CHAPTER 9 THERMOCHEMISTRY ADVANCE CHEMISTRY Purpose Describe the

CHAPTER 9: THERMOCHEMISTRY ADVANCE CHEMISTRY

Purpose � Describe the nature of energy and form of energy. � State the laws of thermodynamic. � Know the Hess’s Law � Calculate the enthalpy � Calculate the internal energy. � Know the relationship between heat, work, and internal energy. � Describe and demonstrate how a calorimeter work. � Construct the enthalpy diagram. � Know what a state function is.
Nature of Energy � Energy is the capacity to cause change or transfer heat. � Work is the energy used to caused an object that has mass to move. � Heat is the energy transfer form one to another. � Temperature is the degree of how hot or cold something is.
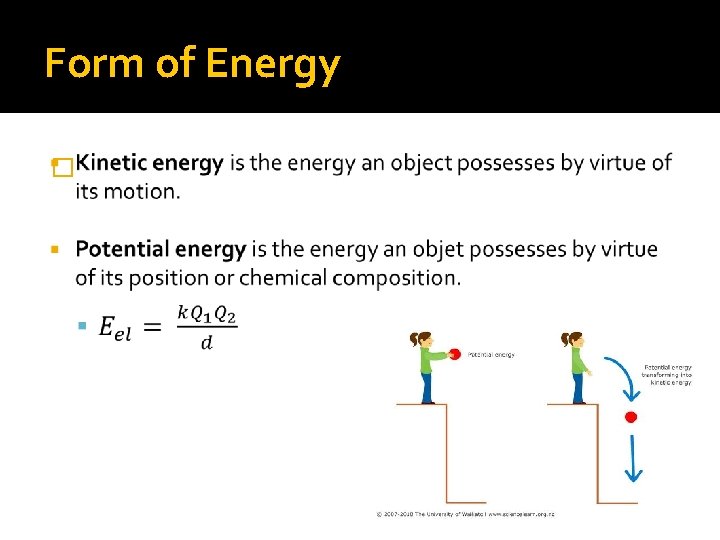
Form of Energy �

Unit of Energy �

System and Surrounding
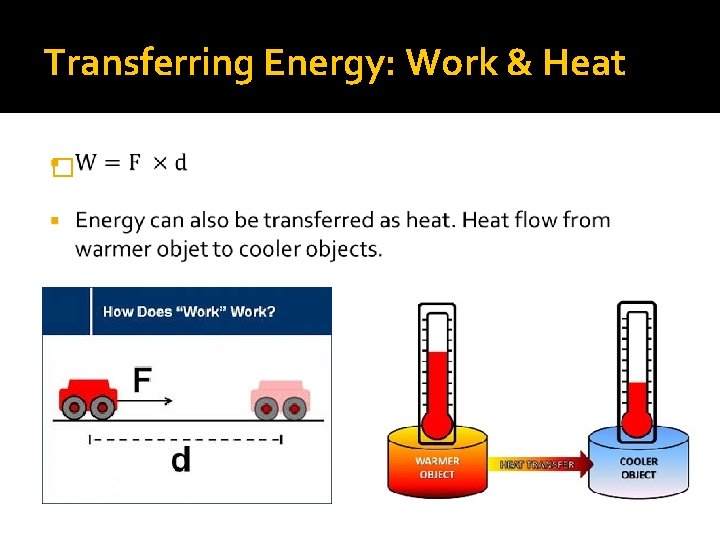
Transferring Energy: Work & Heat �
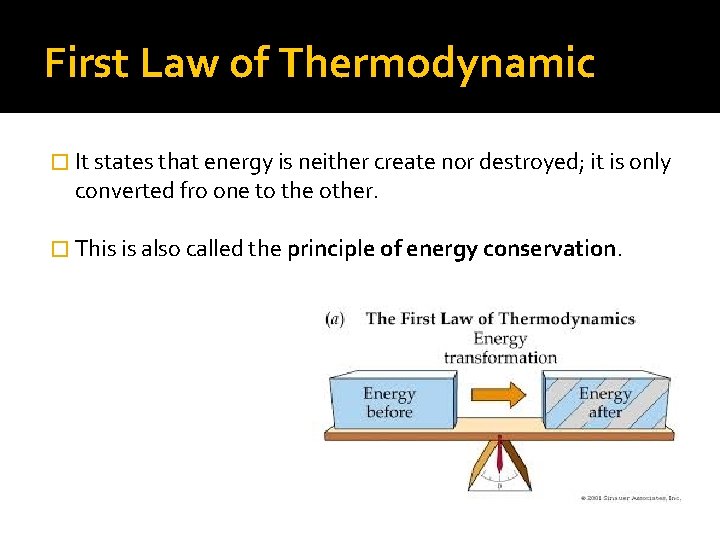
First Law of Thermodynamic � It states that energy is neither create nor destroyed; it is only converted fro one to the other. � This is also called the principle of energy conservation.
