CHAPTER 9 THERMODYNAMICS THERMAL ENERGY PHYSICAL SCIENCE 9










































- Slides: 52

CHAPTER 9: THERMODYNAMICS

THERMAL ENERGY

PHYSICAL SCIENCE 9. 1 p. 178 Introduction • The Bible indicates that God’s purpose for the sun is to benefit mankind • Genesis 1: 16 -18; Deuteronomy 4: 19 • We should learn how best to use this source of clean, renewable energy

PHYSICAL SCIENCE • • • 9. 1 What is heat? How is it produced? How is it measured? How do we use it? Could the sun be a reliable, inexhaustible source of energy? p. 178

PHYSICAL SCIENCE 9. 2 pp. 178 -179 Early Theories of Heat • Sixth century BC: Greek Philosopher Heraclitus claimed there were three natural elements—earth, water, and fire • Thirteenth century AD: philosophers and scientists thought that motion is the essence of heat

PHYSICAL SCIENCE 9. 2 pp. 178 -179 • This concept was reaffirmed by English philosopher and scientist Francis Bacon • 18 th century Joseph Black suggested heat was like an invisible fluid • Solids needed to be filled up with this heat-fluid until they melted

PHYSICAL SCIENCE 9. 2 pp. 178 -179 • French chemist Lavoisier developed this idea and called the heat-fluid “caloric” • The caloric theory was successful in observations and predictions • Theory of steam engines in early 1800 s by Nicholas Sadi Carnot

PHYSICAL SCIENCE 9. 2 pp. 178 -179 • Carnot’s work evolved into the science of modern thermodynamics • The study of thermal energy and heat and how they relate to other kinds of energy and work

PHYSICAL SCIENCE 9. 3 pp. 179 -180 Kinetic-Molecular Theory of Heat • Benjamin Thompson was first to discount the caloric theory • Observed during military training cannons fired without cannonballs became much hotter than those that fired normally

PHYSICAL SCIENCE 9. 3 pp. 179 -180 • If the caloric theory were true, release of caloric and resulting temperature rise should have been unaffected by the cannonball when the gunpowder was fired • Rumsford (Thompson) observed a dull boring bit could generate more heat

PHYSICAL SCIENCE 9. 3 • Julius Robert von Mayer was first to experiment with the idea of heat as energy (not as matter) in 1842 pp. 179 -180

PHYSICAL SCIENCE 9. 3 pp. 179 -180 • Mayer used a horse-powered mechanism to stir a pot of paper pulp • Calculated mechanical energy needed to heat mixture • Demonstrated mechanical energy could be converted to thermal energy

PHYSICAL SCIENCE 9. 3 pp. 179 -180 • James Prescott Joule: clear connection between mechanical energy and heat • Did experiments using mechanical devices that were dropped, shaken, and stirred to produce changes in temperature of liquids and gases

PHYSICAL SCIENCE 9. 3 pp. 179 -180 • Joule concluded that the equivalent of 4. 18 N • m of mechanical work would raise the temperature of 1 g of water 1 °C • Established that energy causes temperature changes in matter

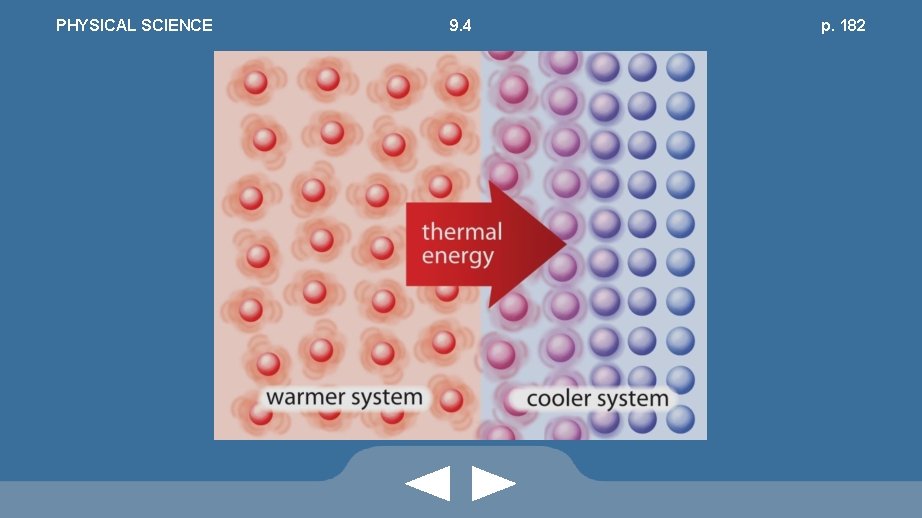
PHYSICAL SCIENCE 9. 4 pp. 181 -182 The Nature of Thermal Energy • Kinetic-molecular model: atoms, molecules, ions, and subatomic particles are in constant motion and have kinetic energy • Internal energy of matter • Cannot be measured

