Chapter Ten Titrimetry Inorganic chemistry Organic chemistry n






















![①. Initially, V =0. 00 ml Na. OH added [H 3 O+]= 0. 1000 ①. Initially, V =0. 00 ml Na. OH added [H 3 O+]= 0. 1000](https://slidetodoc.com/presentation_image_h/224c5f148c1f3283f67c89c57baaace8/image-32.jpg)




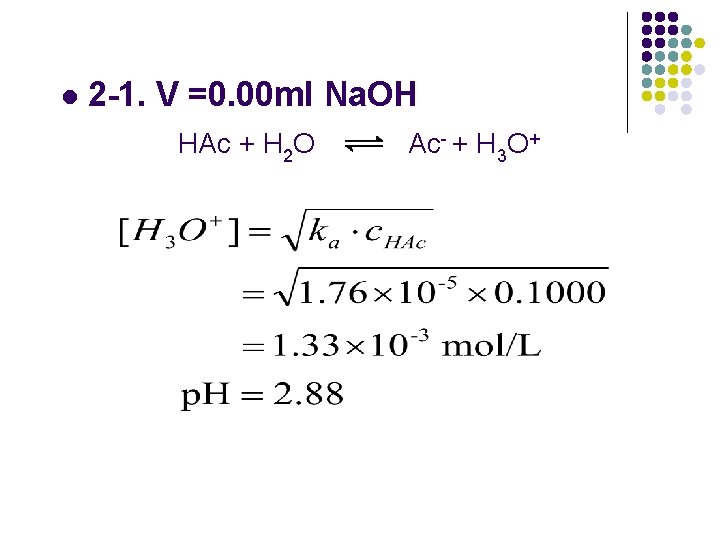
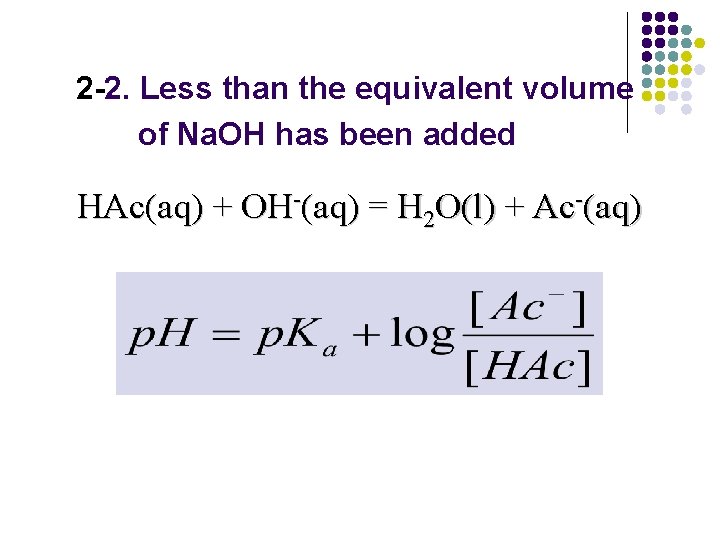











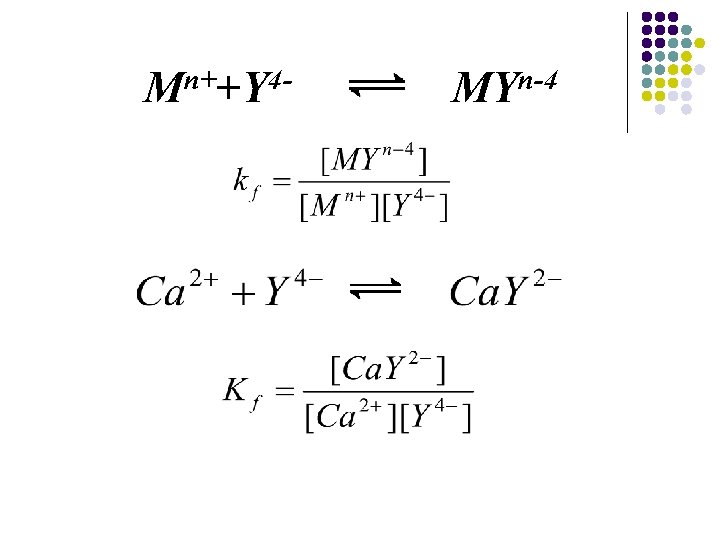



































- Slides: 121

Chapter Ten Titrimetry Inorganic chemistry Organic chemistry n. Chemistry Physical chemistry Analytical chemistry





Quantitative analysis(定量分析) Gravimetric analysis Chemical analysis Volumetric analysis Instrumental analysis Spectrometry Chromatographic method Electrochemical analysis

l. Acid-base titration (neutralization titration) Volumetric analysis (volumetry, titrimetry) l Oxidation-reduction titration l Coordination titration (EDTA titration) l Precipitation titration

10 -1 General Principles of titration 10 -2 Acid-Base Titration 10 -3 EDTA Titration 10 -4 Analytical Error and Significant Figures

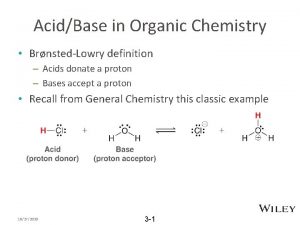
l l l A titration is a standard laboratory method of chemical analysis which can be used to determine the concentration of a unknown reactant. In a titration two reagents are mixed, one with a known concentration and one with an unknown concentration. There is some way to indicate when the two reagents have reacted essentially completely, and at the end of the titration the unknown solution's concentration can be calculated.

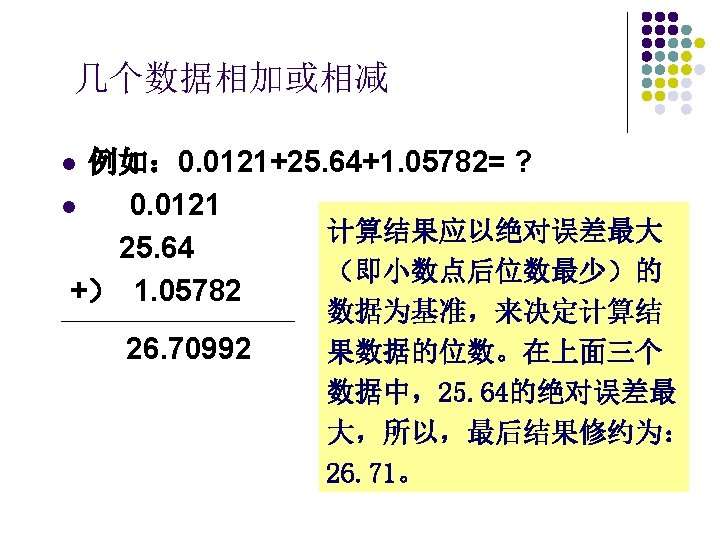
General Principles of titration l Titration consists of adding carefully metered volumes of the solution of known concentration to the other solution of unknown concentration to reach an endpoint.

Typically, one reagent is a solution and is added from a buret. This solution is called the titrant or standard solution. The solution from the buret is added to a conical flask that contains either a measured volume of a solution or a weighed quantity of solid that has been dissolved. The buret has graduations that are used to read the volume of titrant added to the conical flask.

l Titration: Titrant (standard solution) Analyte (sample) Stoichiometric point Indicator End point of titration Error of titration

Titrant in buret Analyte(sample) in conical flask indicator









• titration : fill buret remove bubble fill pipette(Ⅰ、Ⅱ) titration Ⅰ titration Ⅱ

The requirement of a titration are as follows: 1. The reaction should be stoichiometric. 2. The reaction should be rapid. 3. There should be no side reactions, and the reaction should be specific. 4. There should be a marked change in some property of the solution when the reaction is complete. 5. The point at which the reaction is observed to be complete is called the end point. 6. The reaction should be quantitative.

Operation system in titrametric analysis l 1. Prepare the standard solution l 2. standardization of the standard solution l 3. determine the content of the sample

Standard Solution l Standard solution The concentration of a standard solution must be known within the limits of accuracy necessary in the actual analysis for which it is being used. Significant figure Four figures are necessary

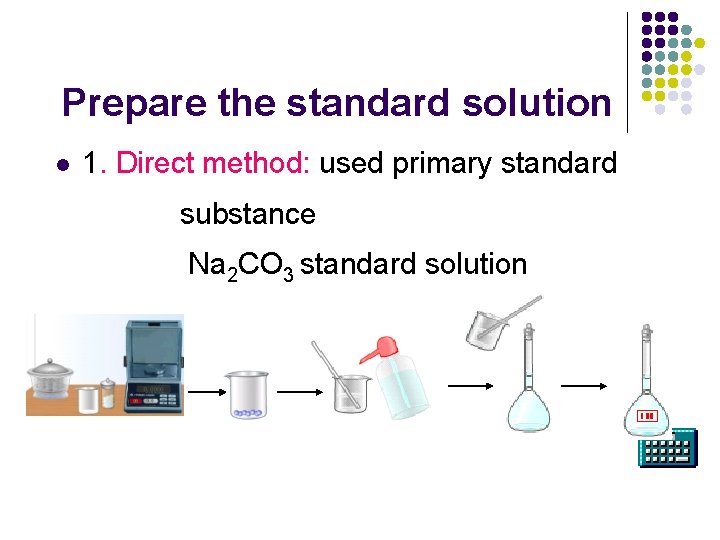
Prepare the standard solution l 1. Direct method: used primary standard substance Na 2 CO 3 standard solution



primary standard substance A primary standard substance should fulfill these requirements: 1. It should be 100. 00% pure 2. It should be stable to drying temperatures 3. It should be readily available. 4. it should have a high formula weight. (large mole weight) 5. Reaction quickly l

