Chapter two Atoms Molecules and Ions The Atomic

















- Slides: 28

Chapter two: Atoms, Molecules and Ions The Atomic Theory The Structure of the Atomic Number, Mass Number and Isotopes Mixtures The Periodic Table Molecules and Ions Chemical Formulas Naming Compounds

B. Pure Substances ö Element w composed of identical atoms w EX: copper wire, aluminum foil

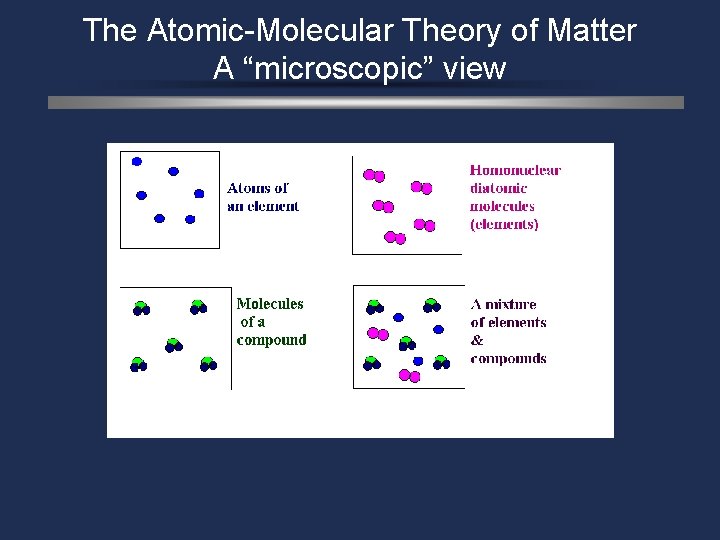
Define Ø Molecule – a combination of 2 0 r more atoms (same or different) that are covalently bonded. Ø A molecule is the smallest particle of a substance which exhibits the physical and chemical characteristics of the substance. Ø Diatomic molecules of elements : H 2 O 2 Cl 2 N 2 F 2 Br 2 I 2

Define Compounds a compound of 2 or more different elements bonded together in a fixed proportion. H 2 O CO 2 Ca. SO 4 HBR Molecules Na 2 O H 2 CO 3 KOH

B. Pure Substances ö Compound w composed of 2 or more elements in a fixed ratio w properties differ from those of individual elements w EX: table salt (Na. Cl)

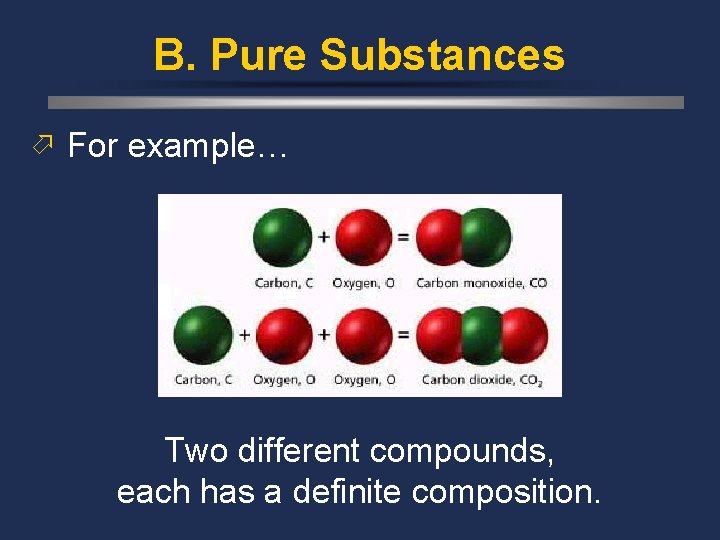
B. Pure Substances ö For example… Two different compounds, each has a definite composition.

Compounds Slight differences in combinations of atoms can have large difference in properties H 2 O- water, H 2 O 2 – hydrogen peroxide C 2 H 6 O – ethanol, drinkable C 2 H 6 O 2 – ethylene glycol, poisonous