PHYSICAL SCIENCE 9. 4 p. 182

PHYSICAL SCIENCE 9. 5 pp. 182 -183 Using Solar Energy to Solve Problems • For a renewable energy source to be valuable, it must be able to convert its energy into a useable form • Solar panels can change sunlight directly into electricity

PHYSICAL SCIENCE 9. 5 pp. 182 -183 • Energy costs continue to rise • Is there a more economical way of converting the free energy of the sun into electricity? • Solar thermal (ST) power plant • Mojave Desert, California

TEMPERATURE

PHYSICAL SCIENCE 9. 6 pp. 184 -185 Thermometric Properties • Scientists need an objective standard for measuring temperature • The temperature of a substance is directly related to the average kinetic energy of its atoms and molecules

PHYSICAL SCIENCE 9. 6 pp. 184 -185 • Measured in a dimensional unit called the degree (°) • “Change of hotness” per degree varies depending on the temperature scale (Fahrenheit, Celsius, or Kelvin) • Measured with a thermometer • Thermometric property

PHYSICAL SCIENCE 9. 7 p. 185 Early Thermometers • Thermoscope was built by Galileo around 1600 • Later scientists revised Galileo's design by replacing air with a combination of alcohol and water in a sealed tube

PHYSICAL SCIENCE 9. 8 pp. 185 -187 Temperature Scales • Need an anchor, or standard, point for a temperature scale • Standards for a measuring scale are called fiducial points

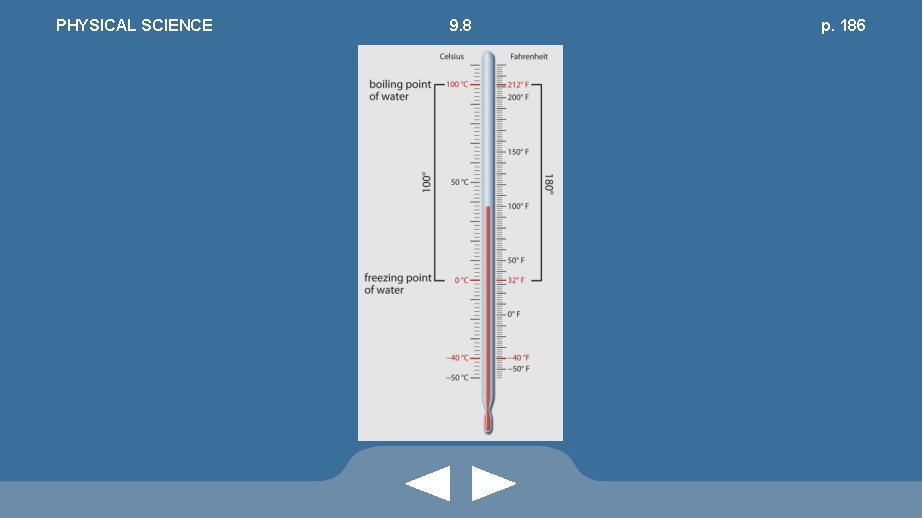
PHYSICAL SCIENCE 9. 8 pp. 185 -187 • The Fahrenheit Scale • Freezing temperature of pure water • Boiling temperature of pure water • On the Fahrenheit scale there are 180 degrees between water’s freezing point, 32 °F, and boiling point, 212 °F

