Chapter Eight Molecular Motion NMR Molecular vibrations All

Chapter Eight Molecular Motion & NMR
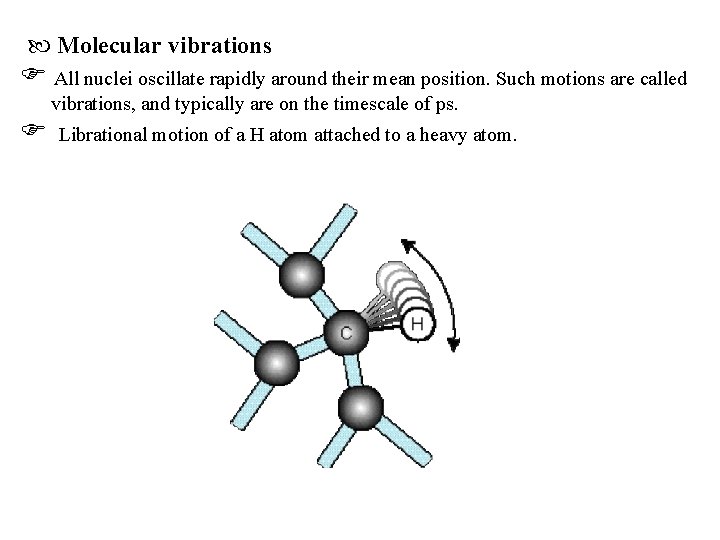
Molecular vibrations All nuclei oscillate rapidly around their mean position. Such motions are called vibrations, and typically are on the timescale of ps. Librational motion of a H atom attached to a heavy atom.
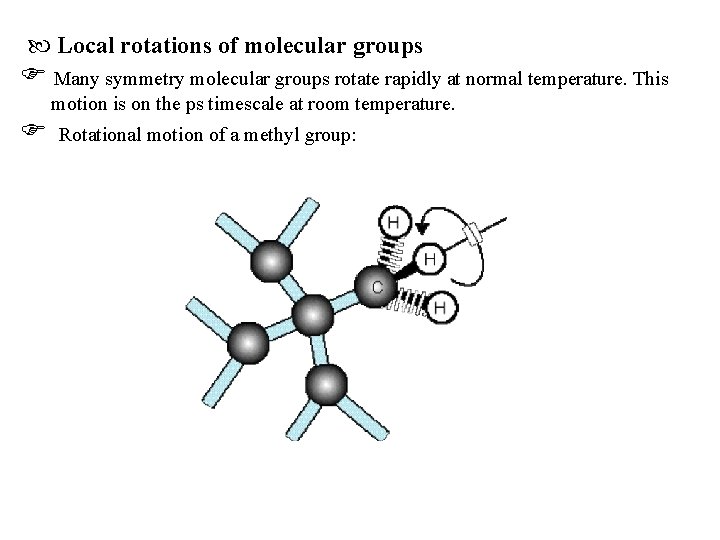
Local rotations of molecular groups Many symmetry molecular groups rotate rapidly at normal temperature. This motion is on the ps timescale at room temperature. Rotational motion of a methyl group:
Molecular flexibility Large molecules like protein have a large degree of internal flexibility, which is in some cases indispensable for their function.
