Chapter Introduction Lesson 1 Classifying Matter Lesson 2







- Slides: 25

Chapter Introduction Lesson 1 Classifying Matter Lesson 2 Physical Properties

Chemistry: The study of matter and the changes it undergoes.

Understanding Matter is anything that has mass and takes up space.

– What is mass? • What is volume? – How is it measured? • Which has more volume a deflated balloon or an inflated balloon?

Understanding Matter (cont. ) 1. Everything you can see is matter. 2. Are there things you cannot see, that are matter? 3. What is the smallest whole part of matter?

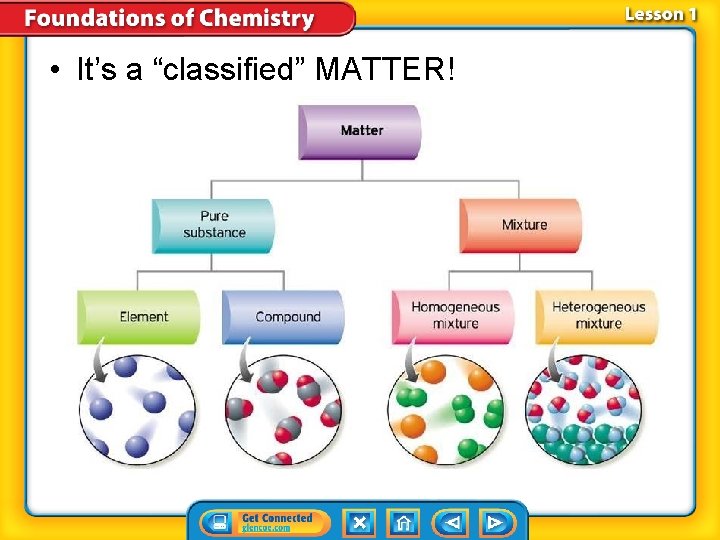
• It’s a “classified” MATTER!

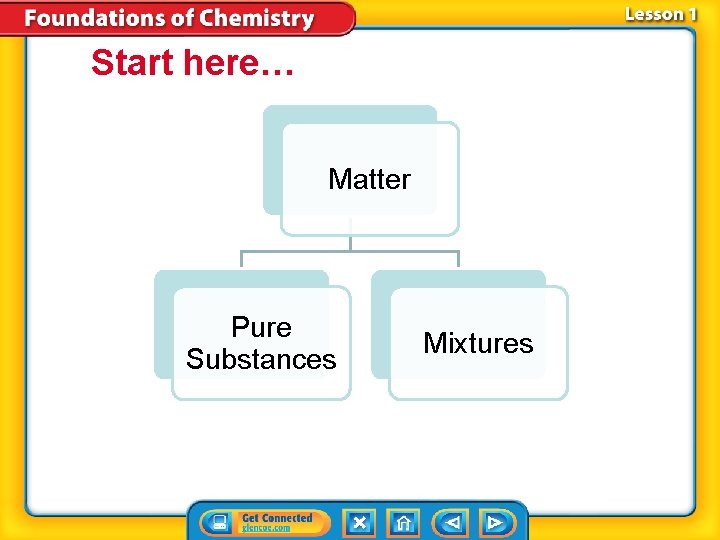
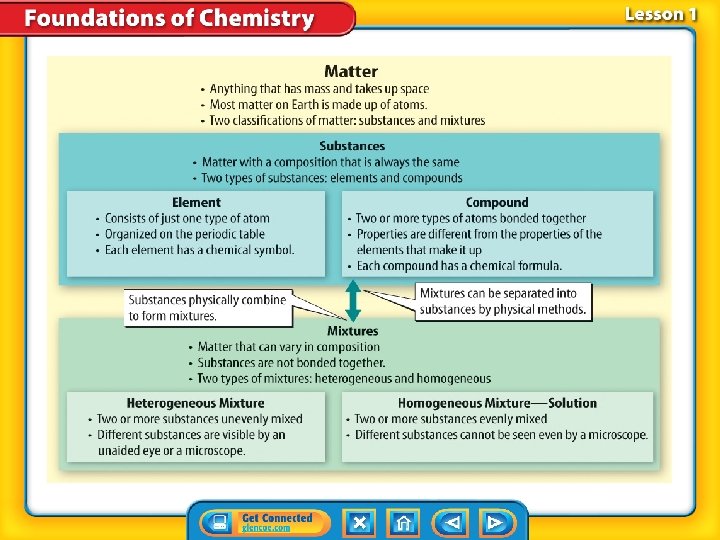
Start here… Matter Pure Substances Mixtures