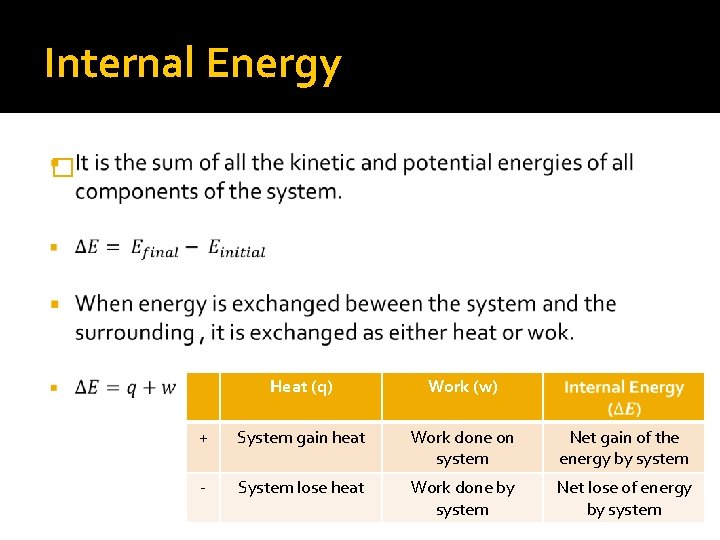
Internal Energy � Heat (q) Work (w) + System gain heat Work done on system Net gain of the energy by system - System lose heat Work done by system Net lose of energy by system
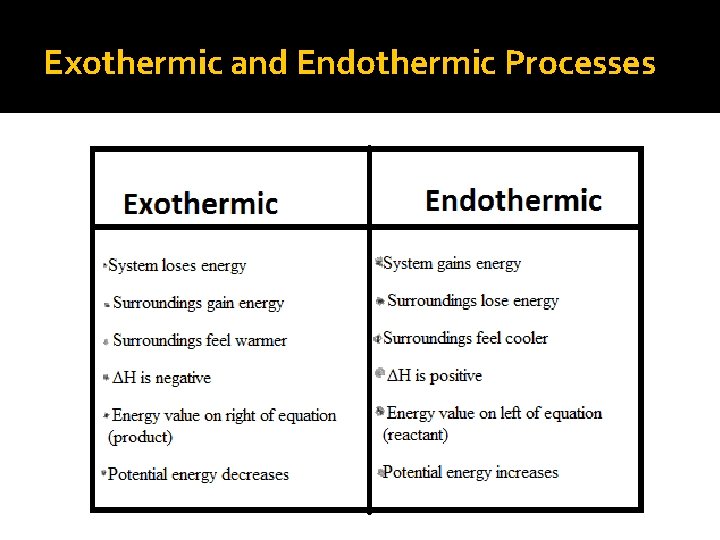
Exothermic and Endothermic Processes
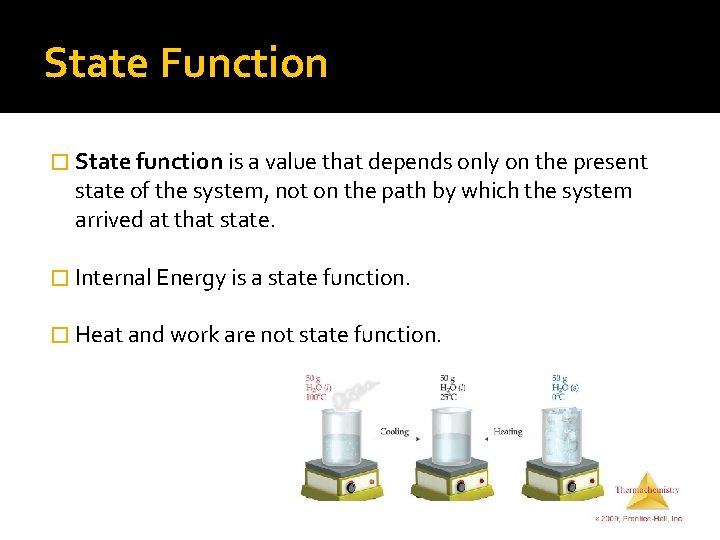
State Function � State function is a value that depends only on the present state of the system, not on the path by which the system arrived at that state. � Internal Energy is a state function. � Heat and work are not state function.