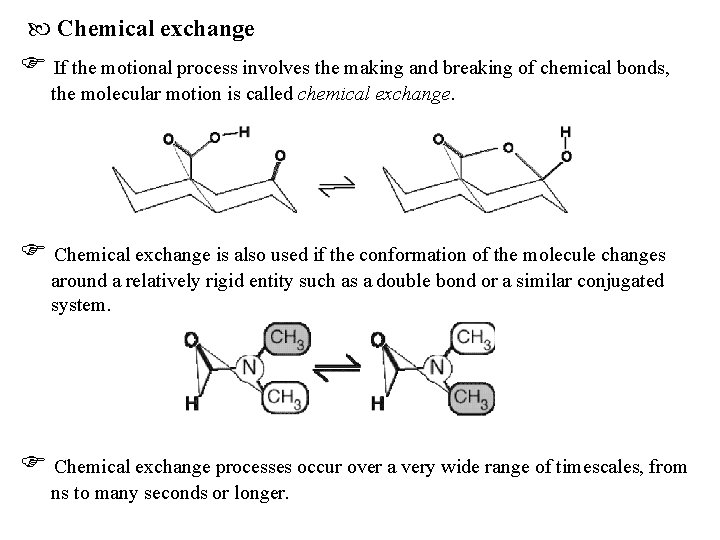
Chemical exchange If the motional process involves the making and breaking of chemical bonds, the molecular motion is called chemical exchange. Chemical exchange is also used if the conformation of the molecule changes around a relatively rigid entity such as a double bond or a similar conjugated system. Chemical exchange processes occur over a very wide range of timescales, from ns to many seconds or longer.
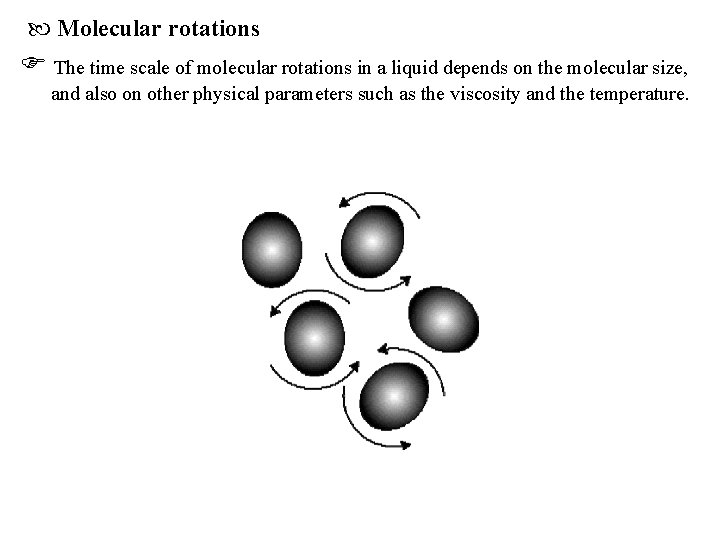
Molecular rotations The time scale of molecular rotations in a liquid depends on the molecular size, and also on other physical parameters such as the viscosity and the temperature.
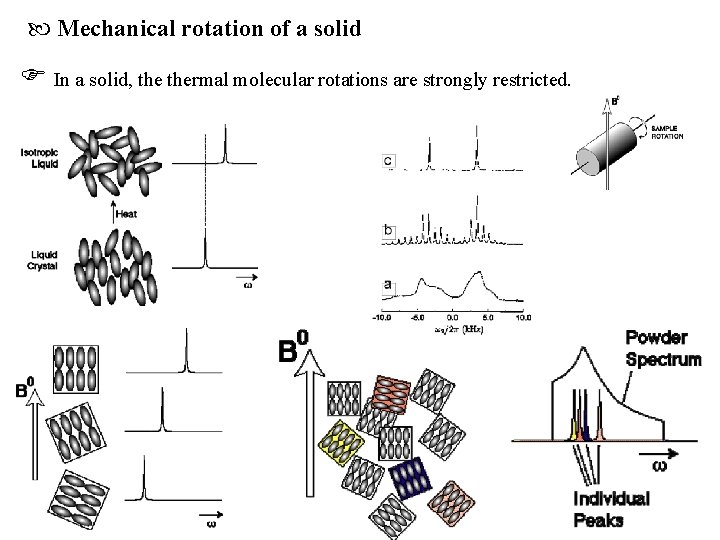
Mechanical rotation of a solid In a solid, thermal molecular rotations are strongly restricted.
