Chapter Seven Quantum Theory and the Electronic Structure

- Slides: 11

Chapter Seven Quantum Theory and the Electronic Structure of Atoms

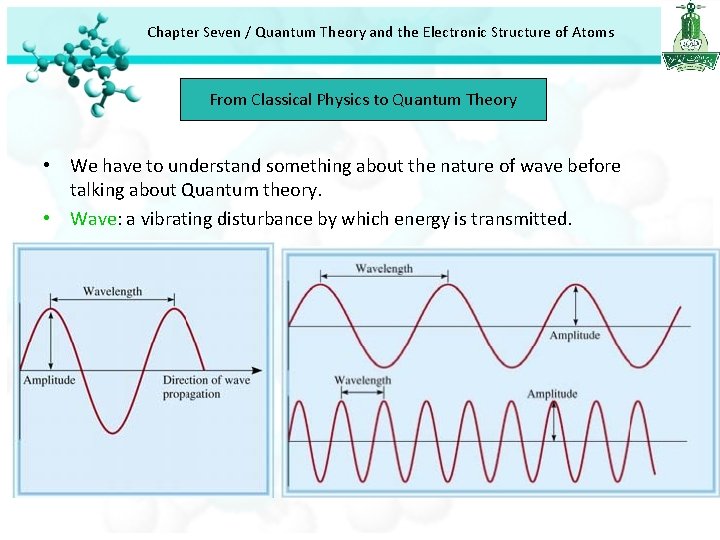
Chapter Seven / Quantum Theory and the Electronic Structure of Atoms From Classical Physics to Quantum Theory • We have to understand something about the nature of wave before talking about Quantum theory. • Wave: a vibrating disturbance by which energy is transmitted.

Chapter Seven / Quantum Theory and the Electronic Structure of Atoms From Classical Physics to Quantum Theory • Wavelength (λ) lambda: is the distance between identical points on successive waves. • Frequency (ν) nu: is the number of waves that pass through a particular point in 1 second. • Amplitude : is the vertical distance from the midline of a wave to the peak. • Wave speed (u): depend on type of wave and the nature of the medium through which the wave is traveling. u = λν • Wavelength usually expressed in units of meter, centimeter, or nanometer. • Frequency is measured in hertz (Hz) 1 Hz = 1 cycle/s Normally the word cycle is left out and we say and we expressed frequency as for example 25/s

Chapter Seven / Quantum Theory and the Electronic Structure of Atoms From Classical Physics to Quantum Theory Example: The wavelength of the green light from a traffic signal is centered at 522 nm. What is the frequency of this radiation? u =λν ν=u/λ The speed of light is known as 3 x 108 m/s Because the speed light in m we have to change the wavelength to m λ = 522 x 10 -9 m ν = 3 x 108 / 522 x 10 -9 = 5. 75 x 1014 Hz.

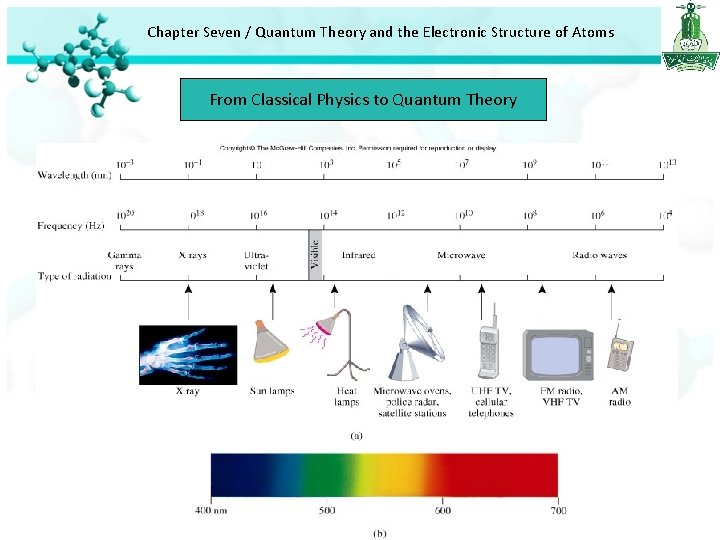
Chapter Seven / Quantum Theory and the Electronic Structure of Atoms From Classical Physics to Quantum Theory • There are many type of waves, such as water waves, sound waves and light waves. • Clerk Maxwell proposed in 1973 that visible light consists of electromagnetic wave has an electric field component and a magnetic filed component. The two components have the same wavelength and frequency, and hence the speed. • Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves. • for all electromagnetic radiation c = λν Where c is the speed of light = 3 x 108 m/s

