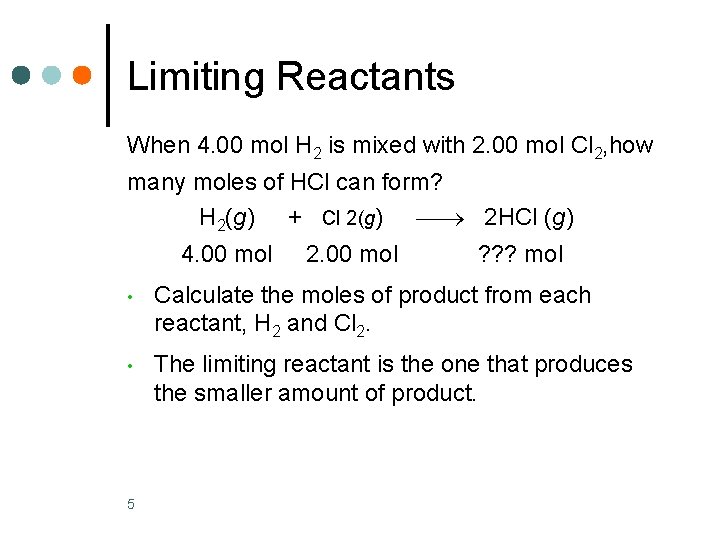
Limiting Reactants When 4. 00 mol H 2 is mixed with 2. 00 mol Cl 2, how many moles of HCl can form? H 2(g) + Cl 2(g) 2 HCl (g) 4. 00 mol 2. 00 mol ? ? ? mol • Calculate the moles of product from each reactant, H 2 and Cl 2. • The limiting reactant is the one that produces the smaller amount of product. 5

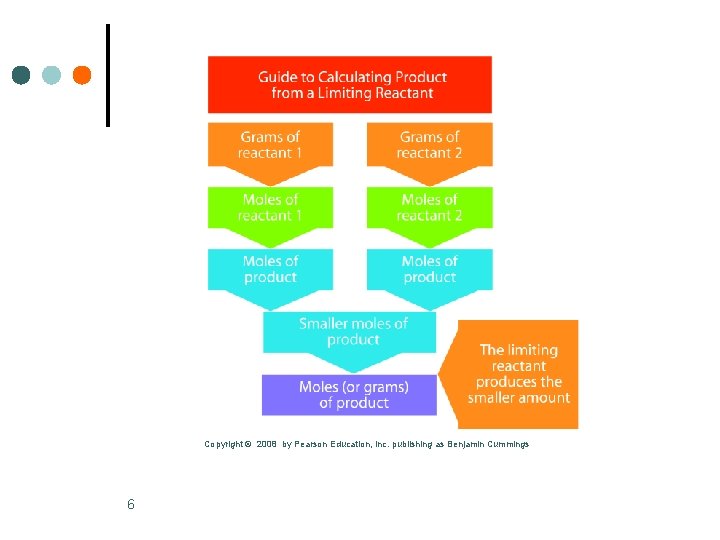
Copyright © 2008 by Pearson Education, Inc. publishing as Benjamin Cummings 6

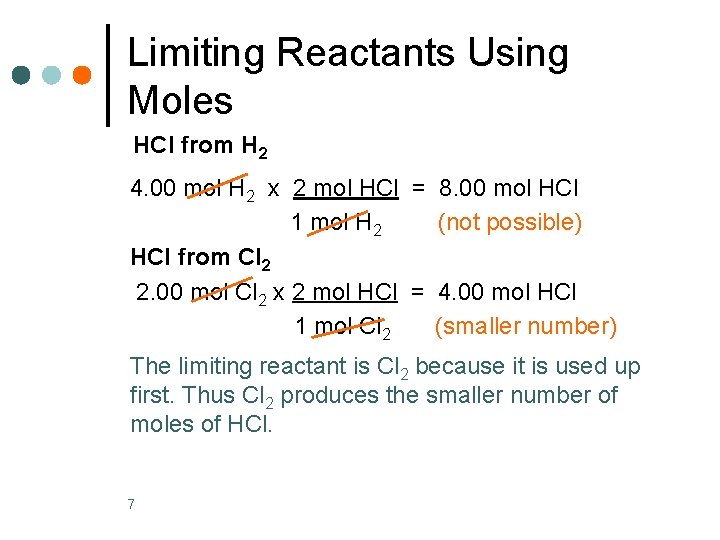
Limiting Reactants Using Moles HCl from H 2 4. 00 mol H 2 x 2 mol HCl = 8. 00 mol HCl 1 mol H 2 (not possible) HCl from Cl 2 2. 00 mol Cl 2 x 2 mol HCl = 4. 00 mol HCl 1 mol Cl 2 (smaller number) The limiting reactant is Cl 2 because it is used up first. Thus Cl 2 produces the smaller number of moles of HCl. 7