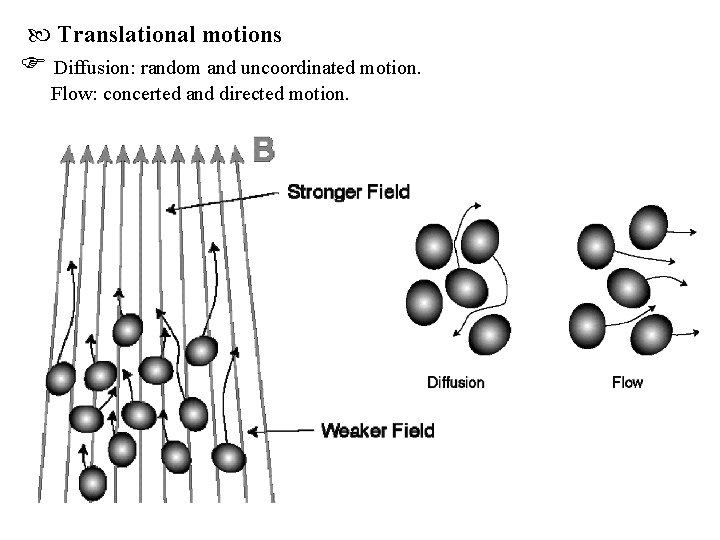
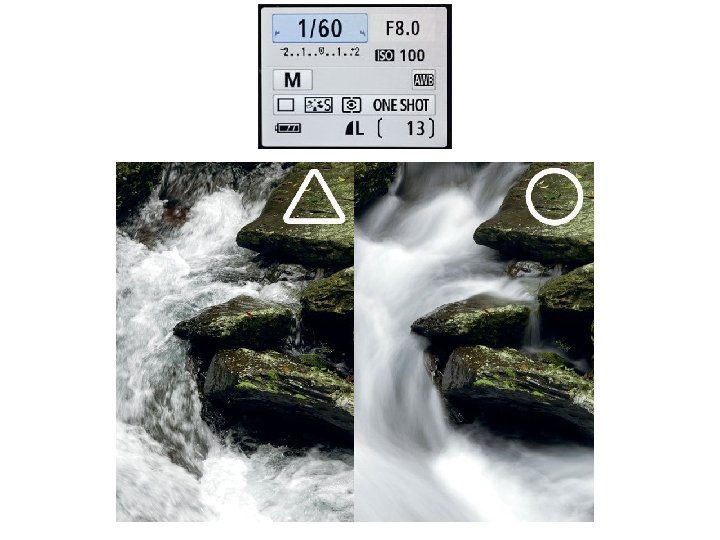
Translational motions Diffusion: random and uncoordinated motion. Flow: concerted and directed motion.
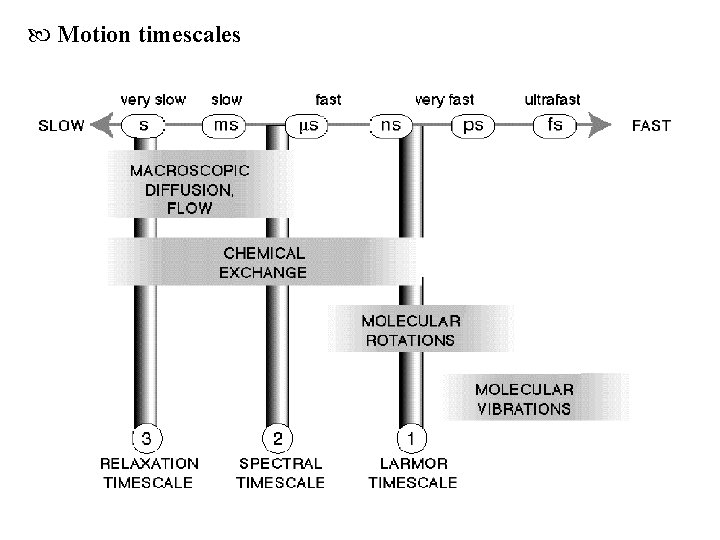
Motion timescales
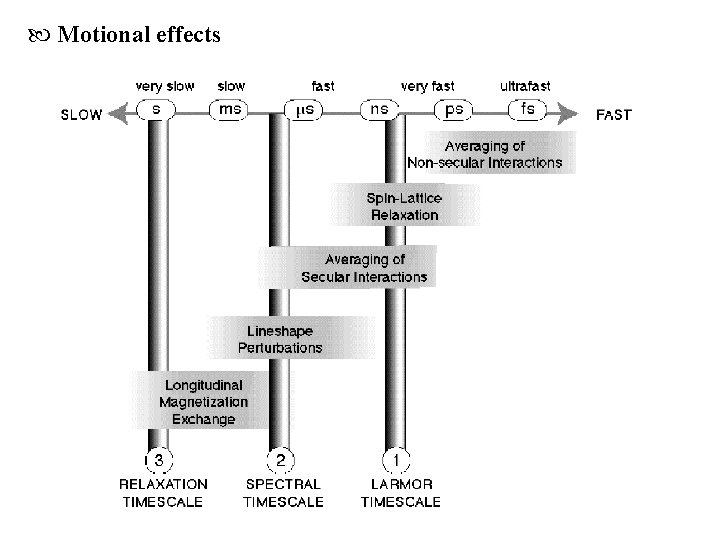