PHYSICAL SCIENCE 9. 8 pp. 185 -187 • The Celsius Scale • Temperature range between freezing and boiling points of water: 100 degrees • Freezing: 0 °C • Boiling: 100 °C

PHYSICAL SCIENCE • Conversions: • t. F = 1. 8 t. C + 32° • t. C = (5/9)(t. F – 32°) 9. 8 pp. 185 -187

PHYSICAL SCIENCE 9. 8 p. 186

PHYSICAL SCIENCE 9. 8 pp. 185 -187 • The Kelvin Scale • Scientists in early 1800 s discovered that gases had interesting properties at very low temperatures • Absolute zero • One fiducial point: triple point of water

PHYSICAL SCIENCE 9. 8 pp. 185 -187 • A substance’s triple point is the temperature and pressure at which the solid, liquid, and gaseous phases of the substance simultaneously exist in a stable condition • On the Kelvin scale the triple point of water is 273. 16 K

PHYSICAL SCIENCE • Conversions: • T = t. C + 273. 15° • t. C = T – 273. 15° 9. 8 pp. 185 -187

PHYSICAL SCIENCE 9. 9 pp. 187 -189 Matter and Temperature • Temperature can have significant effects on important properties of matter • Thermal Expansion • Bridges • Roads and sidewalks

PHYSICAL SCIENCE 9. 9 • Every material has its own unique thermal expansion properties pp. 187 -189

PHYSICAL SCIENCE 9. 9 pp. 187 -189 • Electrical Resistance • Greater electrical resistance means poorer conducting ability • Increased temperature causes greater electrical resistance

PHYSICAL SCIENCE 9. 9 • Viscosity • A fluid’s resistance to flow • Viscosity generally decreases in warmer fluids pp. 187 -187

HEAT

PHYSICAL SCIENCE 9. 10 p. 190 The Nature of Heat • “Heat” is often used for both thermal energy and temperature • Neither is scientifically correct • The amount of thermal energy in an object is a property of the object—it has thermal energy

PHYSICAL SCIENCE 9. 10 • Heating and cooling occur through one of three processes • Conduction • Convection • Radiation p. 190

PHYSICAL SCIENCE 9. 11 pp. 190 -193 Heat Transfer • Conduction—when two objects of different temperatures touch, thermal energy moves from the hotter to the cooler object • Warmer object will cool • Cooler object will warm

PHYSICAL SCIENCE 9. 11 • Thermal equilibrium • Chief process by which thermal energy moves through solids pp. 190 -193

PHYSICAL SCIENCE 9. 11 pp. 190 -193 • Convection—thermal energy is carried from one location to another by a fluid • Important for energy transfer in fluids • Natural convection • Convection current • Forced convection • Atmospheric convection

PHYSICAL SCIENCE 9. 11 pp. 190 -193 • Radiation • Thermal energy moves most efficiently through a vacuum as radiant energy • Converted to electromagnetic energy at its source—then converted back to thermal energy at its destination

PHYSICAL SCIENCE 9. 11 pp. 190 -193 • Radiant energy can transfer thermal energy between two objects that are not in contact • Absorption of radiant energy depends on properties of the material

PHYSICAL SCIENCE 9. 12 pp. 193 -194 Insulation and Thermal Resistance • Thermal insulators: materials that resist the flow of thermal energy • Arrangement of atoms • Aerogels • Vacuums are the best insulators