Enthalpy �
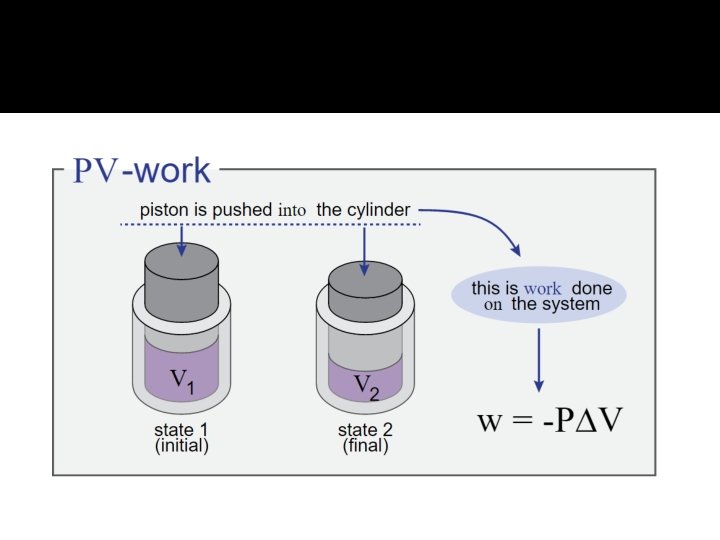
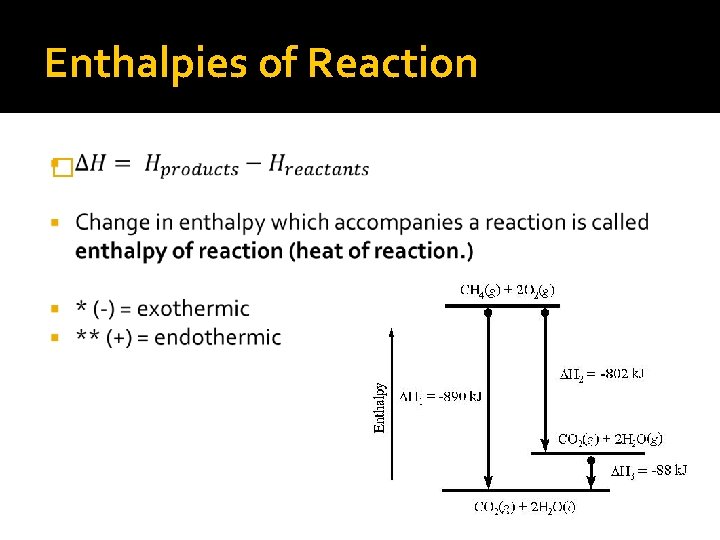
Enthalpies of Reaction �
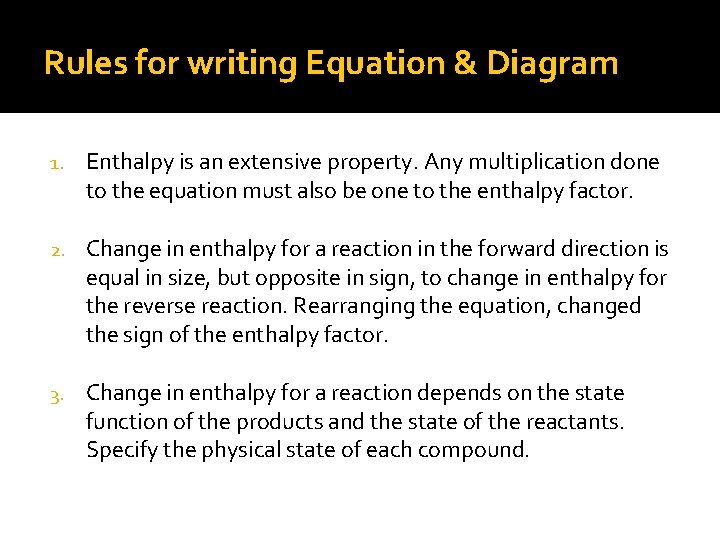
Rules for writing Equation & Diagram 1. Enthalpy is an extensive property. Any multiplication done to the equation must also be one to the enthalpy factor. 2. Change in enthalpy for a reaction in the forward direction is equal in size, but opposite in sign, to change in enthalpy for the reverse reaction. Rearranging the equation, changed the sign of the enthalpy factor. 3. Change in enthalpy for a reaction depends on the state function of the products and the state of the reactants. Specify the physical state of each compound.