Motional effects
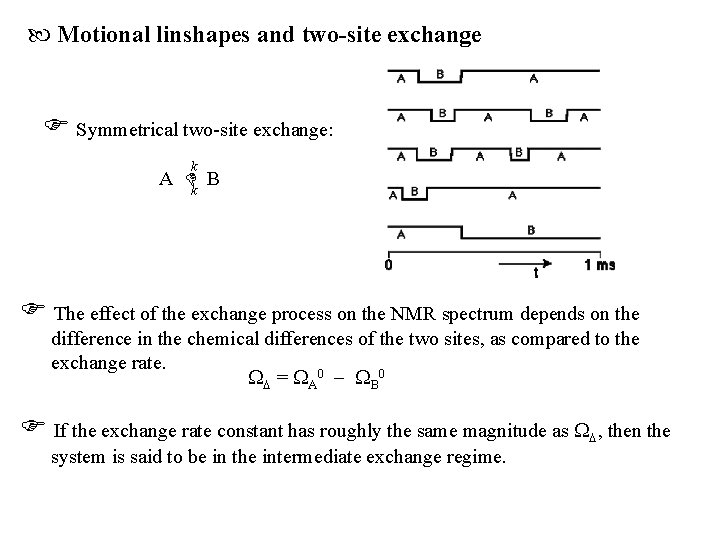
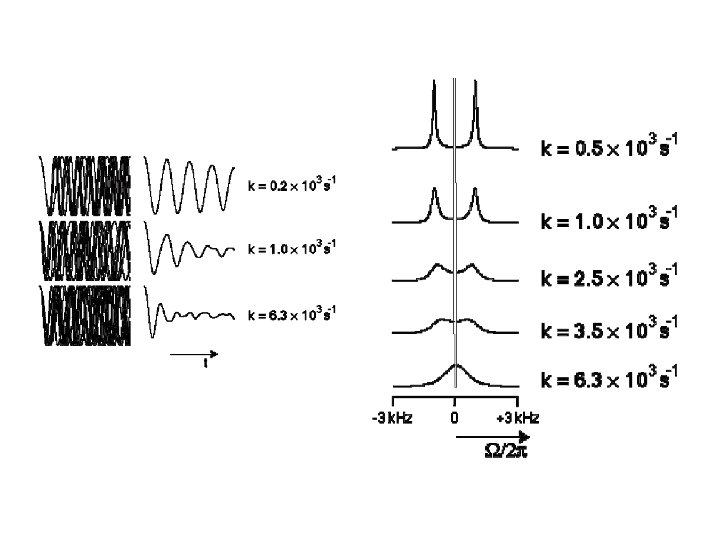
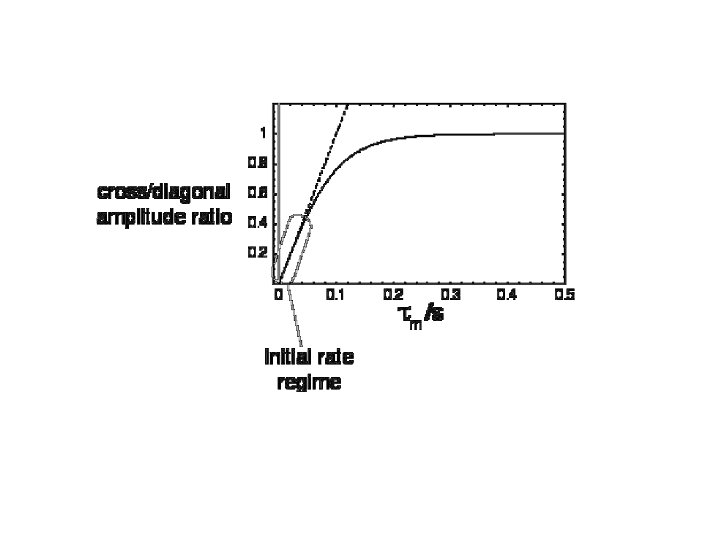
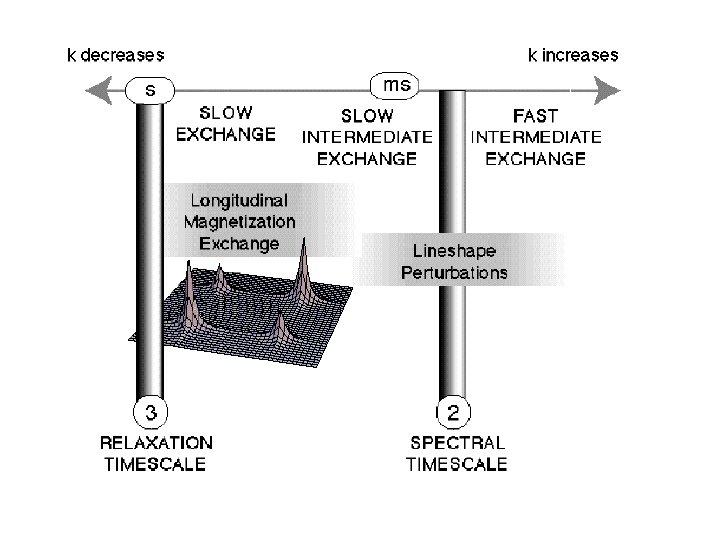
Motional linshapes and two-site exchange Symmetrical two-site exchange: k A k B The effect of the exchange process on the NMR spectrum depends on the difference in the chemical differences of the two sites, as compared to the exchange rate. = A 0 B 0 If the exchange rate constant has roughly the same magnitude as , then the system is said to be in the intermediate exchange regime.