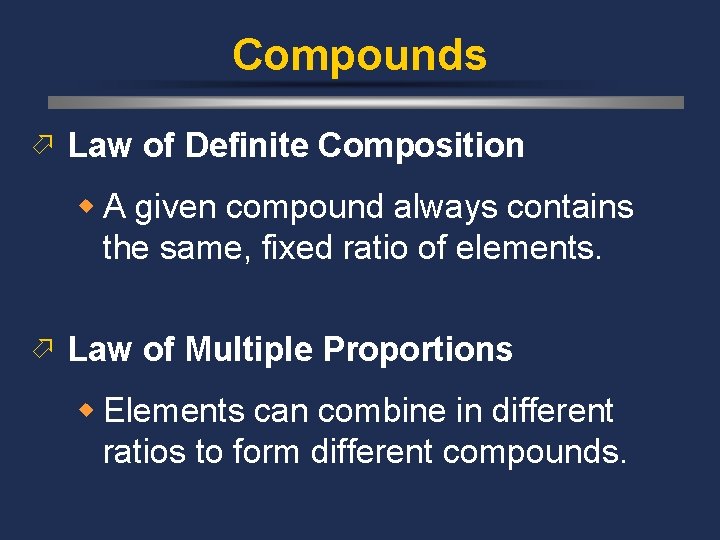
Compounds ö Law of Definite Composition w A given compound always contains the same, fixed ratio of elements. ö Law of Multiple Proportions w Elements can combine in different ratios to form different compounds.

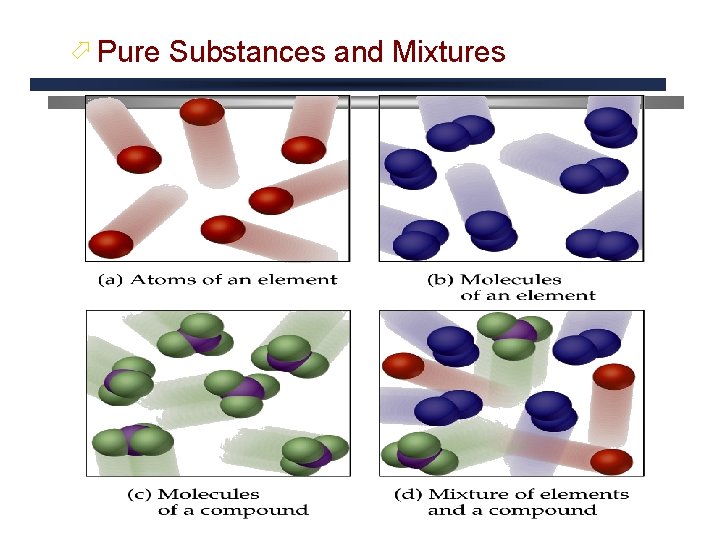
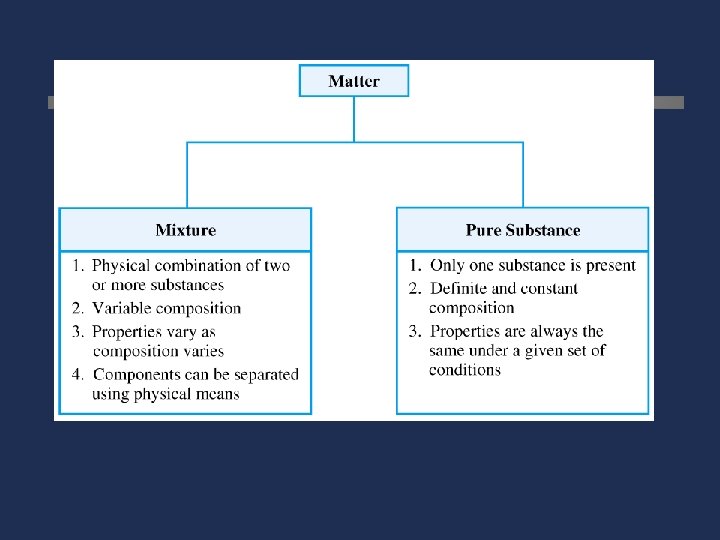
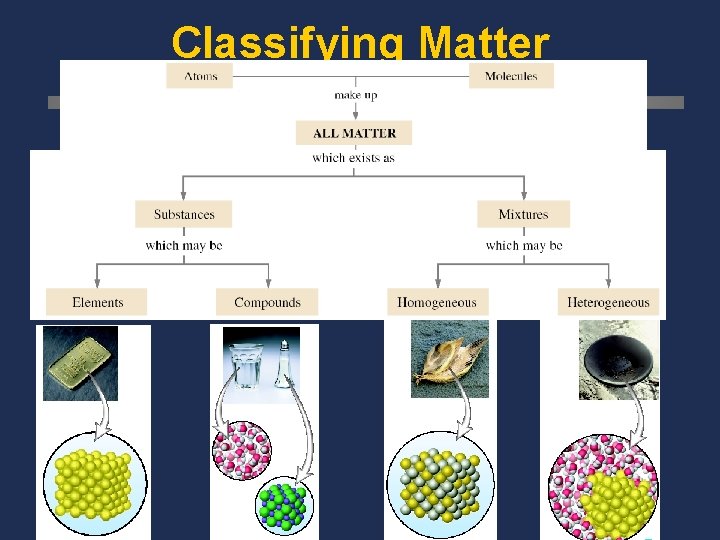
Pure Substances Same kind of particles throughout Compounds Elements Can be decomposed into simpler substances by chemical changes, always in a definite ration cannot be decomposed into simpler substances by chemical changes