Chapter Seven / Quantum Theory and the Electronic Structure of Atoms From Classical Physics to Quantum Theory

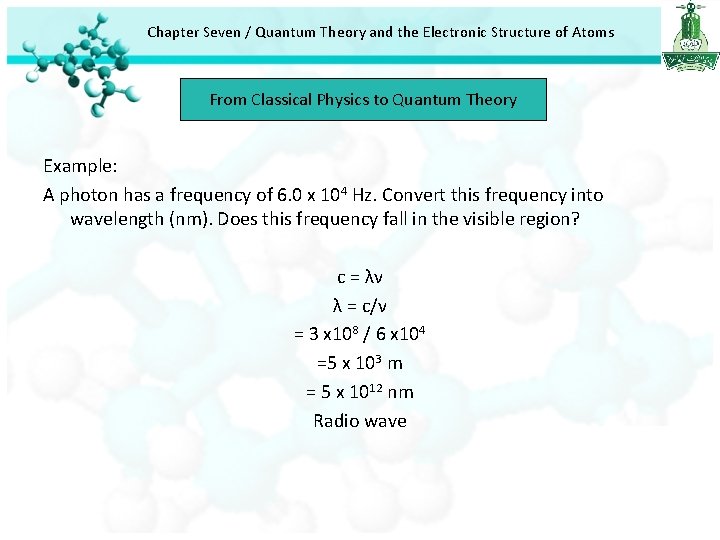
Chapter Seven / Quantum Theory and the Electronic Structure of Atoms From Classical Physics to Quantum Theory Example: A photon has a frequency of 6. 0 x 104 Hz. Convert this frequency into wavelength (nm). Does this frequency fall in the visible region? c = λν λ = c/ν = 3 x 108 / 6 x 104 =5 x 103 m = 5 x 1012 nm Radio wave

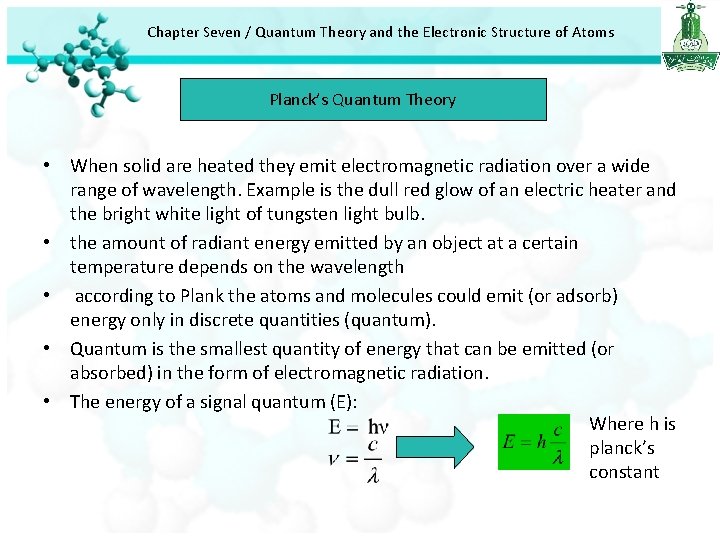
Chapter Seven / Quantum Theory and the Electronic Structure of Atoms Planck’s Quantum Theory • When solid are heated they emit electromagnetic radiation over a wide range of wavelength. Example is the dull red glow of an electric heater and the bright white light of tungsten light bulb. • the amount of radiant energy emitted by an object at a certain temperature depends on the wavelength • according to Plank the atoms and molecules could emit (or adsorb) energy only in discrete quantities (quantum). • Quantum is the smallest quantity of energy that can be emitted (or absorbed) in the form of electromagnetic radiation. • The energy of a signal quantum (E): Where h is planck’s constant