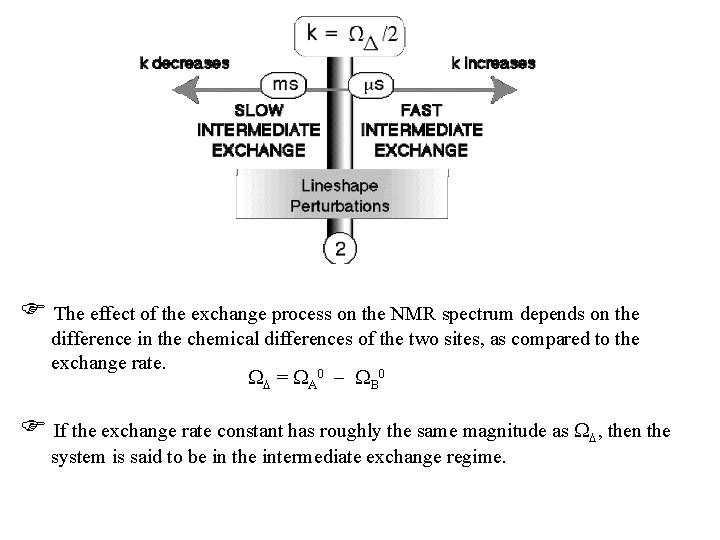
The effect of the exchange process on the NMR spectrum depends on the difference in the chemical differences of the two sites, as compared to the exchange rate. = A 0 B 0 If the exchange rate constant has roughly the same magnitude as , then the system is said to be in the intermediate exchange regime.