Mixture ö Mixtures are two or more substance ö ö that are not chemically combined. Mixtures do not have a fixed composition Mixtures do not have constant boiling points or melting points Variable composition Components retain their characteristic properties

Mixture ö May be separated into pure substances by physical methods ö Mixtures of different compositions may have widely different properties.

ö Pure Substances and Mixtures

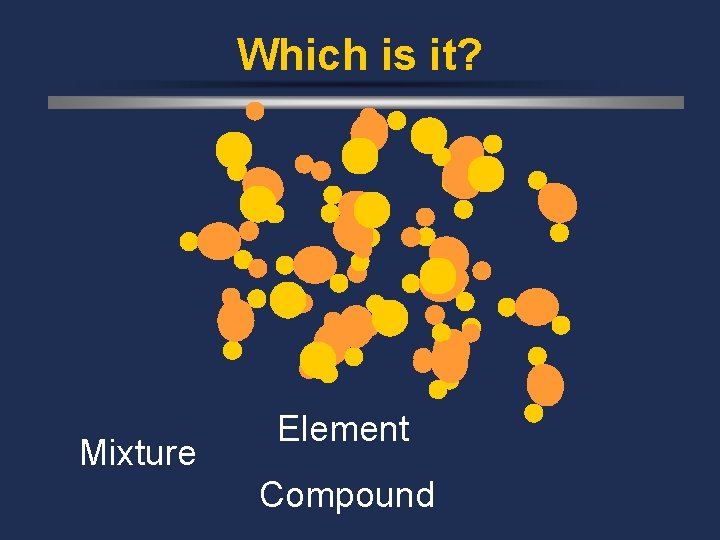
Which is it? Mixture Element Compound

Physical Separation Techniques ö By eye ö Filtration to separate solid and liquid ö Distillation to separate two or more liquids with different boiling points ö Chromatography to separate pure liquids or solutions of compounds

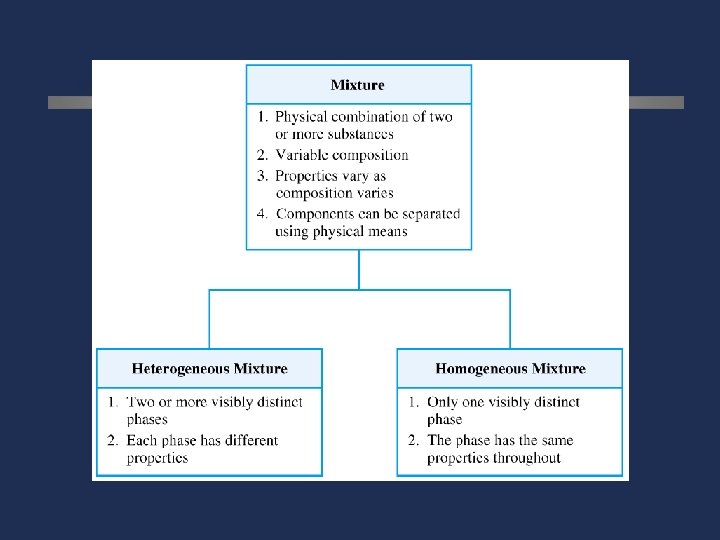
C. Mixtures ö Variable combination of 2 or more pure substances. Heterogeneous Homogeneous



Types of mixtures ö Homogeneous mixture -1 phase -uniform properties in a sample -same composition in a sample eg: sugar and water Heterogeneous mixture -2 or more phases (with same or different physical states) -each phase has different properties eg: oil and water, sand water