Pure Substances 1. A substance is matter where the entire composition is ALWAYS the same. 2. Meaning: – it is always made up of one or more atoms in the same combinations. – Examples are: elements and compounds. » 118 elements and 10 million or more compounds

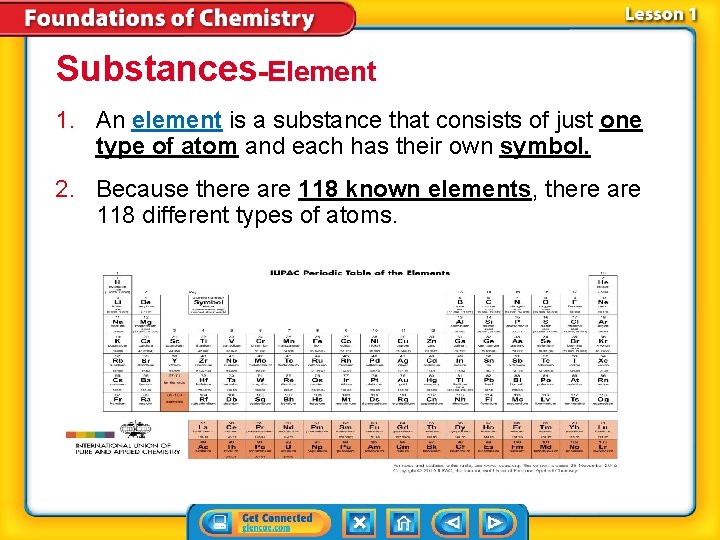
Substances-Element 1. An element is a substance that consists of just one type of atom and each has their own symbol. 2. Because there are 118 known elements, there are 118 different types of atoms.

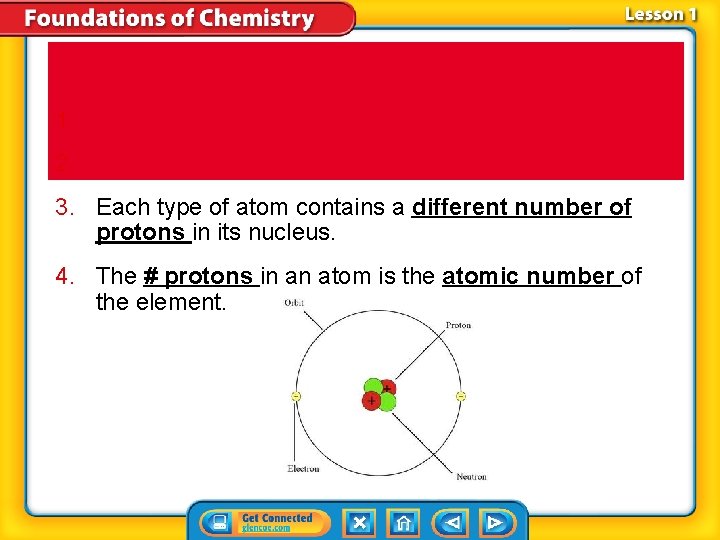
Substances-Element 1. 2. 3. Each type of atom contains a different number of protons in its nucleus. 4. The # protons in an atom is the atomic number of the element.

Substances-Compound 1. A compound is a type of substance containing atoms of two or more different elements chemically bonded together. 2. The combination of symbols and numbers that represents a compound is called a chemical formula. glucose