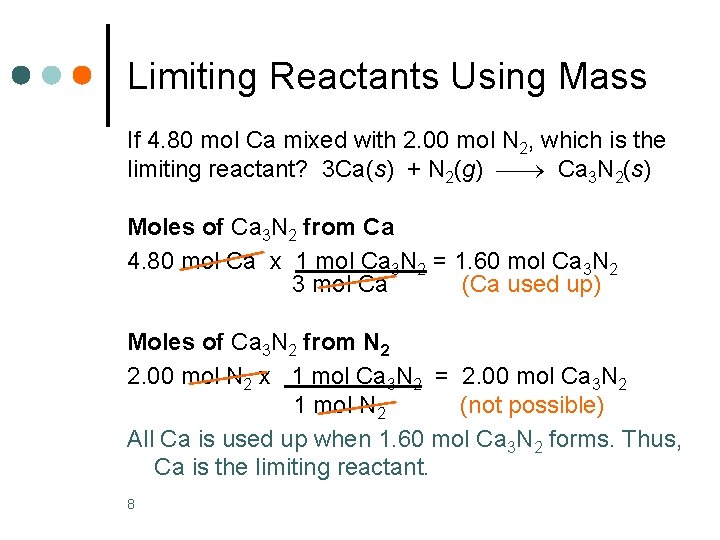
Limiting Reactants Using Mass If 4. 80 mol Ca mixed with 2. 00 mol N 2, which is the limiting reactant? 3 Ca(s) + N 2(g) Ca 3 N 2(s) Moles of Ca 3 N 2 from Ca 4. 80 mol Ca x 1 mol Ca 3 N 2 = 1. 60 mol Ca 3 N 2 3 mol Ca (Ca used up) Moles of Ca 3 N 2 from N 2 2. 00 mol N 2 x 1 mol Ca 3 N 2 = 2. 00 mol Ca 3 N 2 1 mol N 2 (not possible) All Ca is used up when 1. 60 mol Ca 3 N 2 forms. Thus, Ca is the limiting reactant. 8

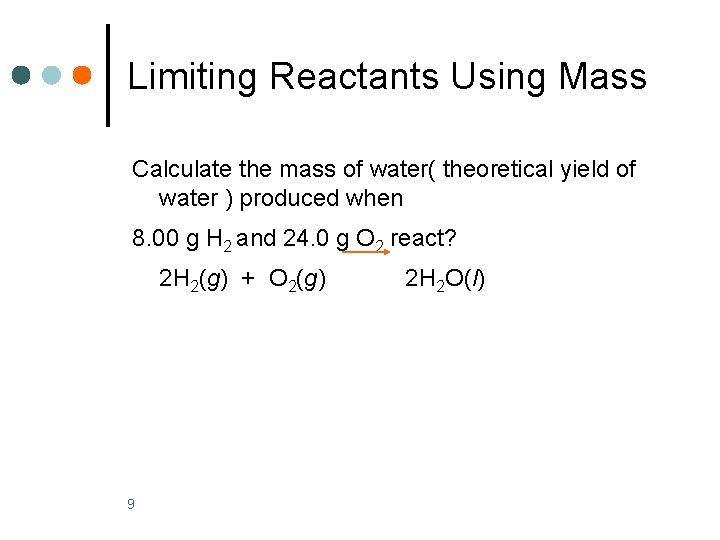
Limiting Reactants Using Mass Calculate the mass of water( theoretical yield of water ) produced when 8. 00 g H 2 and 24. 0 g O 2 react? 2 H 2(g) + O 2(g) 9 2 H 2 O(l)

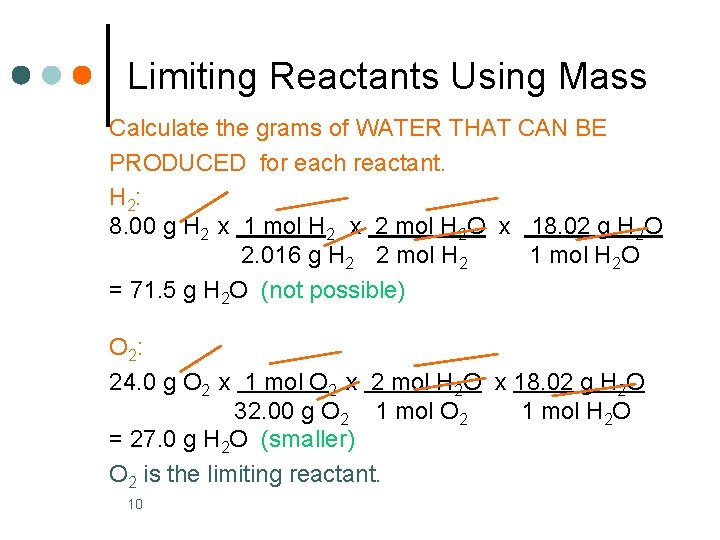
Limiting Reactants Using Mass Calculate the grams of WATER THAT CAN BE PRODUCED for each reactant. H 2: 8. 00 g H 2 x 1 mol H 2 x 2 mol H 2 O x 18. 02 g H 2 O 2. 016 g H 2 2 mol H 2 1 mol H 2 O = 71. 5 g H 2 O (not possible) O 2: 24. 0 g O 2 x 1 mol O 2 x 2 mol H 2 O x 18. 02 g H 2 O 32. 00 g O 2 1 mol H 2 O = 27. 0 g H 2 O (smaller) O 2 is the limiting reactant. 10