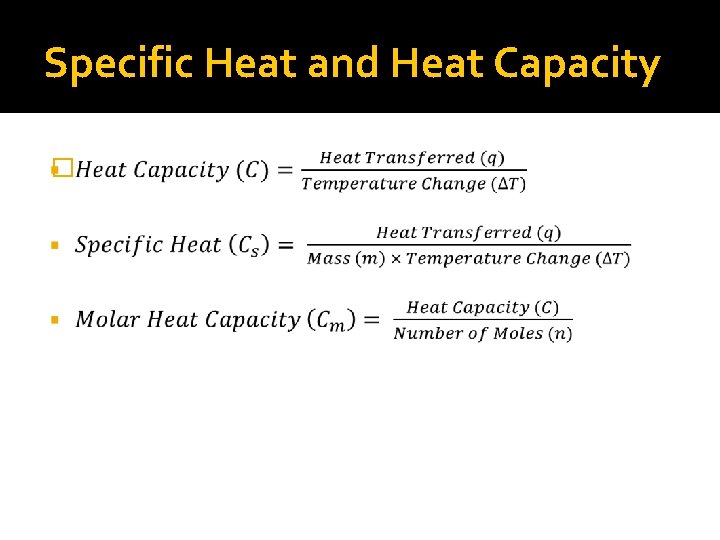
Specific Heat and Heat Capacity �
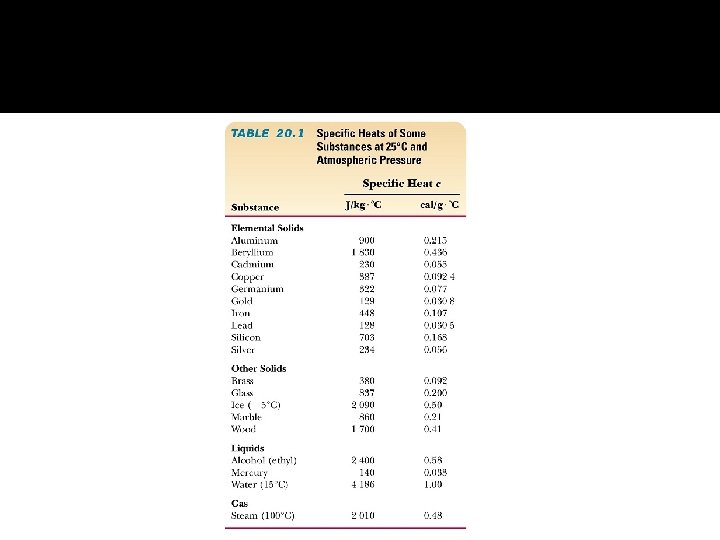
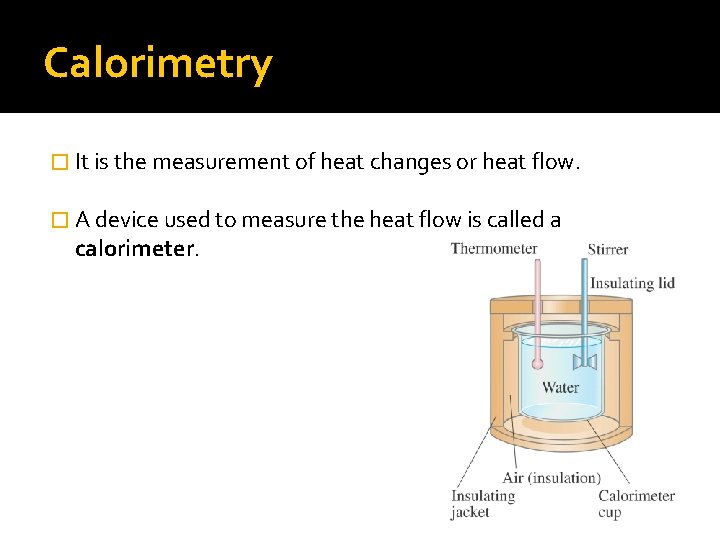
Calorimetry � It is the measurement of heat changes or heat flow. � A device used to measure the heat flow is called a calorimeter.