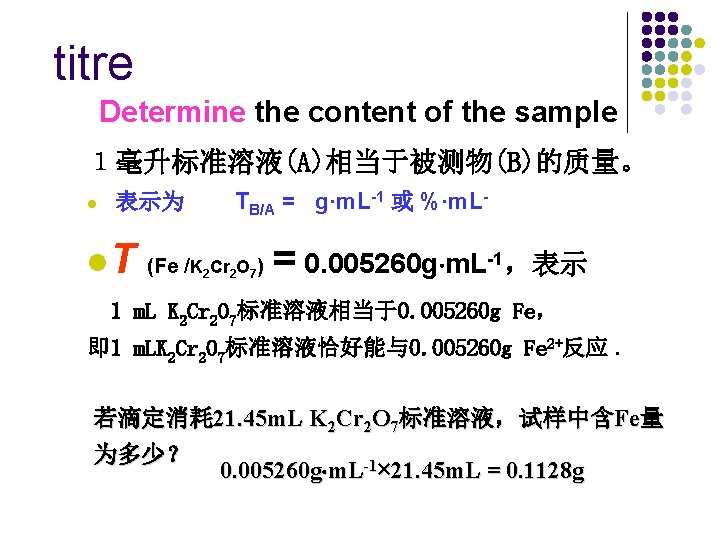
titre Determine the content of the sample 1毫升标准溶液(A)相当于被测物(B)的质量。 l 表示为 TB/A = g·m. L-1 或 %·m. L- l. T (Fe /K 2 Cr 2 O 7) = 0. 005260 g m. L-1,表示 1 m. L K 2 Cr 2 O 7标准溶液相当于0. 005260 g Fe, 即 1 m. LK 2 Cr 2 O 7标准溶液恰好能与0. 005260 g Fe 2+反应. 若滴定消耗 21. 45 m. L K 2 Cr 2 O 7标准溶液,试样中含Fe量 为多少? 0. 005260 g m. L-1× 21. 45 m. L = 0. 1128 g

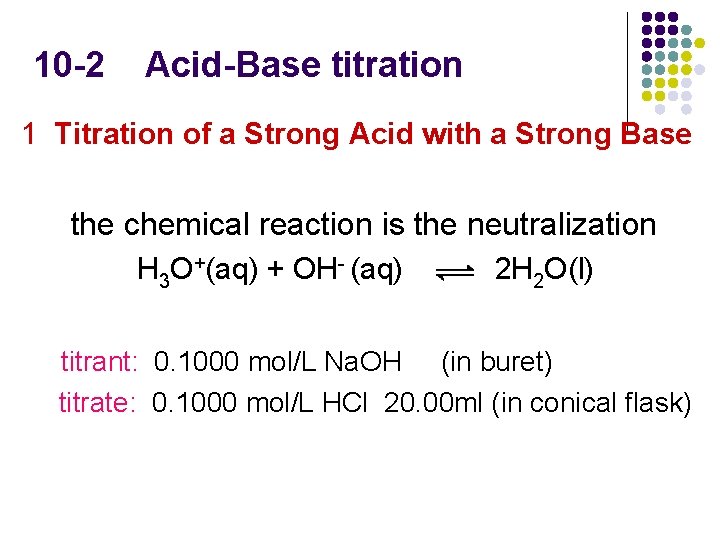
10 -2 Acid-Base titration 1 Titration of a Strong Acid with a Strong Base the chemical reaction is the neutralization H 3 O+(aq) + OH- (aq) 2 H 2 O(l) titrant: 0. 1000 mol/L Na. OH (in buret) titrate: 0. 1000 mol/L HCl 20. 00 ml (in conical flask)
![① Initially V 0 00 ml Na OH added H 3 O 0 1000 ①. Initially, V =0. 00 ml Na. OH added [H 3 O+]= 0. 1000](https://slidetodoc.com/presentation_image_h/224c5f148c1f3283f67c89c57baaace8/image-32.jpg)
①. Initially, V =0. 00 ml Na. OH added [H 3 O+]= 0. 1000 mol/L p. H = 1. 00 ②. V=18. 00 ml Na. OH mol/L added mmoles acid left unreacted=2. 000 -1. 800 =0. 2000 volume solution =20. 00+18. 00=38. 00 ml p. H = 2. 28

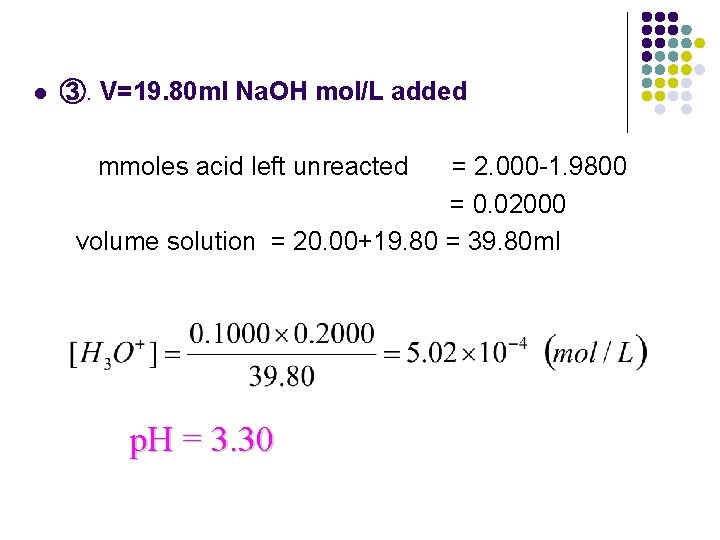
l ③. V=19. 80 ml Na. OH mol/L added mmoles acid left unreacted = 2. 000 -1. 9800 = 0. 02000 volume solution = 20. 00+19. 80 = 39. 80 ml p. H = 3. 30

l ④. V=19. 98 ml Na. OH mol/L added mmoles acid left unreacted = 2. 000 - 1. 9800 =0. 02000 volume solution = 20. 00+19. 98 = 39. 98 ml p. H = 4. 3

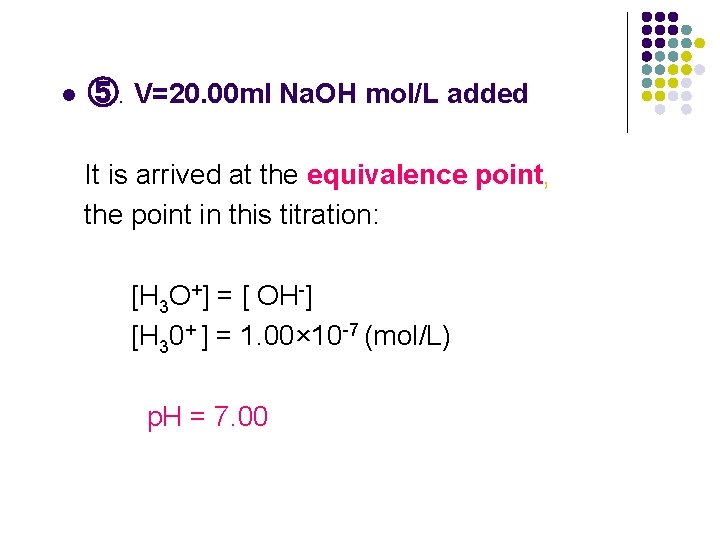
l ⑤. V=20. 00 ml Na. OH mol/L added It is arrived at the equivalence point, the point in this titration: [H 3 O+] = [ OH-] [H 30+ ] = 1. 00× 10 -7 (mol/L) p. H = 7. 00

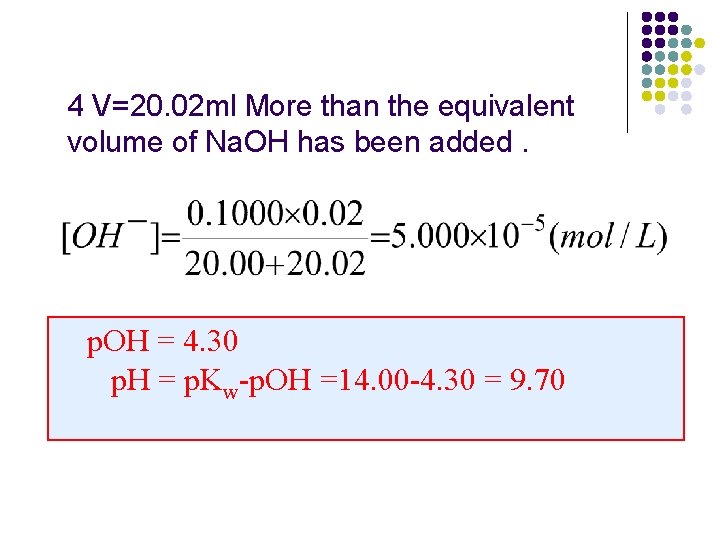
l ⑥. V=20. 02 ml Na. OH mol/L added after the equivalence point mmoles base unreacted = 2. 002 -2. 000 = 0. 02000 volume solution = 20. 00+20. 02 = 40. 02 ml p. OH = 4. 30 p. H =14 -4. 3=9. 7