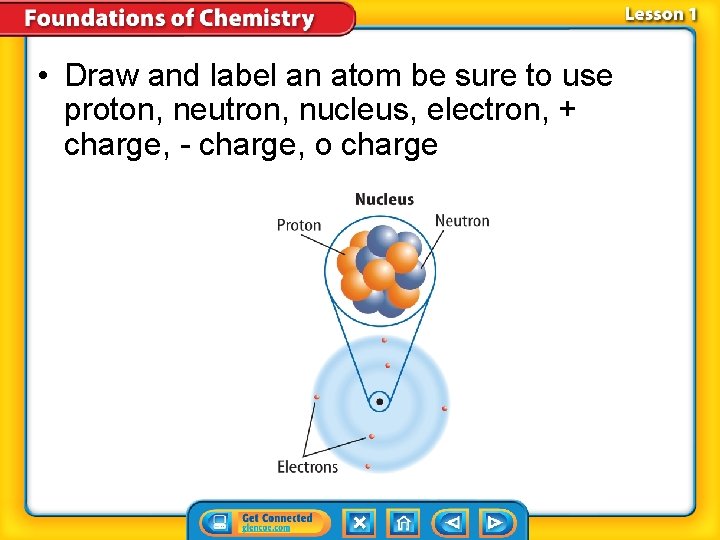
• Draw and label an atom be sure to use proton, neutron, nucleus, electron, + charge, - charge, o charge

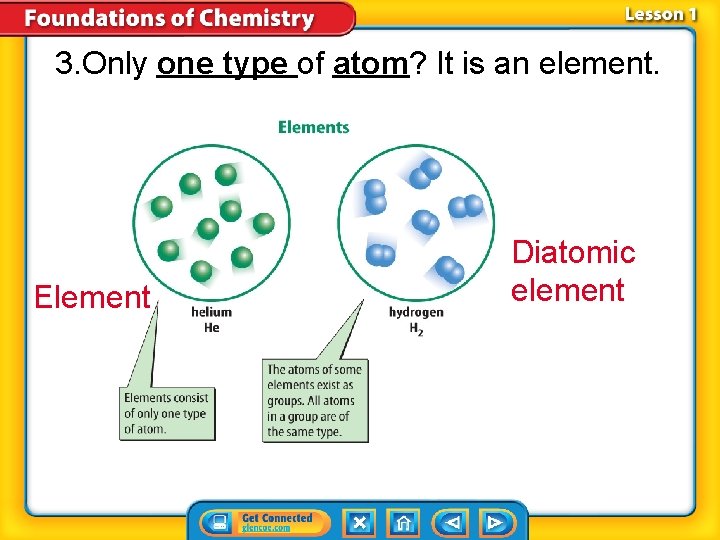
3. Only one type of atom? It is an element. Element Diatomic element

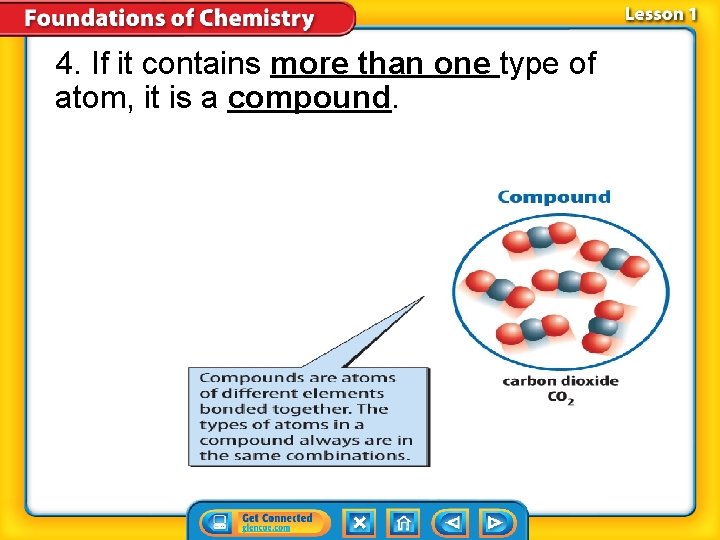
4. If it contains more than one type of atom, it is a compound.

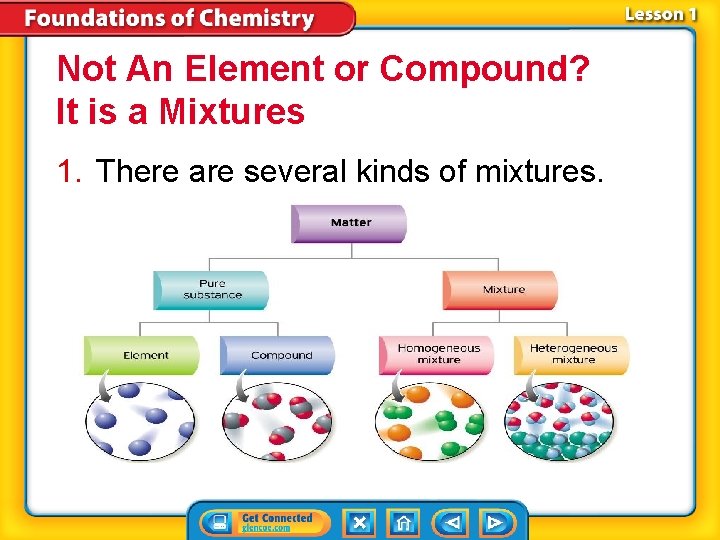
Not An Element or Compound? It is a Mixtures 1. There are several kinds of mixtures.