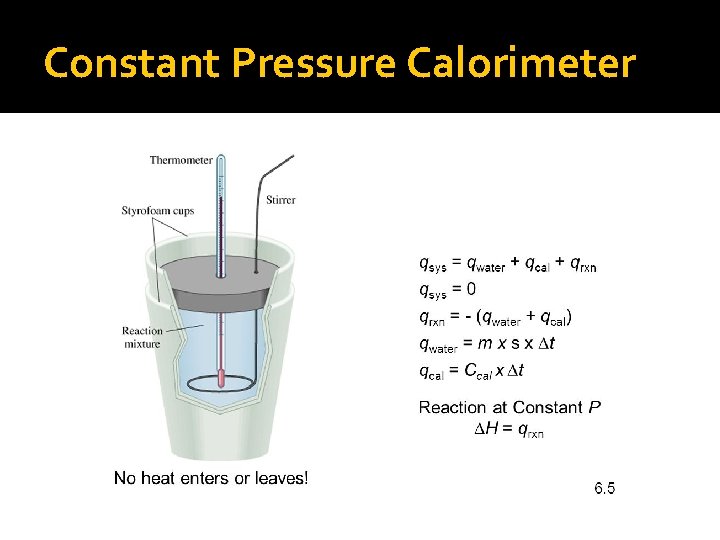
Constant Pressure Calorimeter
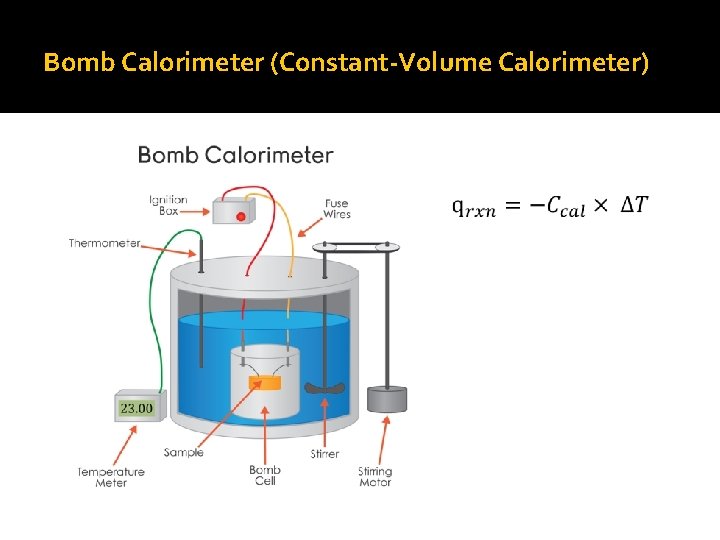
Bomb Calorimeter (Constant-Volume Calorimeter)
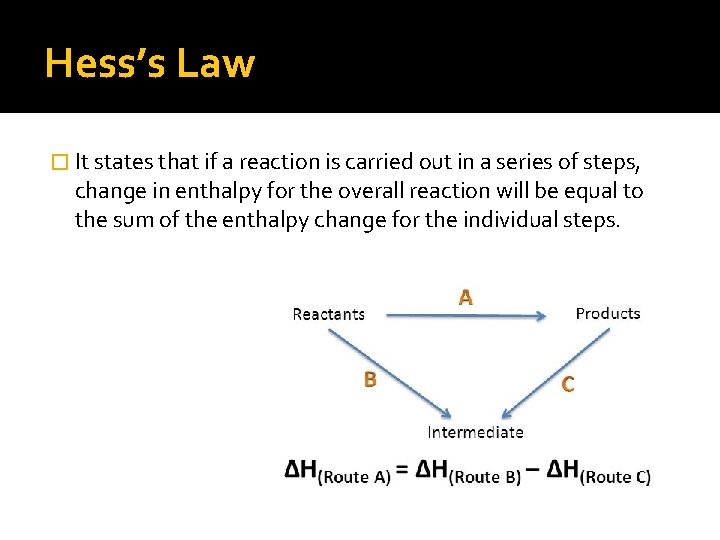
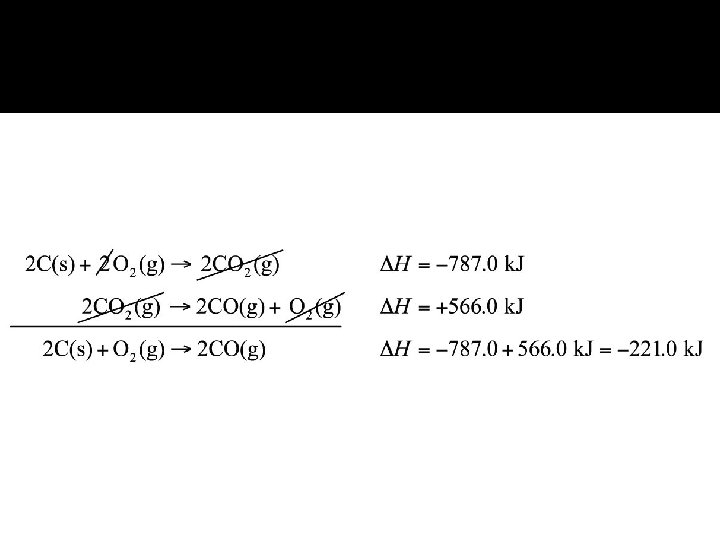
Hess’s Law � It states that if a reaction is carried out