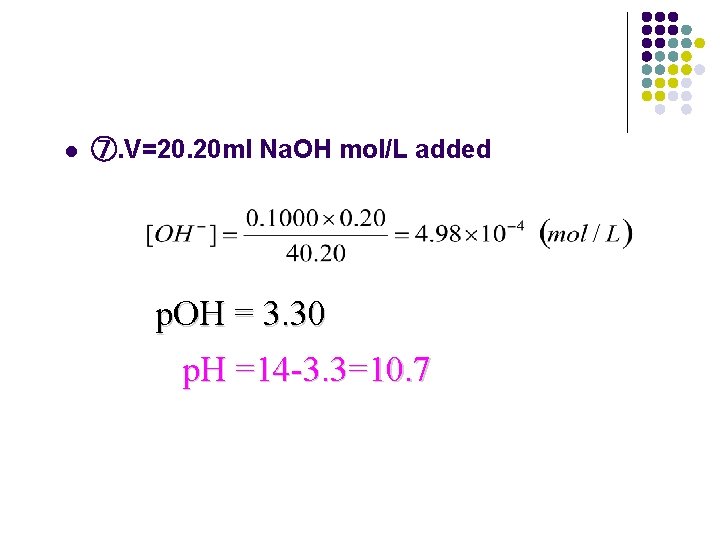
l ⑦. V=20. 20 ml Na. OH mol/L added p. OH = 3. 30 p. H =14 -3. 3=10. 7

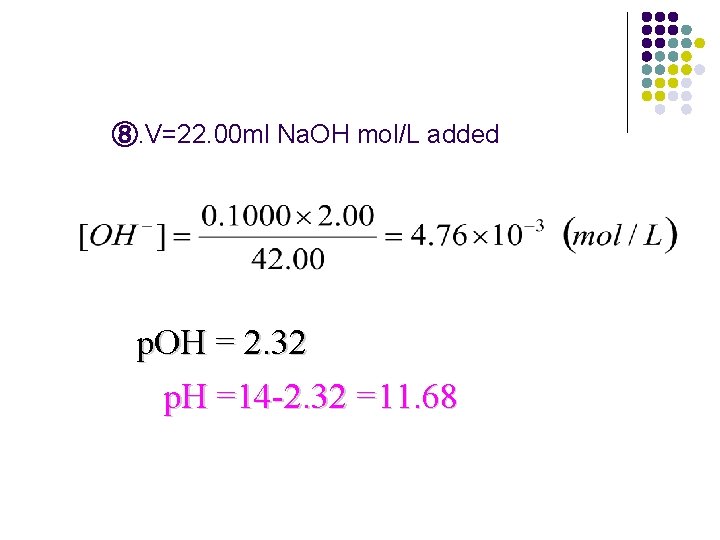
⑧. V=22. 00 ml Na. OH mol/L added p. OH = 2. 32 p. H =14 -2. 32 =11. 68

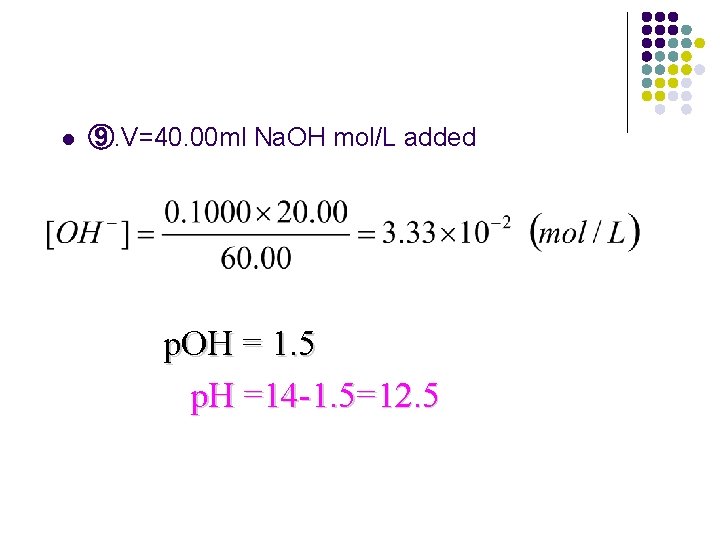
l ⑨. V=40. 00 ml Na. OH mol/L added p. OH = 1. 5 p. H =14 -1. 5=12. 5

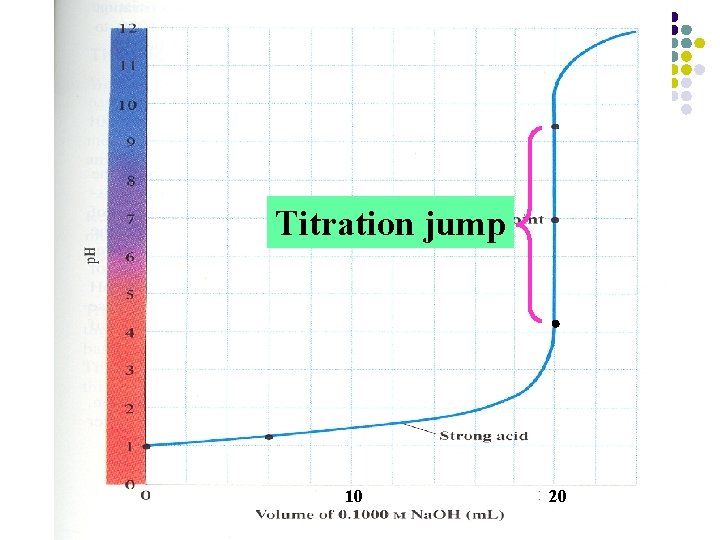
Titration jump 10 20

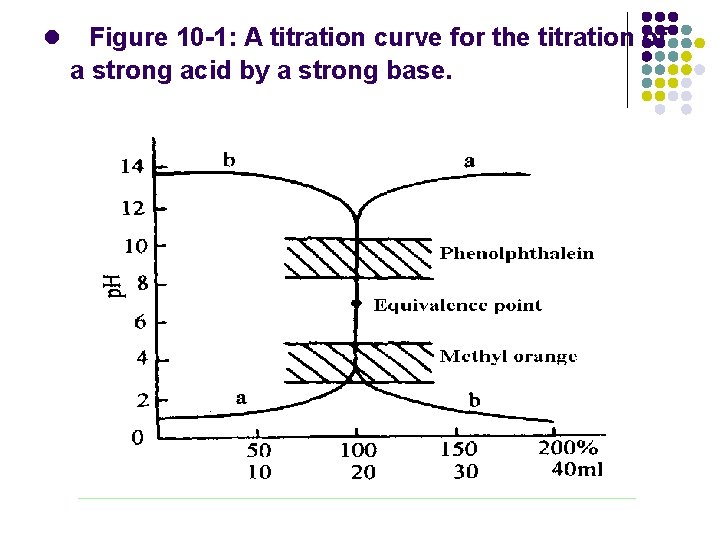
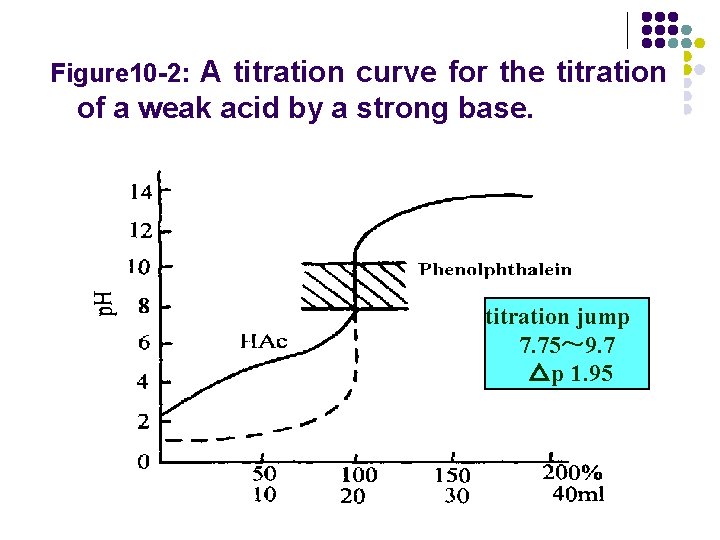
l Figure 10 -1: A titration curve for the titration of a strong acid by a strong base.

