Chapter Two Standards for Measurement Scientific Notation Measurement











- Slides: 25

Chapter Two � Standards ◦ ◦ ◦ ◦ ◦ for Measurement Scientific Notation Measurement and Uncertainty Significant Figures in Calculations Metric System Problem-Solving Measuring Mass and Volume Measurement of Temperature Density

Observations in Science � Qualitative Observation – a less specific observation describing the quality of something. � Quantitative Observations – a measurement of a specific quantity of something. � Question: What are some qualitative and quantitative observations of our classroom?

Scientific Notation � Science often must use very large and very small numbers. ◦ The average distance from the Sun to the Earth is 150, 000 kilometers. ◦ The diameter of an atom is about 0. 000001 m. � Writing these out is not always easy.

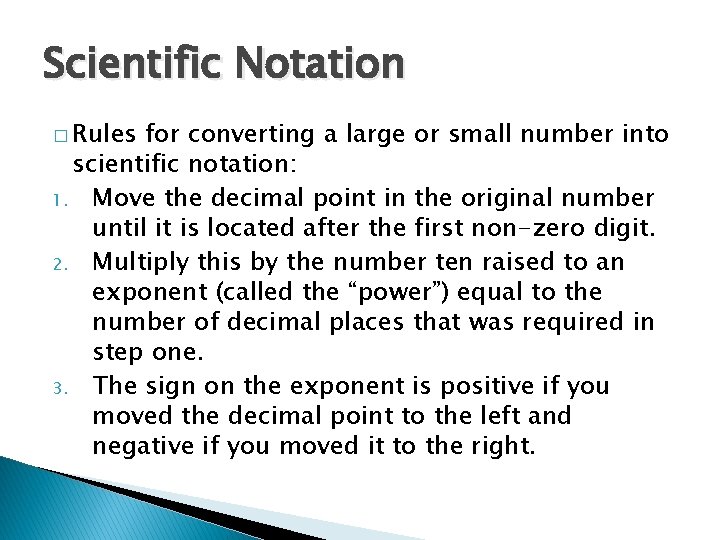
Scientific Notation � Rules for converting a large or small number into scientific notation: 1. Move the decimal point in the original number until it is located after the first non-zero digit. 2. Multiply this by the number ten raised to an exponent (called the “power”) equal to the number of decimal places that was required in step one. 3. The sign on the exponent is positive if you moved the decimal point to the left and negative if you moved it to the right.

Learning Check � Write each of the numbers into scientific notation. ◦ Ex) 150, 000 km ◦ Ex) 0. 000001 m

Scientific Notation � To convert a number from scientific notation back to a decimal number: ◦ Move the decimal point back to the right for a positive exponent – will need to add zero’s at the end of the number. ◦ Move the decimal point back to the left for a negative exponent – will need to add zero’s before the number.

Learning Check � Write the following scientific notation numbers as a decimal equivalent. ◦ Ex) 4. 5 x 105 ◦ Ex) 6. 3 x 10 -6

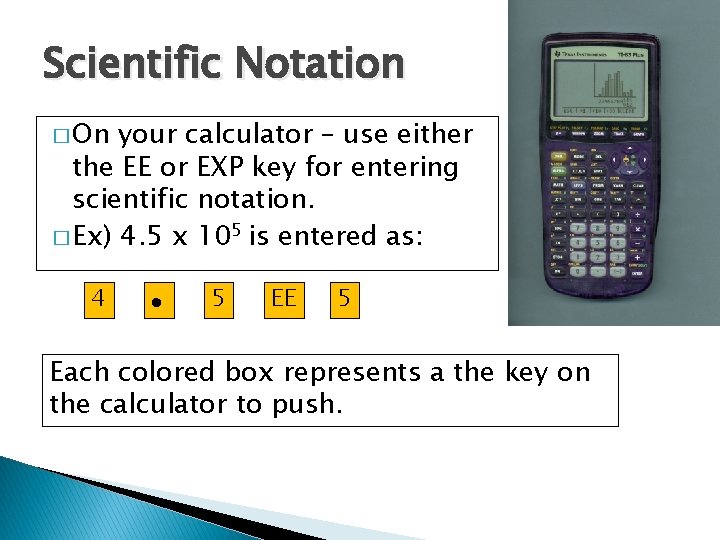
Scientific Notation � On your calculator – use either the EE or EXP key for entering scientific notation. � Ex) 4. 5 x 105 is entered as: 4 5 EE 5 Each colored box represents a the key on the calculator to push.