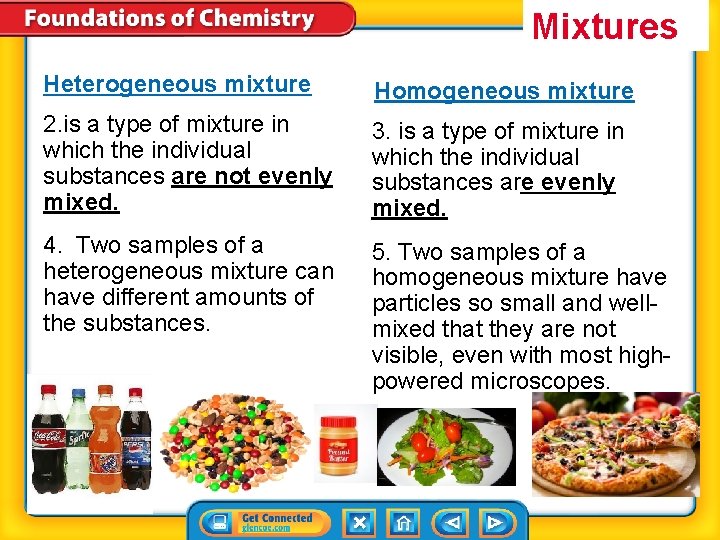
Mixtures Heterogeneous mixture Homogeneous mixture 2. is a type of mixture in which the individual substances are not evenly mixed. 3. is a type of mixture in which the individual substances are evenly mixed. 4. Two samples of a heterogeneous mixture can have different amounts of the substances. 5. Two samples of a homogeneous mixture have particles so small and wellmixed that they are not visible, even with most highpowered microscopes.

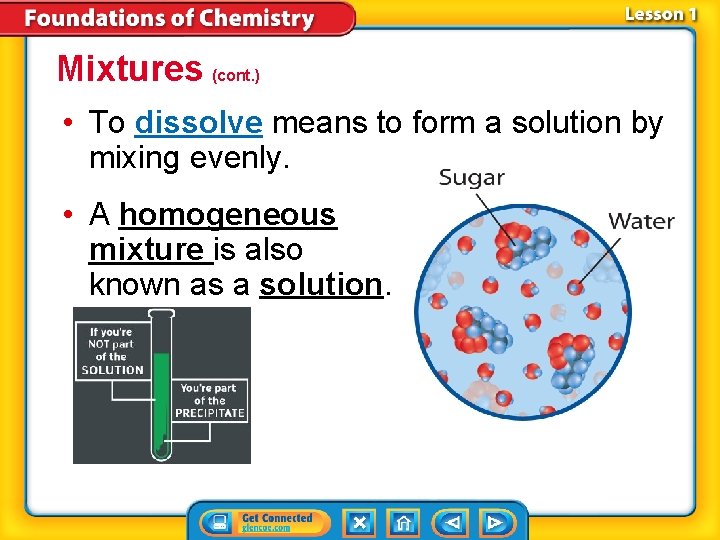
Mixtures (cont. ) • To dissolve means to form a solution by mixing evenly. • A homogeneous mixture is also known as a solution.

Compounds v. Solutions • The composition in a compound does not vary. Therefore, a chemical formula can be used to describe the compound. • Because composition in a mixture can vary, a chemical formula cannot be used to describe mixtures.