• The magnitude of the titration jump will depend on both the concentration of the acid and the concentration of the base. • Usually, the concentration of standard solution should be 0. 1000~0. 5000 mol/L

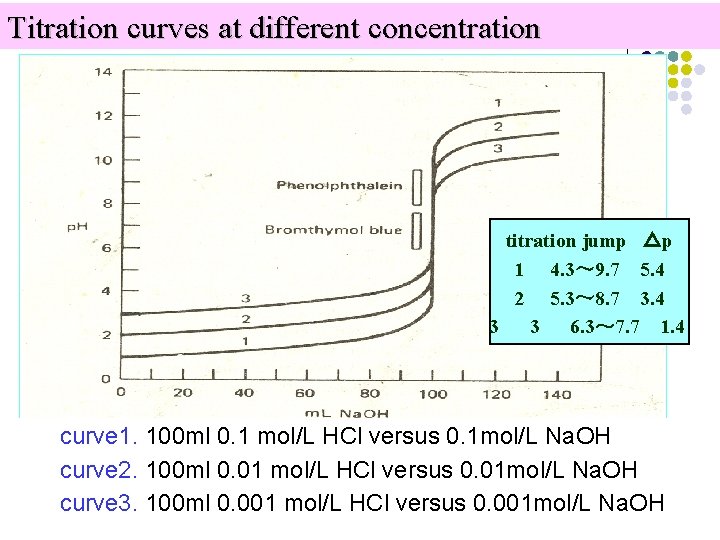
Titration curves at different concentration titration jump △p 1 4. 3~ 9. 7 5. 4 2 5. 3~ 8. 7 3. 4 3 3 6. 3~ 7. 7 1. 4 curve 1. 100 ml 0. 1 mol/L HCl versus 0. 1 mol/L Na. OH curve 2. 100 ml 0. 01 mol/L HCl versus 0. 01 mol/L Na. OH curve 3. 100 ml 0. 001 mol/L HCl versus 0. 001 mol/L Na. OH


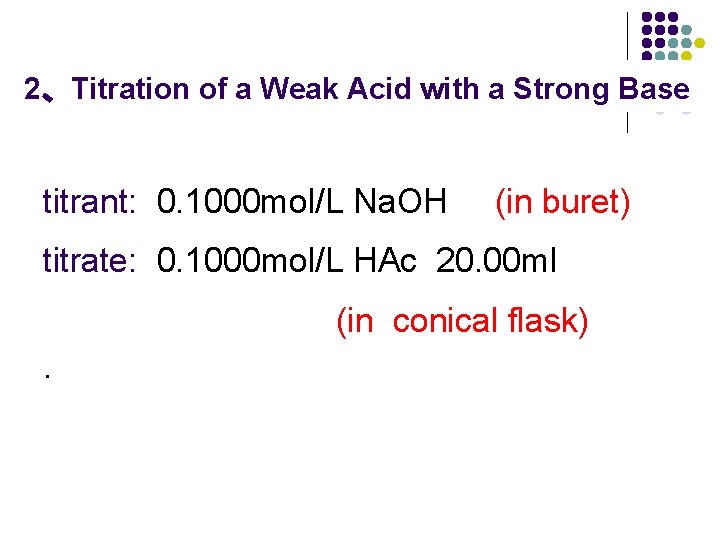
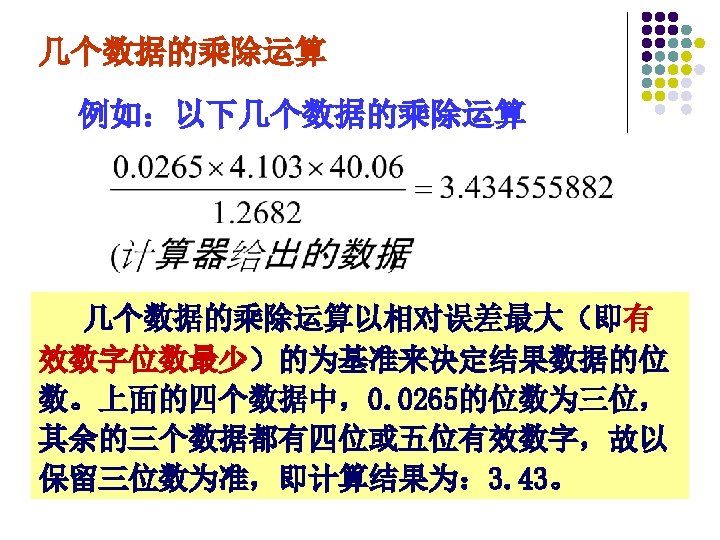
2、Titration of a Weak Acid with a Strong Base titrant: 0. 1000 mol/L Na. OH (in buret) titrate: 0. 1000 mol/L HAc 20. 00 ml (in conical flask).
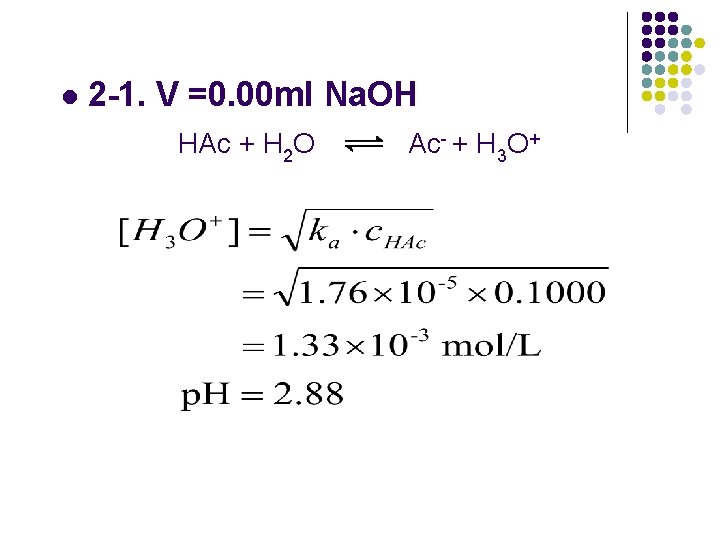
l 2 -1. V =0. 00 ml Na. OH HAc + H 2 O Ac- + H 3 O+
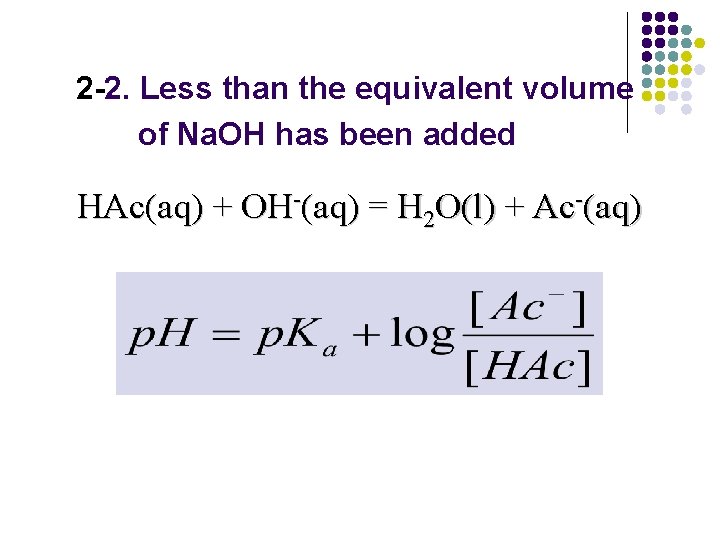
2 -2. Less than the equivalent volume of Na. OH has been added HAc(aq) + OH-(aq) = H 2 O(l) + Ac-(aq)

1). V = 18. 00 ml of 0. 1000 mol/LNa. OH has been added

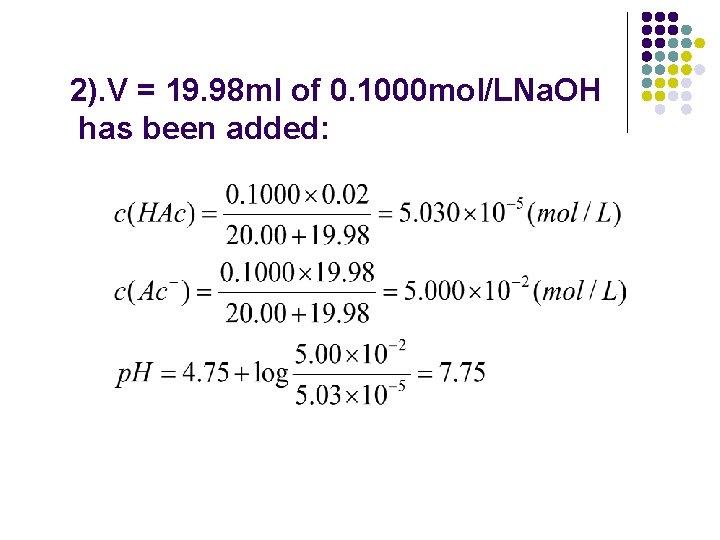
2). V = 19. 98 ml of 0. 1000 mol/LNa. OH has been added:

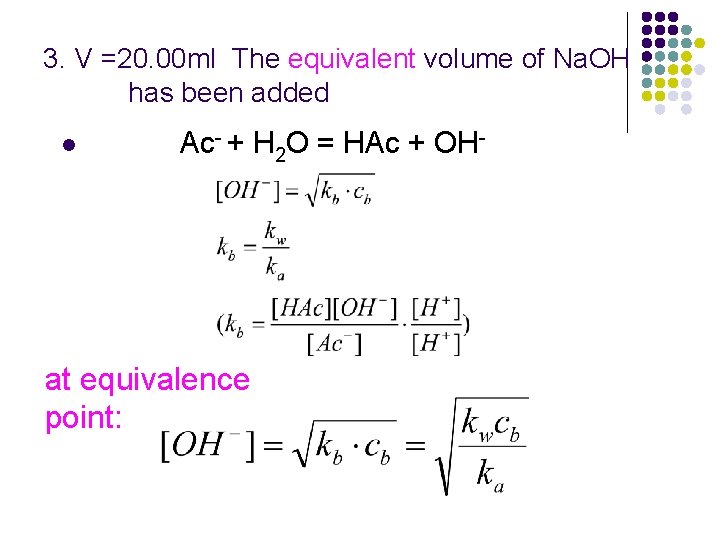
3. V =20. 00 ml The equivalent volume of Na. OH has been added l Ac- + H 2 O = HAc + OH- at equivalence point:

l at equivalence point p. OH = 5. 27 p. H = p. Kw – p. OH =14 -5. 27 = 8. 73

4 V=20. 02 ml More than the equivalent volume of Na. OH has been added. p. OH = 4. 30 p. H = p. Kw-p. OH =14. 00 -4. 30 = 9. 70