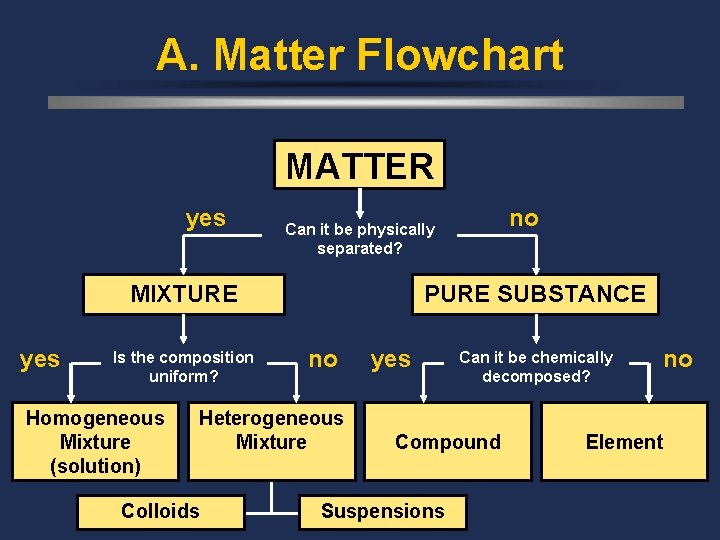
A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

C. Mixtures ö Solution w homogeneous w very small particles w no Tyndall effect w particles don’t settle w EX: rubbing alcohol Tyndall Effect

C. Mixtures ö Colloid w heterogeneous w medium-sized particles w Tyndall effect w particles don’t settle w EX: milk

C. Mixtures ö Suspension w heterogeneous w large particles w Tyndall effect w particles settle w EX: fresh-squeezed lemonade

C. Mixtures ö Examples: w mayonnaise colloid w muddy water suspension w fog colloid w saltwater solution w Italian salad dressing suspension

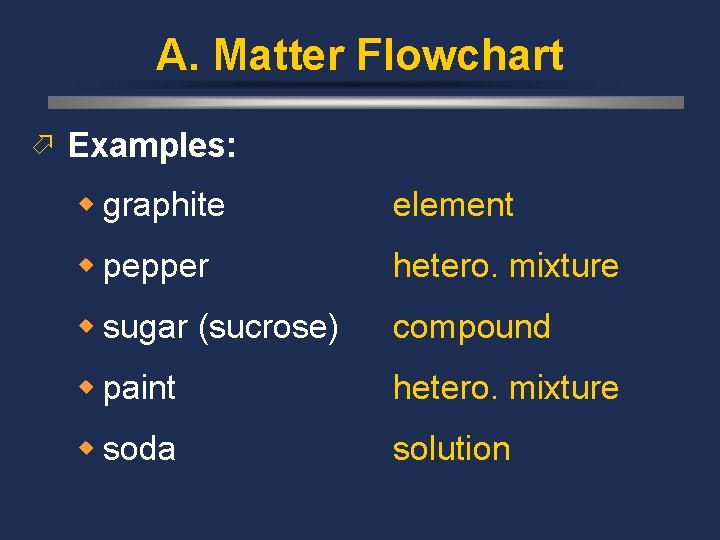
A. Matter Flowchart ö Examples: w graphite element w pepper hetero. mixture w sugar (sucrose) compound w paint hetero. mixture w soda solution

Classifying Matter

The Atomic-Molecular Theory of Matter A “microscopic” view

PRACTICE PROBLEMS #4 1. Classify the following as an element, compound, or mixture (heterogeneous or homogeneous). E HO ö _____ air _____ oxygen ö _____ tin can _____ sugar E C HO HE ö _____ Windex _____ crude oil HO HE ö _____ suntan lotion _____ gummi bear 2. A white solid is dissolved in water. The resulting colorless, clear liquid is boiled in a beaker until dryness. White crystals remain in the beaker. The Homogeneous mixture liquid can be classified as a(n) _______. 3. Classify the following as physical or chemical changes. CC CC ö _____ photosynthesis _____ baking PC PC ö _____ writing with pencil _____ snowing

GROUP STUDY PROBLEM #4 1. Classify the following as an element, compound, or mixture (heterogeneous or homogeneous). ö _____ wine _____ root beer ö _____ penny _____ table salt ö _____ Bleach _____ wood ö _____ diamond _____ vinegar 2. A clear blue liquid in an open beaker was left in the hood. After 1 week, the beaker contained only blue crystals. The original liquid can be classified as a(n) _______. 3. Classify the following as physical or chemical changes. ö _____ perspiration _____ sugar dissolving ö _____ fermentation _____ aging