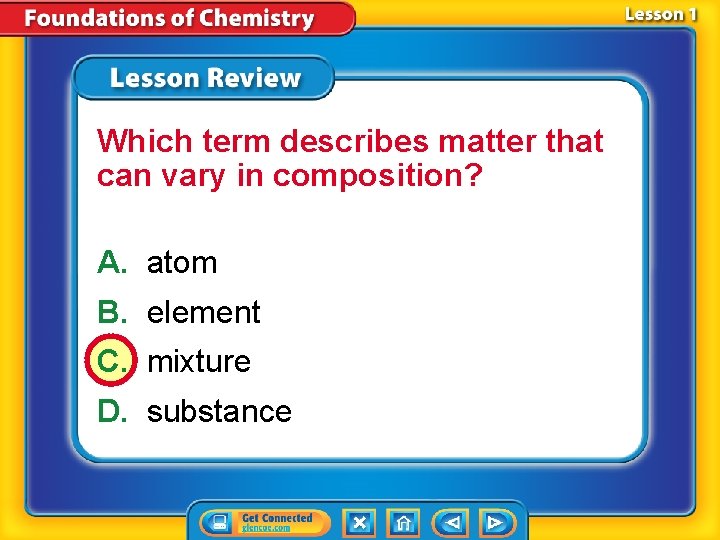
Which term describes matter that can vary in composition? A. atom B. element C. mixture D. substance

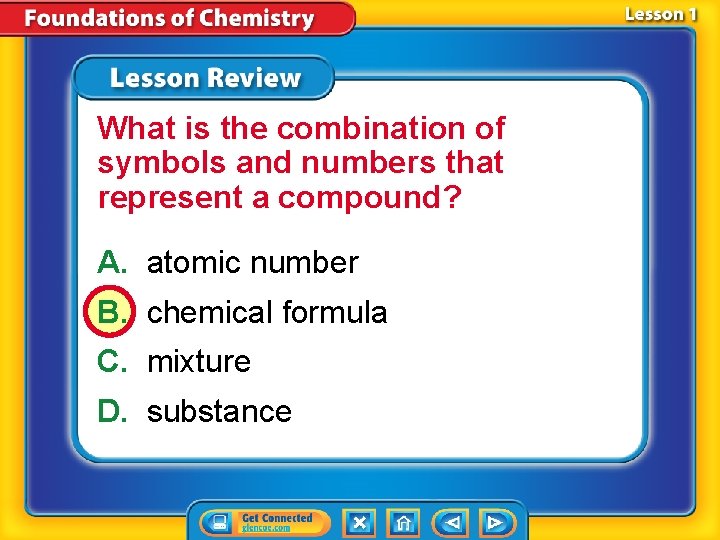
What is the combination of symbols and numbers that represent a compound? A. atomic number B. chemical formula C. mixture D. substance

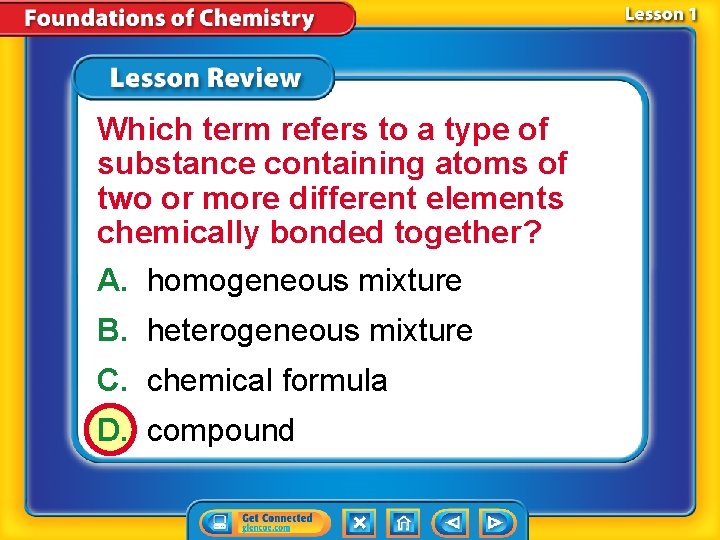
Which term refers to a type of substance containing atoms of two or more different elements chemically bonded together? A. homogeneous mixture B. heterogeneous mixture C. chemical formula D. compound

Do you agree or disagree? 1. The atoms in all objects are the same. 2. You cannot always tell by an object’s appearance whether it is made of more than one type of atom.

Physical Properties • What are some physical properties of matter? • How are physical properties used to separate mixtures?

Physical Properties • physical property • mass • density • solubility