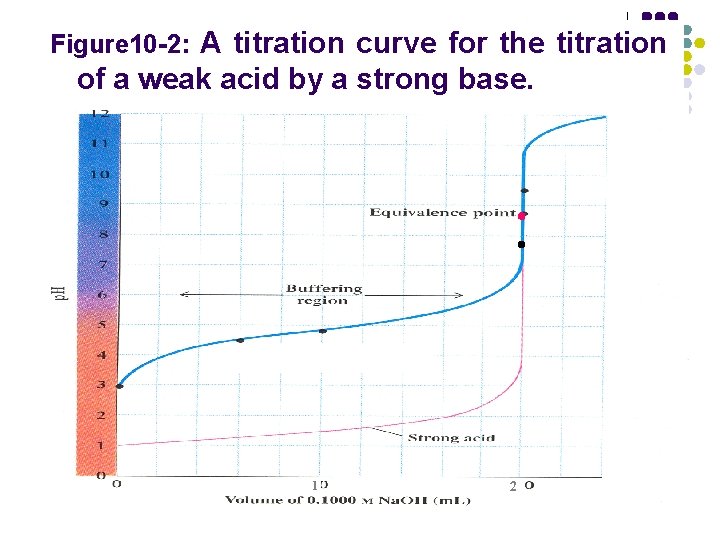
Figure 10 -2: A titration curve for the titration of a weak acid by a strong base. titration jump 7. 75~ 9. 7 △p 1. 95

Figure 10 -2: A titration curve for the titration of a weak acid by a strong base. 1 2

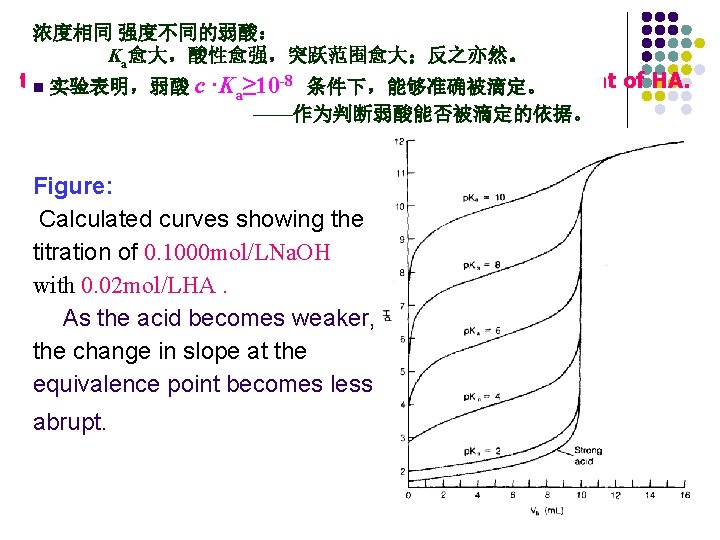
浓度相同 强度不同的弱酸: Ka愈大,酸性愈强,突跃范围愈大;反之亦然。 the titration curve acid dissociation constant of HA. n 实验表明,弱酸 c ·Kwith ≥ 10 -8 the 条件下,能够准确被滴定。 a ——作为判断弱酸能否被滴定的依据。 Figure: Calculated curves showing the titration of 0. 1000 mol/LNa. OH with 0. 02 mol/LHA. As the acid becomes weaker, the change in slope at the equivalence point becomes less abrupt.

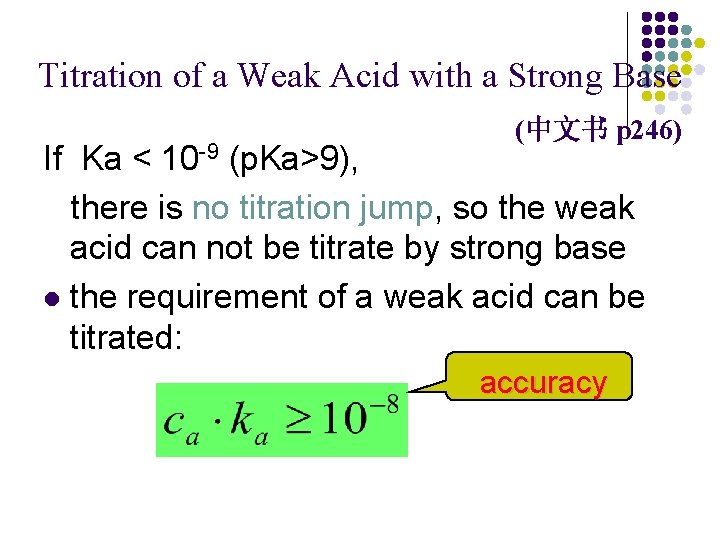
Titration of a Weak Acid with a Strong Base (中文书 p 246) If Ka < 10 -9 (p. Ka>9), there is no titration jump, so the weak acid can not be titrate by strong base l the requirement of a weak acid can be titrated: accuracy

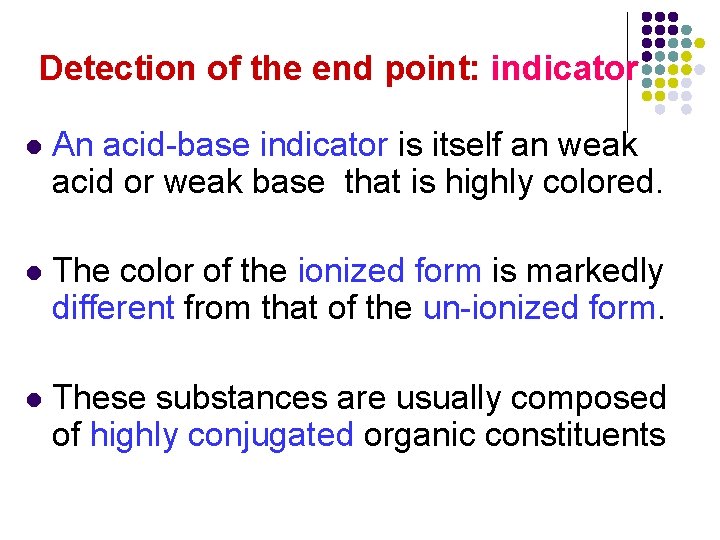
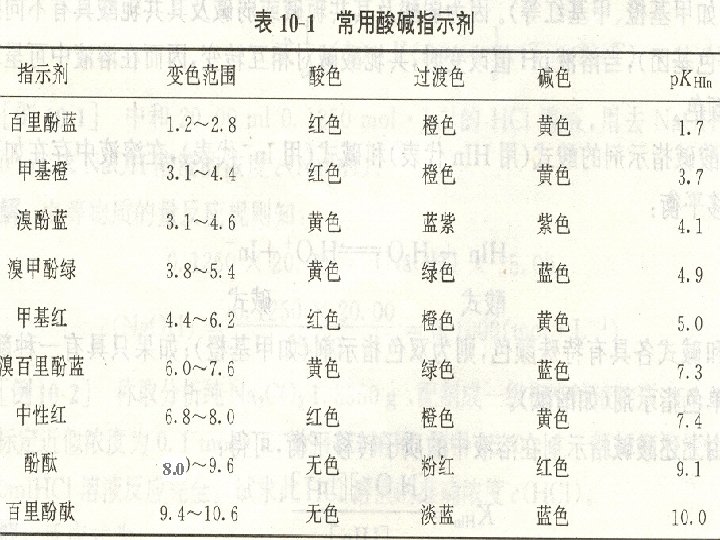
Detection of the end point: indicator l An acid-base indicator is itself an weak acid or weak base that is highly colored. l The color of the ionized form is markedly different from that of the un-ionized form. l These substances are usually composed of highly conjugated organic constituents


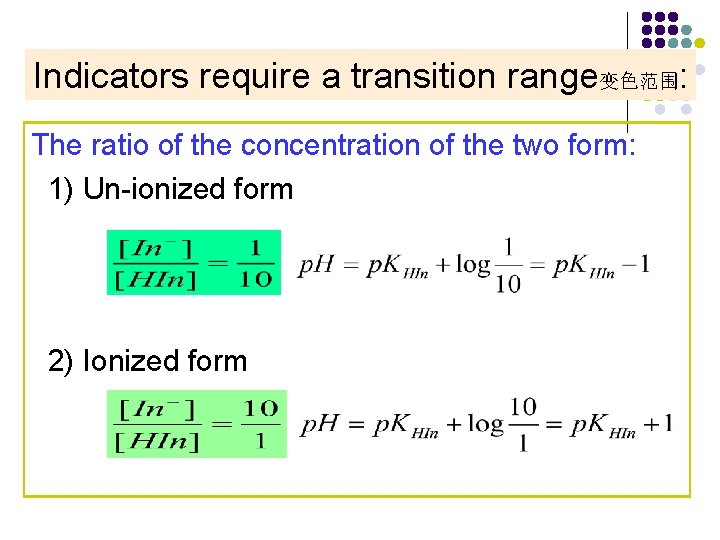
Indicators require a transition range变色范围: The ratio of the concentration of the two form: 1) Un-ionized form 2) Ionized form