in a series of steps, change in enthalpy for the overall reaction will be equal to the sum of the enthalpy change for the individual steps.
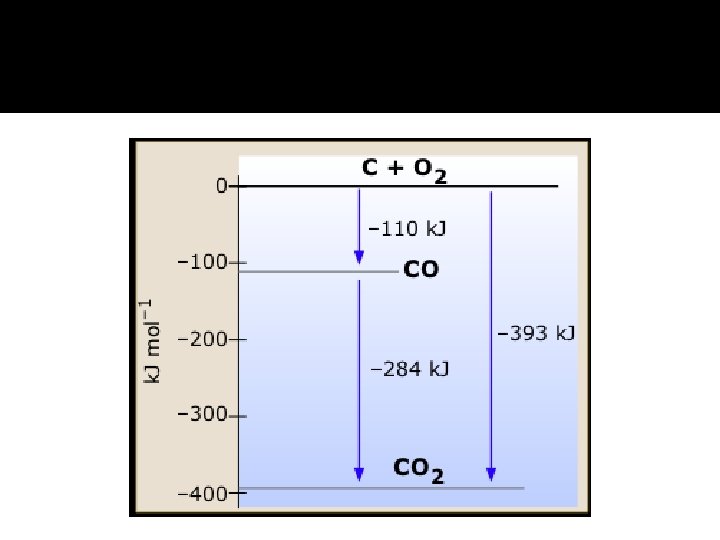
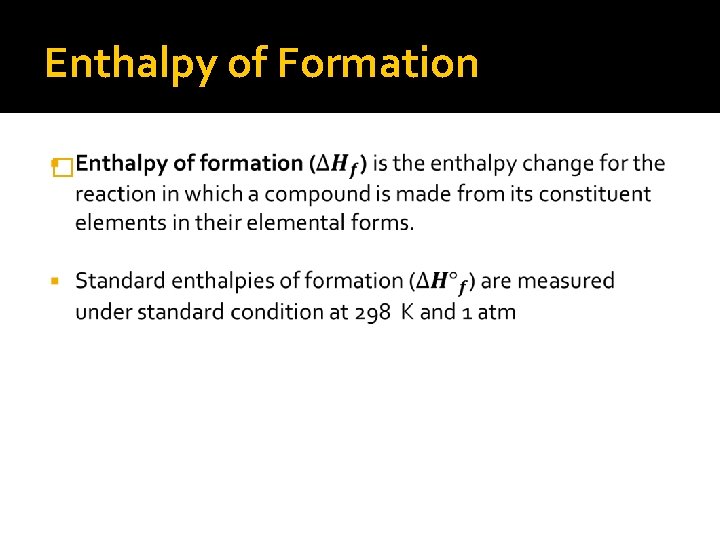
Enthalpy of Formation �
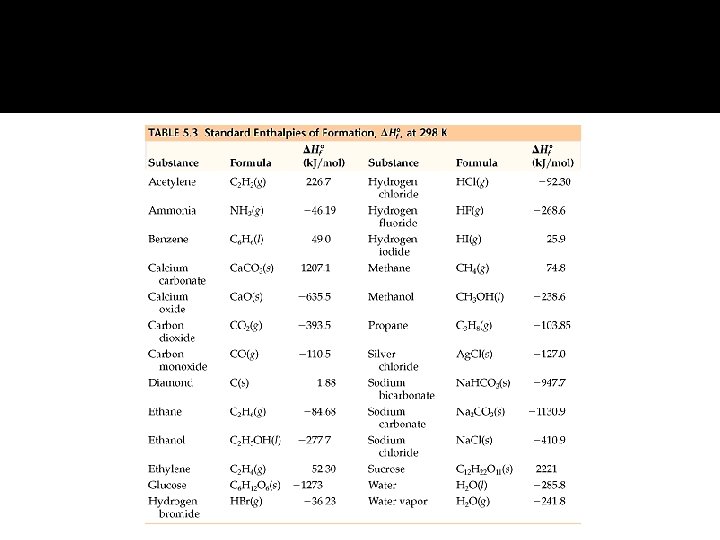