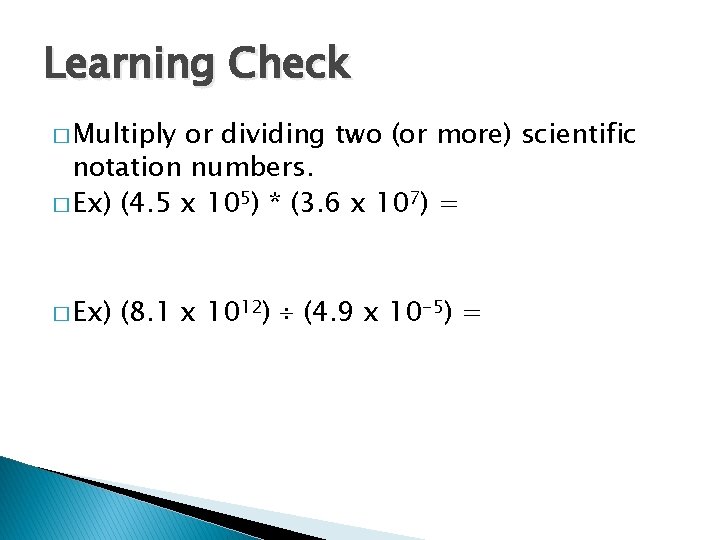
Learning Check � Multiply or dividing two (or more) scientific notation numbers. � Ex) (4. 5 x 105) * (3. 6 x 107) = � Ex) (8. 1 x 1012) (4. 9 x 10 -5) =

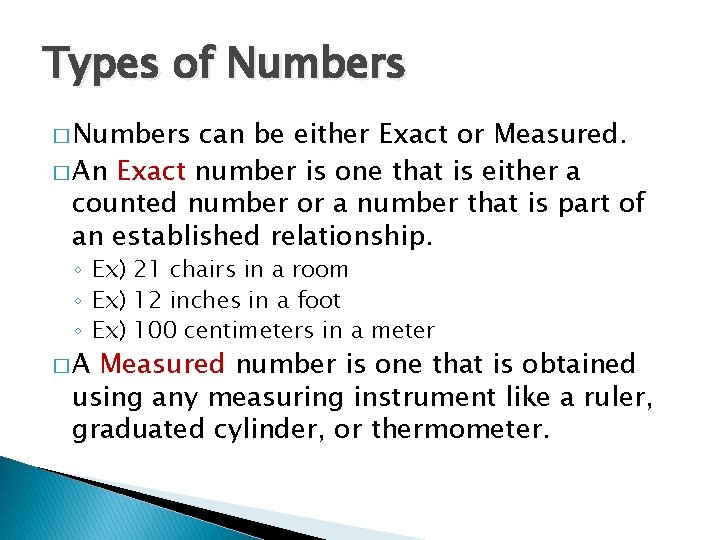
Types of Numbers � Numbers can be either Exact or Measured. � An Exact number is one that is either a counted number or a number that is part of an established relationship. ◦ Ex) 21 chairs in a room ◦ Ex) 12 inches in a foot ◦ Ex) 100 centimeters in a meter �A Measured number is one that is obtained using any measuring instrument like a ruler, graduated cylinder, or thermometer.

Learning Check � Describe number. ◦ ◦ ◦ Ex) Ex) Ex) as either an Exact or Measured 12 apples in a bag 25. 0 m. L of water in a 25 -m. L graduated cylinder 16 ounces in a pound The mass of a U. S. quarter is 5. 67 grams 1000 grams in one kilogram The indoor temperature is 74. 8 o. F

Uncertainty in Measurements � All measured numbers are made up of certain and one uncertain digit. ◦ Certain digits – all will agree. That is: we all agree that is at least 45 but not 46 degrees Fahrenheit. ◦ Uncertain digit – not all will agree! It is each person’s best “guess” between two markings. ◦ Can only have ONE uncertain digit!

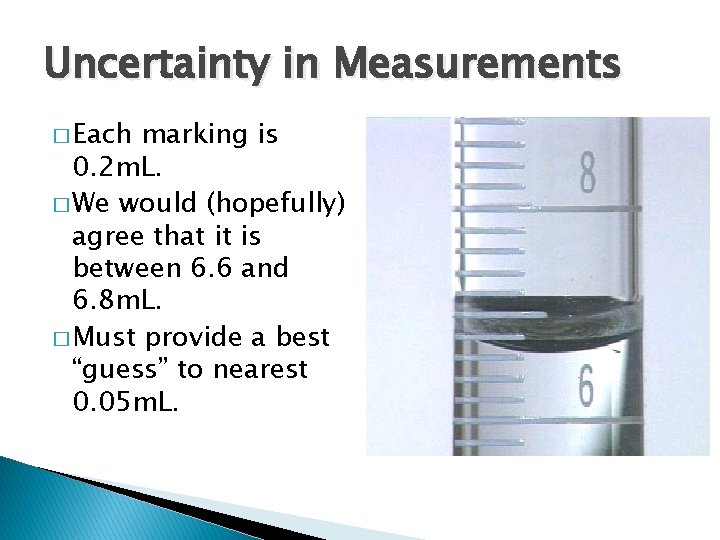
Uncertainty in Measurements � Each marking is 0. 2 m. L. � We would (hopefully) agree that it is between 6. 6 and 6. 8 m. L. � Must provide a best “guess” to nearest 0. 05 m. L.