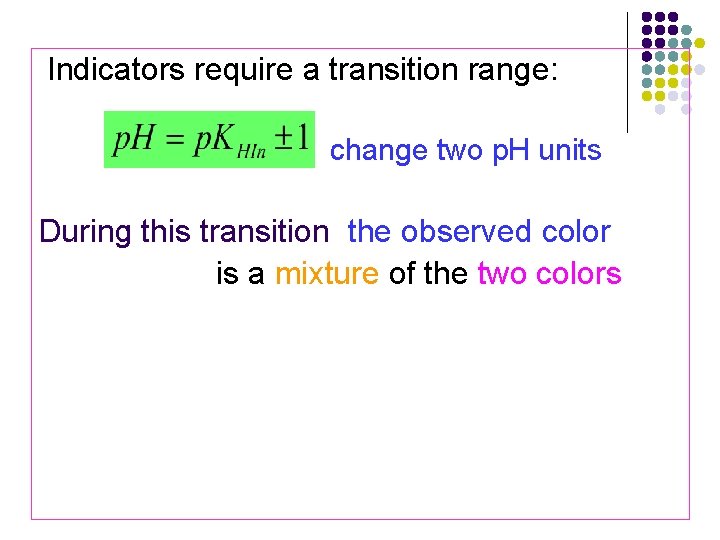
Indicators require a transition range: change two p. H units During this transition the observed color is a mixture of the two colors The indicator changes colore over a p. H range, generally one color is observed p. H=p. KHIn (transition point 变色点of indicator )


8. 0

Choosing an indicator l The p. KHIn of the indicator should be close to the p. H of the equivalence point. 1. we seek an indicator whose transition range overlaps the steepest part of the titration jump as closely as possible. 2. Some colors are easier to see than others

8. 0

20




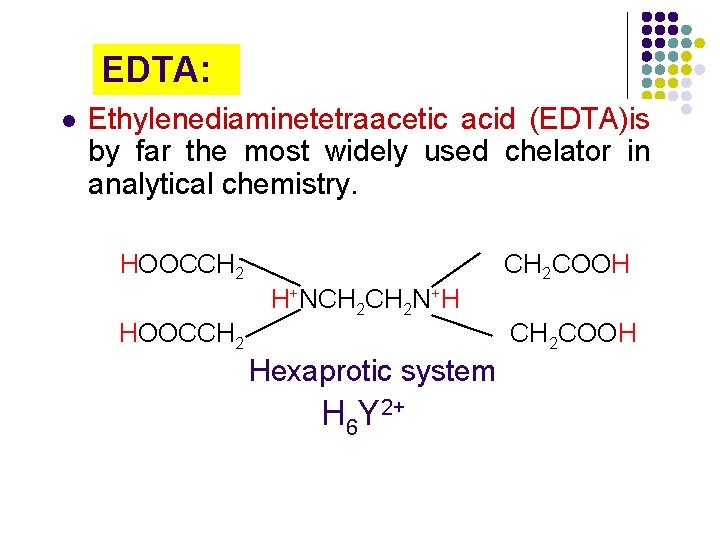
10 -3 Coordination Titrations Complexometric titration (coordination titration or EDTA titration): A titration based on formation of a complex ion is called. Chelating agent: An organic agent that has two or more groups capable of complexing with a metal ion is called a chelating agent. Chelate: the complex formed is called a chelate. Ligand: the chelating agent is called the ligand.

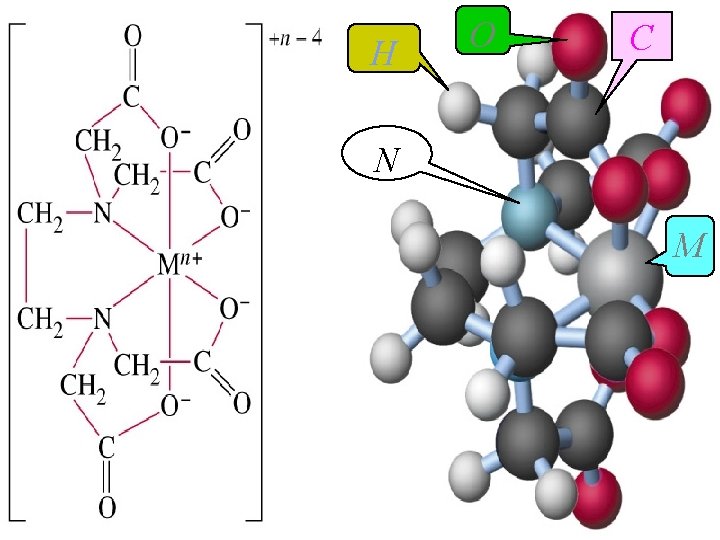
EDTA: l Ethylenediaminetetraacetic acid (EDTA)is by far the most widely used chelator in analytical chemistry. HOOCCH 2 COOH H+NCH 2 N+H HOOCCH 2 COOH Hexaprotic system H 6 Y 2+

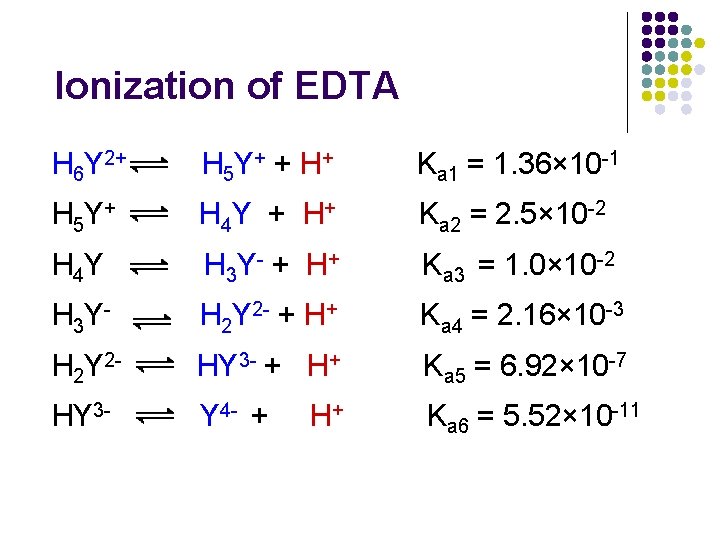
Ionization of EDTA H 6 Y 2+ H 5 Y + + H + Ka 1 = 1. 36× 10 -1 H 5 Y + H 4 Y + H + Ka 2 = 2. 5× 10 -2 H 4 Y H 3 Y - + H + Ka 3 = 1. 0× 10 -2 H 3 Y - H 2 Y 2 - + H+ Ka 4 = 2. 16× 10 -3 H 2 Y 2 - HY 3 - + H+ Ka 5 = 6. 92× 10 -7 HY 3 - Y 4 - + Ka 6 = 5. 52× 10 -11 H+

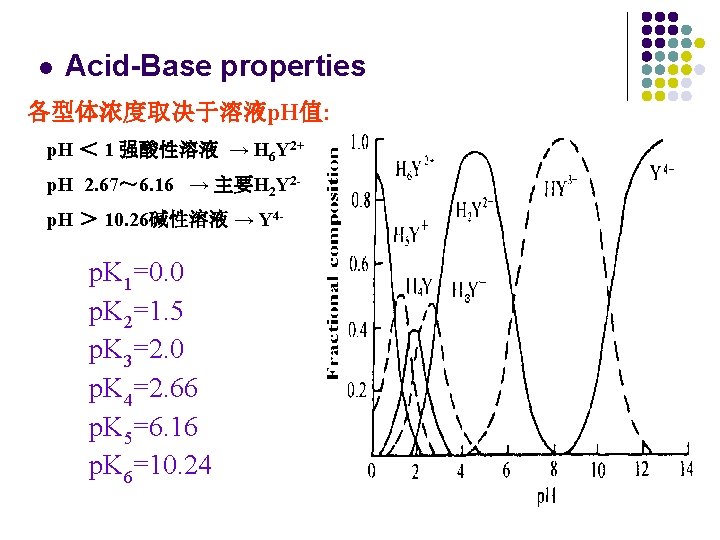
l Acid-Base properties 各型体浓度取决于溶液p. H值: p. H < 1 强酸性溶液 → H 6 Y 2+ p. H 2. 67~ 6. 16 → 主要H 2 Y 2 p. H > 10. 26碱性溶液 → Y 4 - p. K 1=0. 0 p. K 2=1. 5 p. K 3=2. 0 p. K 4=2. 66 p. K 5=6. 16 p. K 6=10. 24

l Characteristics of EDTA complexes 1) It forms strong 1: 1 complexes with most metal ions. 2) Metal complexes is easy to dissolve. 3) Stability. octahedral compound