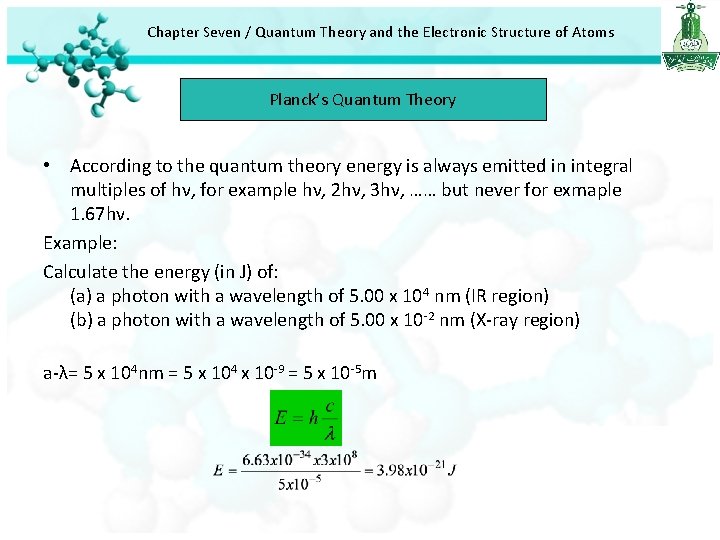
Chapter Seven / Quantum Theory and the Electronic Structure of Atoms Planck’s Quantum Theory • According to the quantum theory energy is always emitted in integral multiples of hν, for example hν, 2 hν, 3 hν, …… but never for exmaple 1. 67 hν. Example: Calculate the energy (in J) of: (a) a photon with a wavelength of 5. 00 x 104 nm (IR region) (b) a photon with a wavelength of 5. 00 x 10 -2 nm (X-ray region) a-λ= 5 x 104 nm = 5 x 104 x 10 -9 = 5 x 10 -5 m

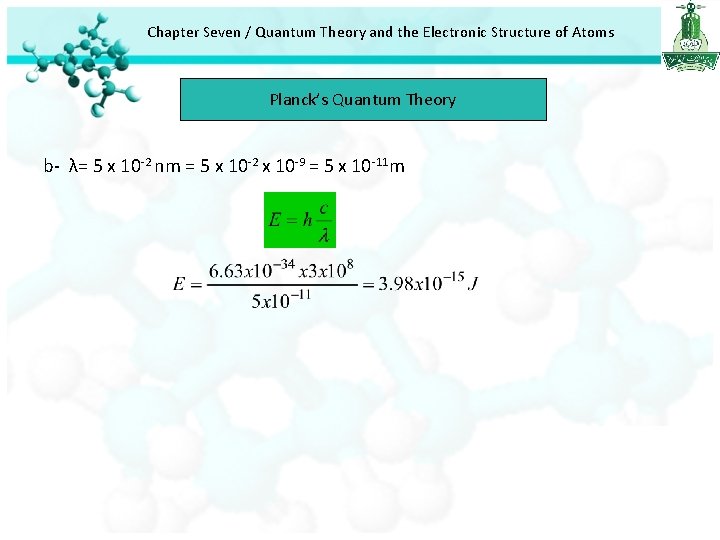
Chapter Seven / Quantum Theory and the Electronic Structure of Atoms Planck’s Quantum Theory b- λ= 5 x 10 -2 nm = 5 x 10 -2 x 10 -9 = 5 x 10 -11 m