Uncertainty in Measurements � What if it seems to be “exactly” on a mark? ? ?

Uncertainty in Measurements � Many of the measuring devices used today are electronic like a scale or a digital thermometer. � The last digit is always the uncertain digit and should always be included even when it is a zero.

Significant Figures � All of the certain digits plus the one uncertain digit are called significant digits. ◦ Thus, our volume from earlier – 6. 65 m. L would have three significant figures. ◦ Our temperature, 36. 60 o. C, would have four significant figures. ◦ And our scale reading, 45. 22 g, would also have four significant figures. � Note: Exact numbers have an infinite significance because they are exact.

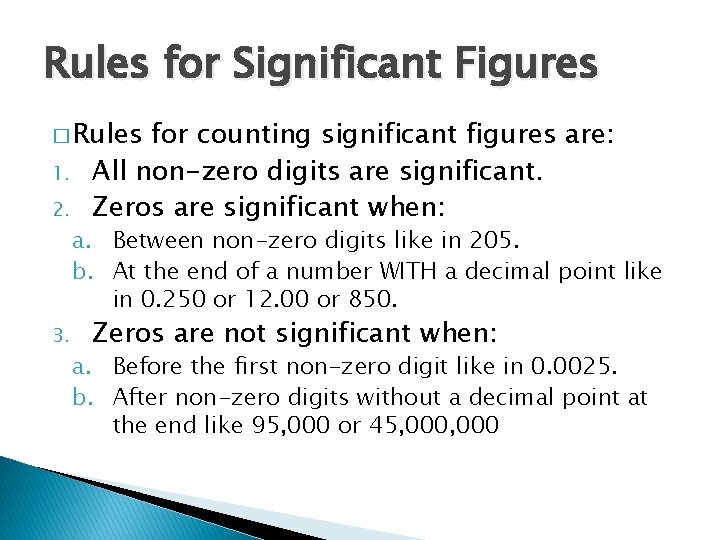
Rules for Significant Figures � Rules 1. 2. 3. for counting significant figures are: All non-zero digits are significant. Zeros are significant when: a. Between non-zero digits like in 205. b. At the end of a number WITH a decimal point like in 0. 250 or 12. 00 or 850. Zeros are not significant when: a. Before the first non-zero digit like in 0. 0025. b. After non-zero digits without a decimal point at the end like 95, 000 or 45, 000

Learning Check � How many significant digits does each measured number have? ◦ ◦ ◦ ◦ 0. 000305 125. 0 1. 0 0. 023012 0. 0001 100 45. 050

Rounding �A calculation involving measured numbers will almost always need to be rounded to a certain place. � Rules: 1. When the first digit to be rounded after those that will be retained is a 4 or less, then drop all of those extra digits. 2. When the first digit to be rounded is 5+, the drop all of those digits and adjust the last digit retained up by one.

Rounding 3. � � If it is EXACTLY 5, then round the final retained digit to an even number. Ex) 45. 62 rounded to two digits is ______ Ex) 89. 145 rounded to three digits is ______ Ex) 9. 15 rounded to two digits is _____ Ex) 84. 5 rounded to two digits is _____

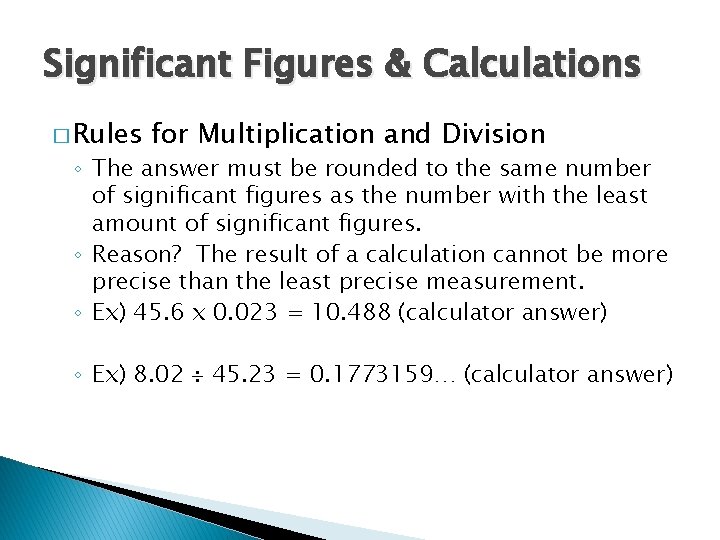
Significant Figures & Calculations � Rules for Multiplication and Division ◦ The answer must be rounded to the same number of significant figures as the number with the least amount of significant figures. ◦ Reason? The result of a calculation cannot be more precise than the least precise measurement. ◦ Ex) 45. 6 x 0. 023 = 10. 488 (calculator answer) ◦ Ex) 8. 02 45. 23 = 0. 1773159… (calculator answer)