H O C N M
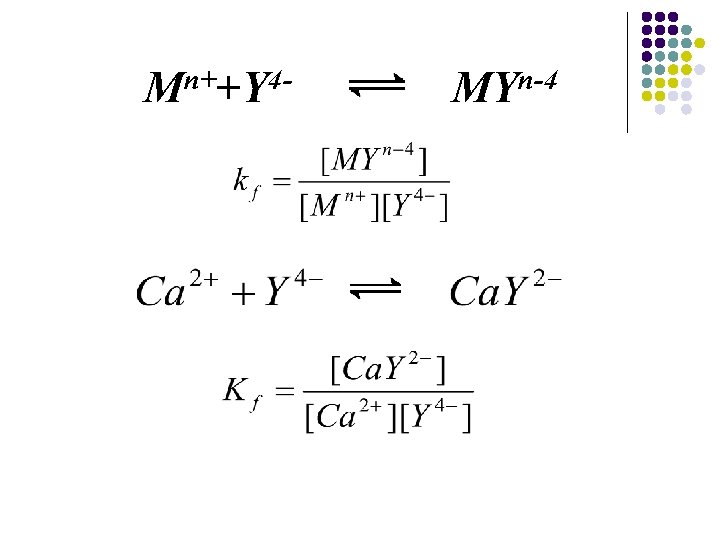
Mn++Y 4 - MYn-4

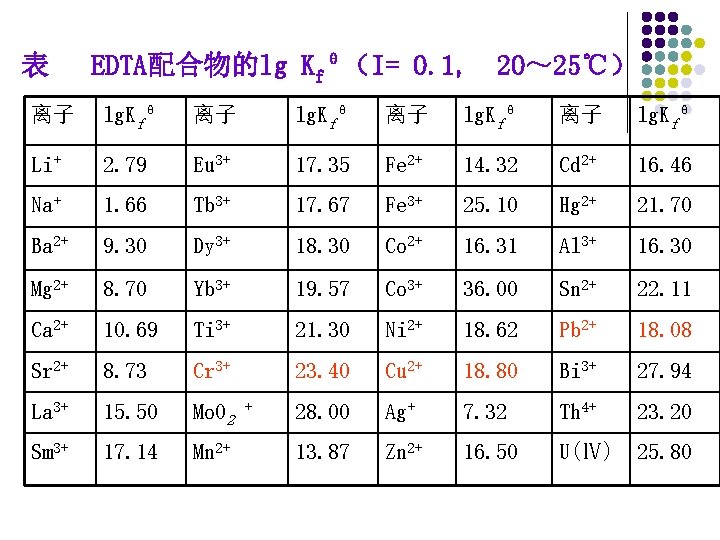
表 EDTA配合物的lg Kfθ(I= 0. 1, 20~ 25℃) 离子 lg. Kfθ Li+ 2. 79 Eu 3+ 17. 35 Fe 2+ 14. 32 Cd 2+ 16. 46 Na+ 1. 66 Tb 3+ 17. 67 Fe 3+ 25. 10 Hg 2+ 21. 70 Ba 2+ 9. 30 Dy 3+ 18. 30 Co 2+ 16. 31 Al 3+ 16. 30 Mg 2+ 8. 70 Yb 3+ 19. 57 Co 3+ 36. 00 Sn 2+ 22. 11 Ca 2+ 10. 69 Ti 3+ 21. 30 Ni 2+ 18. 62 Pb 2+ 18. 08 Sr 2+ 8. 73 Cr 3+ 23. 40 Cu 2+ 18. 80 Bi 3+ 27. 94 La 3+ 15. 50 Mo. O 2 28. 00 Ag+ 7. 32 Th 4+ 23. 20 Sm 3+ 17. 14 Mn 2+ 13. 87 Zn 2+ 16. 50 U(Ⅳ) 25. 80 +


配位滴定中酸度的控制 EDTA的酸效应曲线 Figure (10 -7) gives the minimum p. H needed for titration of many metal ions.





Metal Ion Indicators Eriochrome Black T is a typical indicator. It contains three ionizable protons, so we will represent it by H 3 In. -H+ H 2 In. HIn 2 In 3 l p. Ka 2=6. 3 p. H<6 purple p. Ka 3=11. 6 p. H=8~10 blue 铬黑T p. H>12 orange

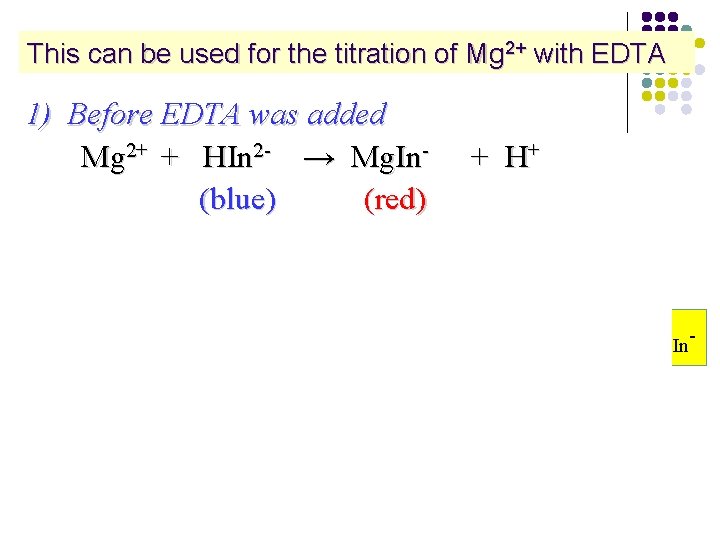
This can be used for the titration of Mg 2+ with EDTA 1) Before EDTA was added Mg 2+ + HIn 2 - → Mg. In- + H+ (blue) (red) 2) Among titration Mg 2+ + HY 3 - → Mg. Y 2 - + H+ (colorless) KMg. Y 2 - > KMg. In- 3) End point Mg. In- + HY 3 - → Mg. Y 2 - + HIn 2(red) (colorless) (blue)

Of couse, the metal indicator complex must be less stable than that of the metal-EDTA complex, or else the EDTA will not displace it from the metal. on the other hand, it must not be too weak, or the EDTA will stare replacing it at the beginning of the titration, and a diffuse end point will result. In general, the metal indicator complex should be 10 to 100 time less stable than the metal-titrant complex.

Application of EDTA titration Determine overall hardness of water • Hard water and soft water • Overall hardness of water is amount of Ca 2+ and Mg 2+ in water. • 1°= 1 mmol/L Ca 2+ and Mg 2+ • Titrant : EDTA (Na 2 H 2 Y) • p. H = 8 ~10 (NH 3 -NH 4 Cl buffer) • Indicator : EBT

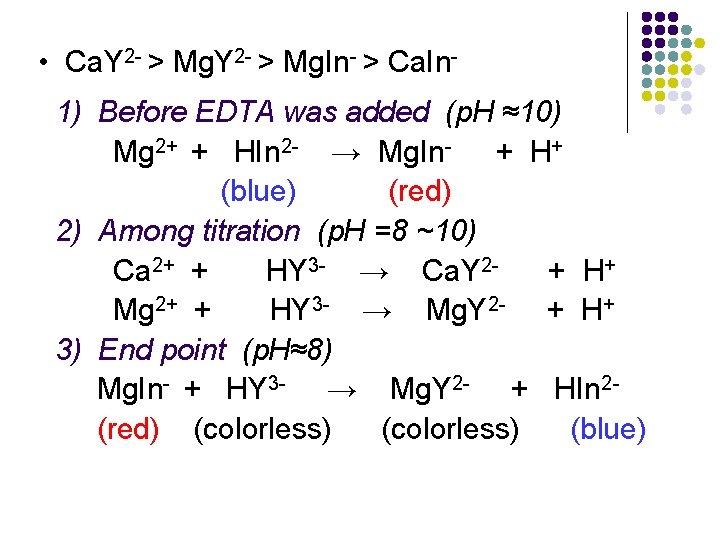
• Ca. Y 2 - > Mg. In- > Ca. In 1) Before EDTA was added (p. H ≈10) Mg 2+ + HIn 2 - → Mg. In- + H+ (blue) (red) 2) Among titration (p. H =8 ~10) Ca 2+ + HY 3 - → Ca. Y 2+ H+ Mg 2+ + HY 3 - → Mg. Y 2 - + H+ 3) End point (p. H≈8) Mg. In- + HY 3 - → Mg. Y 2 - + HIn 2(red) (colorless) (blue)

10 -4 Analytical Error and Significant Figures



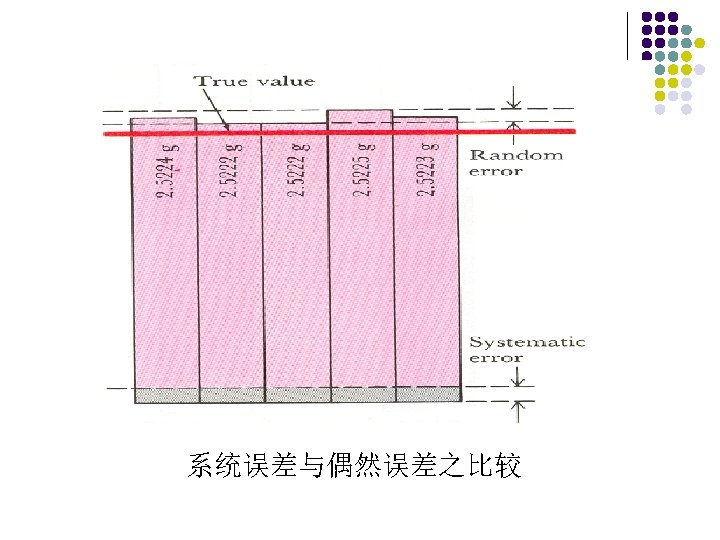
Analytical Error 1. Systematic Error or Determinate Error: It can in principle be discovered and corrected. Errors of the method Operative errors Instrumental errors Reagent errors


Indeterminate Errors or Random Errors: 2. which represent the experimental uncertainty that occurs in any measurement. 偶然误差又称不定误差或随机误差,由于一些难以察觉的 或不可控制的随机因素导致的误差。 纠正方法:“多次测量,取平均值” 以减少偶然误差。


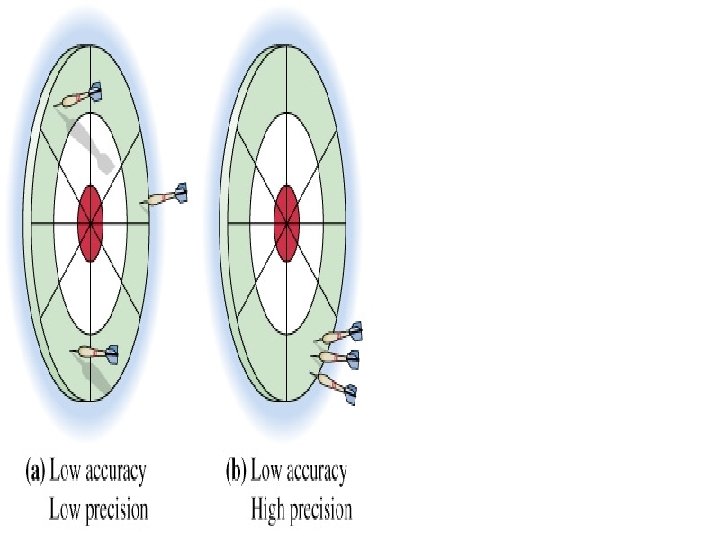
Accuracy And Precision l Accuracy is the degree of agreement between the measured value and the true value. l Precision is defined as the degree of agreement between replicate measurements of the same quantity.