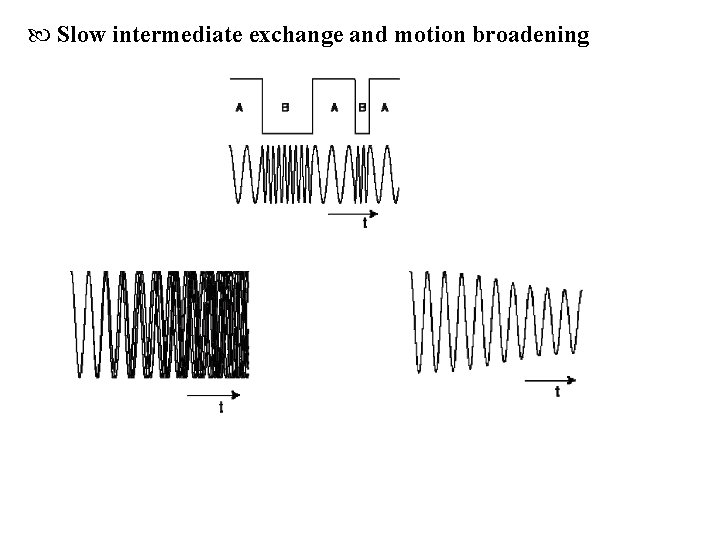
Slow intermediate exchange and motion broadening


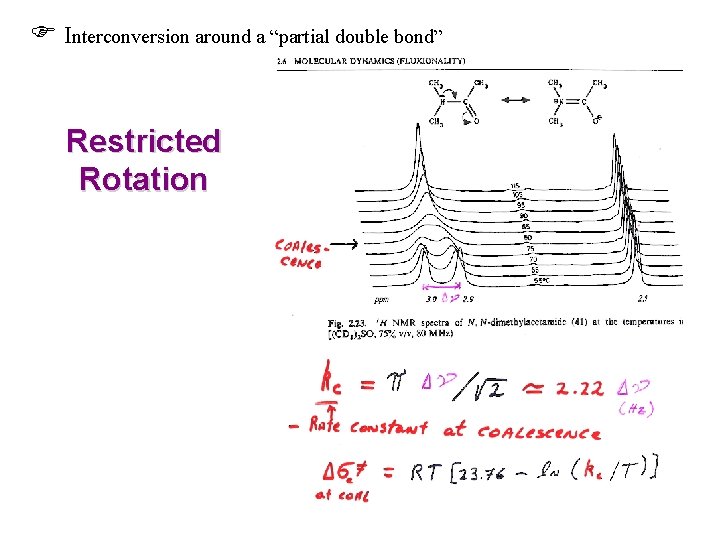
Interconversion around a “partial double bond” Restricted Rotation
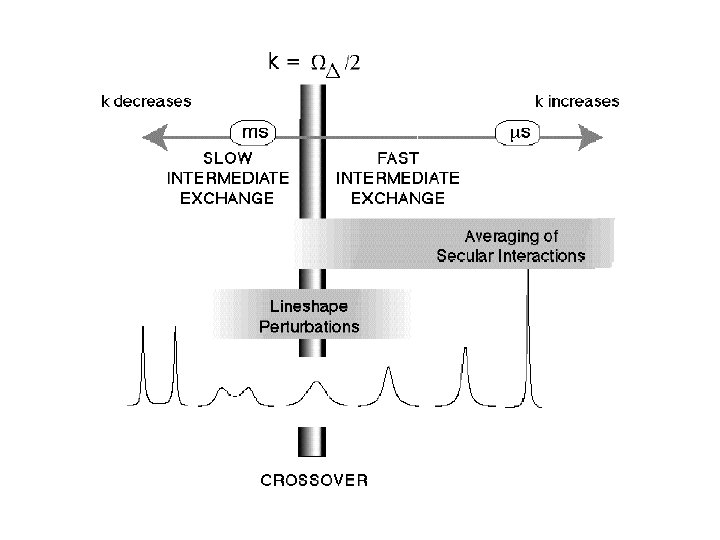
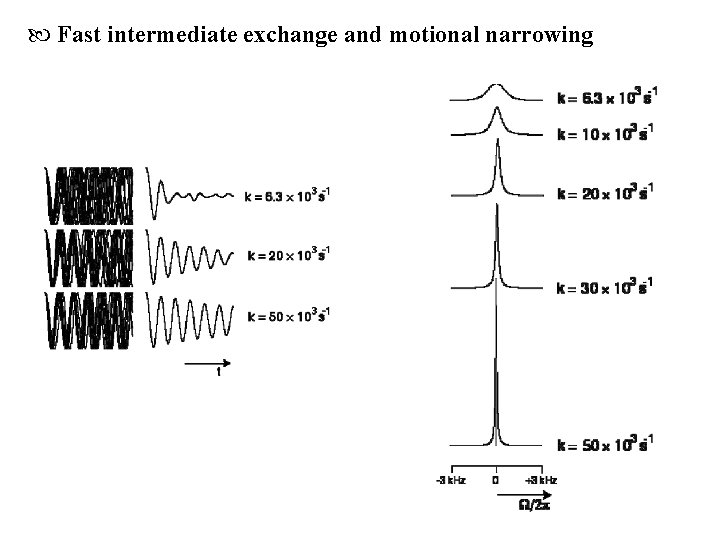