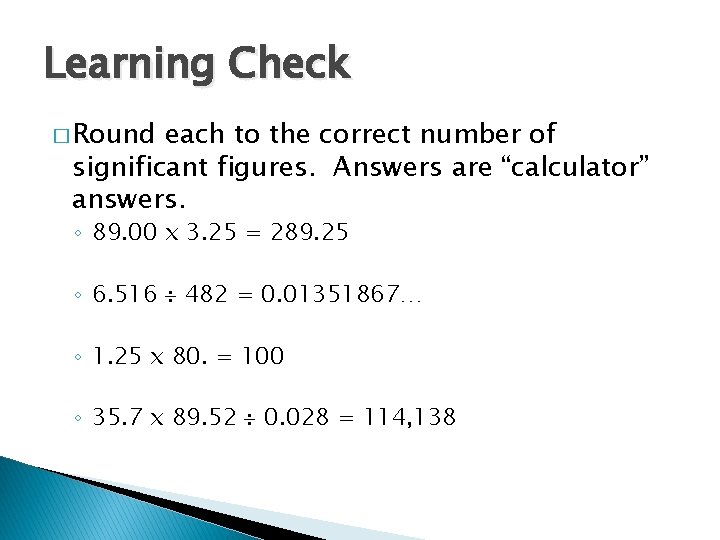
Learning Check � Round each to the correct number of significant figures. Answers are “calculator” answers. ◦ 89. 00 x 3. 25 = 289. 25 ◦ 6. 516 482 = 0. 01351867… ◦ 1. 25 x 80. = 100 ◦ 35. 7 x 89. 52 0. 028 = 114, 138

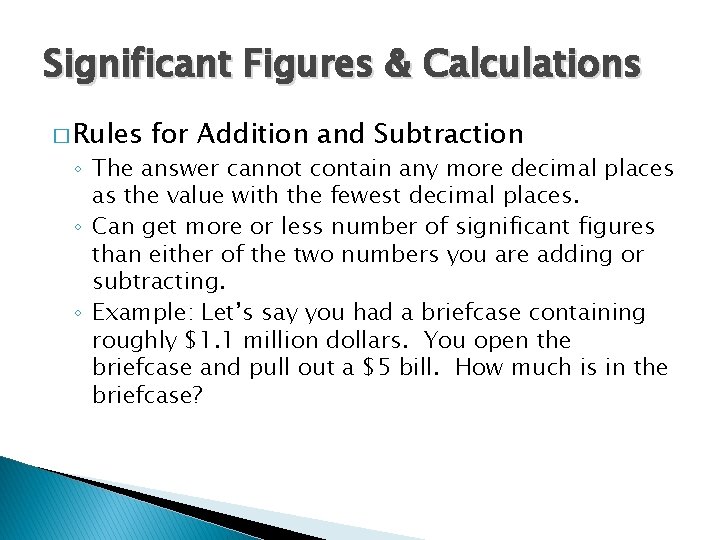
Significant Figures & Calculations � Rules for Addition and Subtraction ◦ The answer cannot contain any more decimal places as the value with the fewest decimal places. ◦ Can get more or less number of significant figures than either of the two numbers you are adding or subtracting. ◦ Example: Let’s say you had a briefcase containing roughly $1. 1 million dollars. You open the briefcase and pull out a $5 bill. How much is in the briefcase?

Learning Check � Round each to the correct number of significant figures. Answers are “calculator” answers. ◦ 45. 2 + 3. 5 = 48. 7 ◦ 73. 21 – 71. 58 = 1. 63 ◦ 151 + 3. 28 = 154. 28 ◦ 173. 18 – 167. 98 = 5. 2

Mixed Calculations � For a mixed calculation, you must follow each of two rules individually and follow order of operations. � Ex) (45. 23 – 3. 15) 4825 = 0. 87212435… ◦ You must do the subtraction first! � Ex) (11. 35 + 2. 55) x 0. 12 = 1. 668 � Ex) (23. 33 – 22. 93) x 4. 58 = 1. 832 ◦ You must do the addition first!