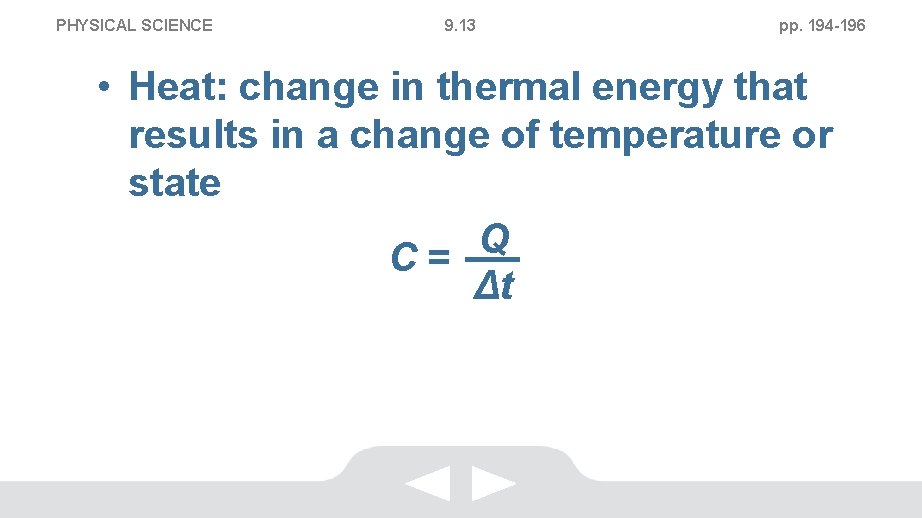
PHYSICAL SCIENCE 9. 13 pp. 194 -196 Measuring Heat • Heat Capacity • Thermal energy, in joules, an object must gain or lose to cause a temperature change of 1 °C • Units: J/°C or J/K

PHYSICAL SCIENCE 9. 13 pp. 194 -196 • Heat: change in thermal energy that results in a change of temperature or state Q C= Δt

PHYSICAL SCIENCE 9. 13 pp. 194 -196 • Specific Heat Capacity • Specific heat (csp) is the heat capacity per gram of material—the amount of thermal energy that must be gained or lost to change the temperature of 1 g of the substance 1°C

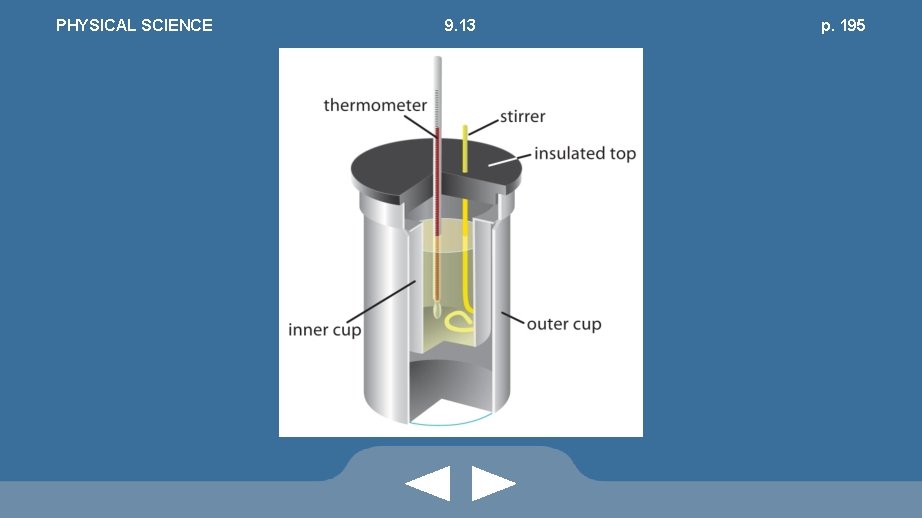
PHYSICAL SCIENCE • Formula: 9. 13 Q csp = m × Δt • An object’s specific heat can be determined with a calorimeter pp. 194 -196

PHYSICAL SCIENCE 9. 13 p. 195

PHYSICAL SCIENCE 9. 13 Water’s heat capacity allows perspiration to be an effective cooling mechanism for our bodies. pp. 194 -196

PHYSICAL SCIENCE 9. 14 pp. 196 -197 Heat and Phase Changes • When thermal energy is added to or taken from a material, its temperature usually changes because kinetic energy of its atoms and molecules changes • Sometimes the temperature doesn’t change

PHYSICAL SCIENCE 9. 14 pp. 196 -197 • Latent heat of fusion (Lf): the amount of thermal energy exchanged per gram of material during melting or freezing • Latent heat of vaporization (Lv): the amount of thermal energy exchanged per gram of material during boiling or condensation

PHYSICAL SCIENCE 9. 14 pp. 196 -197 • Every substance has a distinctive specific heat for each of its states • Every substance has a characteristic latent heat of fusion and of vaporization