Fast intermediate exchange and motional narrowing
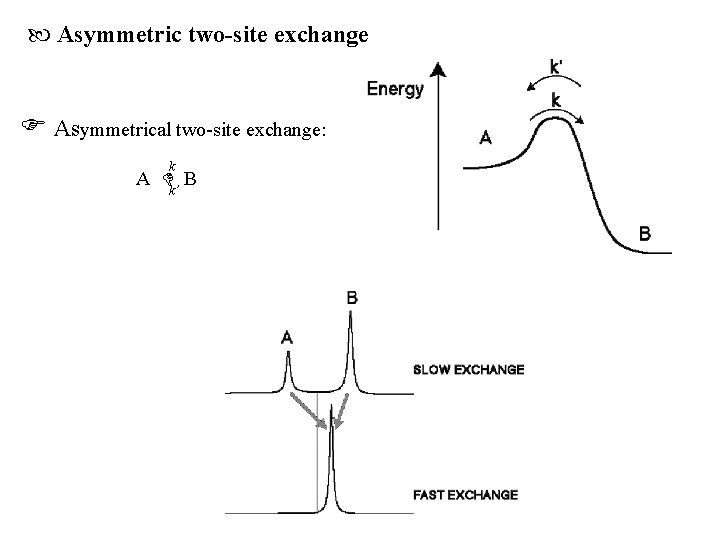
Asymmetric two-site exchange Asymmetrical two-site exchange: k A k’ B
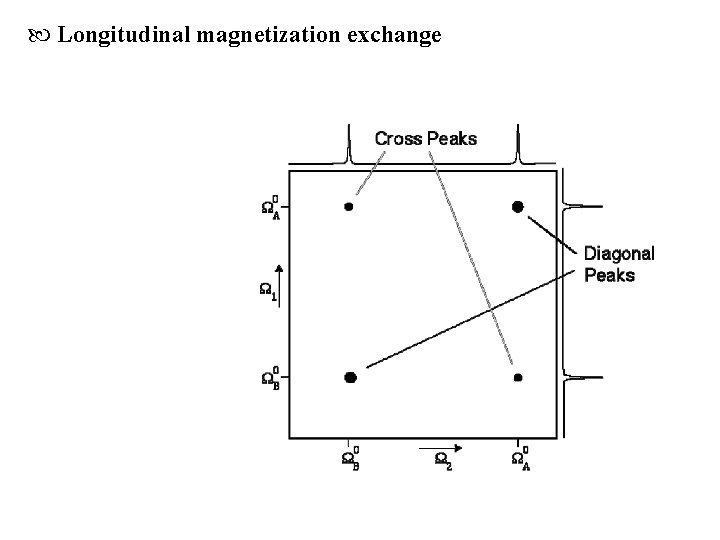
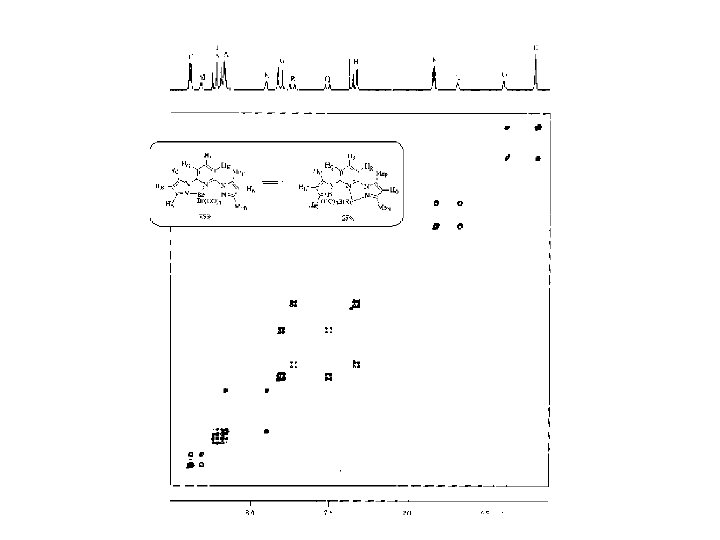
Longitudinal magnetization exchange
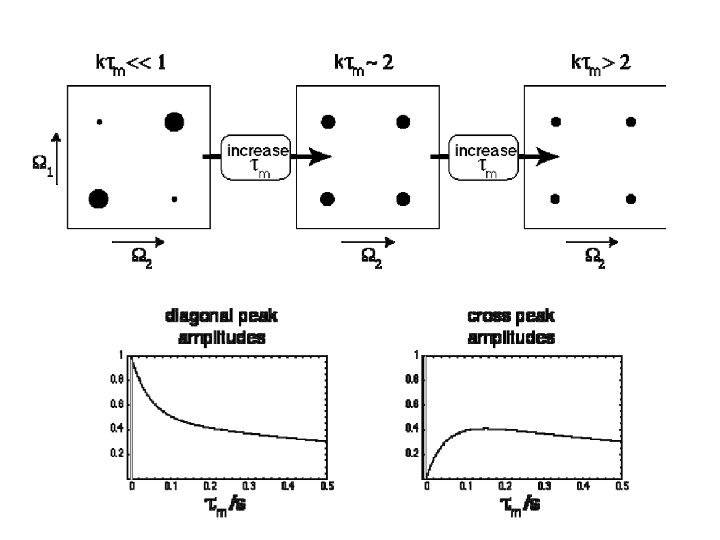
- Slides: 26