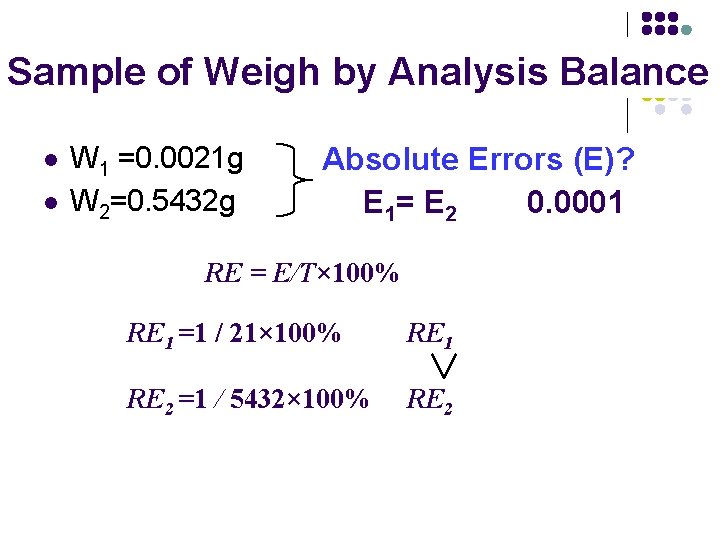
1. Absolute Errors (E): The difference between the true value (T) and the measured value (x)。 E = x – T (测定值与真值之差) 2. Relative Error (RE) The absolute error expressed as a percentage of the true value is the relative error. RE = E/T× 100% (绝对误差在真值中所占的百分率)


Sample of Weigh by Analysis Balance l l W 1 =0. 0021 g W 2=0. 5432 g Absolute Errors (E)? E 1= E 2 0. 0001 RE = E/T× 100% RE 1 =1 / 21× 100% RE 1 RE 2 =1 / 5432× 100% RE 2


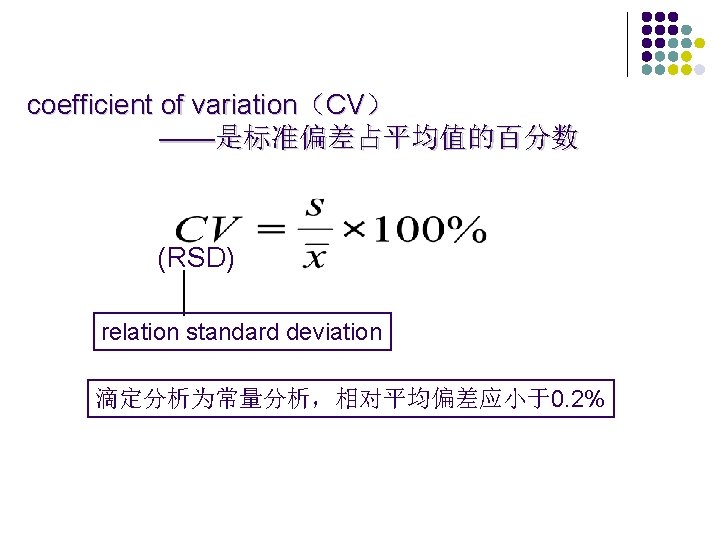
精密度(precision) —多次重复测定某一量时所得测量值的离散程度, 常以偏差来表示。 Absolute deviation (d) l Relative deviation(Rd) l Absolute average deviation (d ) l Relative average deviation (Rd) l





例: 在分析某一样品中Cl含量为: 39. 87, 39. 94, 40. 10, 39. 74, 39. 90, 39. 88,求各偏差值 X 39. 87 39. 94 40. 10 39. 74 39. 90 39. 88 X d d d/X S 39. 905 -0. 035 +0. 195 -0. 165 -0. 005 -0. 025 0. 077 1. 92‰ 0. 117 • 相对标准偏差 也称变异系数(CV),其计算式为: CV =S / x 100% 本例中 CV = 0. 29%














 Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic vs inorganic
Organic vs inorganic Smear clear composition
Smear clear composition Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Which compound is inorganic
Which compound is inorganic Importance of organic compounds
Importance of organic compounds Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Organic and inorganic cofactors
Organic and inorganic cofactors Organic vs inorganic compounds
Organic vs inorganic compounds Organic and inorganic cofactors
Organic and inorganic cofactors Iupac nomenclature of cycloalkanes
Iupac nomenclature of cycloalkanes Organic vs inorganic compounds
Organic vs inorganic compounds Organic growth vs inorganic growth
Organic growth vs inorganic growth Organic and inorganic nutrients
Organic and inorganic nutrients Asam oksalat rumus kimia
Asam oksalat rumus kimia Organic vs inorganic
Organic vs inorganic Organic versus inorganic compounds
Organic versus inorganic compounds Organic vs inorganic
Organic vs inorganic What is monograph
What is monograph Ib organic chemistry functional groups
Ib organic chemistry functional groups Pericyclic
Pericyclic Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Nonene
Nonene Analytical chemistry chapters
Analytical chemistry chapters Halohydrin
Halohydrin Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Introduction to inorganic chemistry
Introduction to inorganic chemistry Is inorganic chemistry hard
Is inorganic chemistry hard It's twenty to ten.
It's twenty to ten. Ten ten siempre fuerzas y esperanza
Ten ten siempre fuerzas y esperanza Amia 10 x 10
Amia 10 x 10 Father of organic chemistry
Father of organic chemistry Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Functional group vs homologous series
Functional group vs homologous series Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Ario practice problems
Ario practice problems Ch3ch2ch2cooch(ch3)2 name
Ch3ch2ch2cooch(ch3)2 name Organic chemistry lab report example
Organic chemistry lab report example Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Cyclo organic chemistry
Cyclo organic chemistry Organic chemistry wade
Organic chemistry wade Prefix for alkanes
Prefix for alkanes Cracking organic chemistry
Cracking organic chemistry Met et prop but pent hex hept oct non dec undec
Met et prop but pent hex hept oct non dec undec Organic chemistry myanmar
Organic chemistry myanmar Hhcchh
Hhcchh Alpha cleavage
Alpha cleavage Hono organic chemistry
Hono organic chemistry Geminal and vicinal
Geminal and vicinal Topic 11 organic chemistry
Topic 11 organic chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Alkene alcohol naming
Alkene alcohol naming What is organic chemistry like
What is organic chemistry like Organic chemistry vocabulary
Organic chemistry vocabulary Separation scheme of caffeine from vivarin tablets
Separation scheme of caffeine from vivarin tablets A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Difference between fats and oil
Difference between fats and oil Ario+
Ario+ How to calculate percentage yield in organic chemistry
How to calculate percentage yield in organic chemistry Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Radicals
Radicals Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Klein
Klein Organic chemistry case studies
Organic chemistry case studies Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Octane lewis structure
Octane lewis structure Conformation of sugars ppt
Conformation of sugars ppt Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Macromolecules cheat sheet
Macromolecules cheat sheet Mindup mind map
Mindup mind map Organic chemistry stuart warren
Organic chemistry stuart warren Ir spectroscopy
Ir spectroscopy The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms Rhodopsin cgmp
Rhodopsin cgmp Organic chemistry
Organic chemistry Condensed structure of cyclohexane
Condensed structure of cyclohexane Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? Structure name
Structure name Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry David klein
David klein Organic chemistry
Organic chemistry Chemistry
Chemistry Nbs reaction
Nbs reaction Organic composition definition
Organic composition definition Butan 2 on
Butan 2 on Functional groups organic chemistry table
Functional groups organic chemistry table Organic chemistry class 11 notes
Organic chemistry class 11 notes Hybridisation
Hybridisation What is a branched hydrocarbon
What is a branched hydrocarbon Iodine test for starch
Iodine test for starch Conjugation organic chemistry
Conjugation organic chemistry Organic chemistry nomenclature
Organic chemistry nomenclature Functional groups
Functional groups Organic chemistry
Organic chemistry Hex hept oct non dec
Hex hept oct non dec Ferrox test for oxygen
Ferrox test for oxygen Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane Acetoacetic ester synthesis mechanism
Acetoacetic ester synthesis mechanism Organic chemistry
Organic chemistry Brooklyn college organic chemistry
Brooklyn college organic chemistry What is this
What is this Inorganic plants
Inorganic plants Veins are often formed from hot water solutions
Veins are often formed from hot water solutions Prosthetic group example
Prosthetic group example Emulsifying agent pharmaceutics
Emulsifying agent pharmaceutics Inorganic catalyst vs enzyme
Inorganic catalyst vs enzyme Inorganic matrix of bone
Inorganic matrix of bone