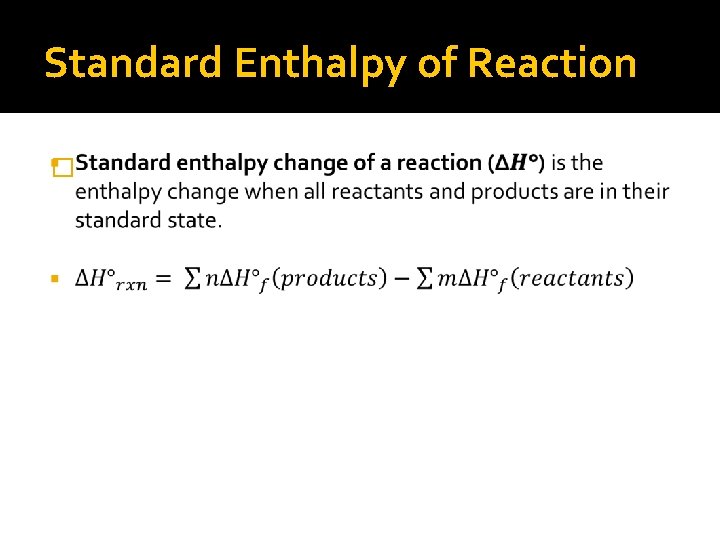
Standard Enthalpy of Reaction �
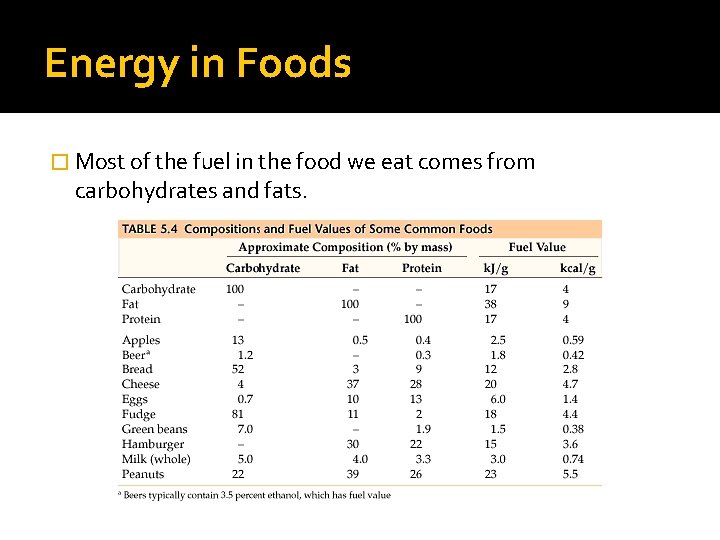
Energy in Foods � Most of the fuel in the food we eat comes from carbohydrates and fats.

Energy in Fuels � The vast majority of the energy consumed in many counties comes from fossil fuels.
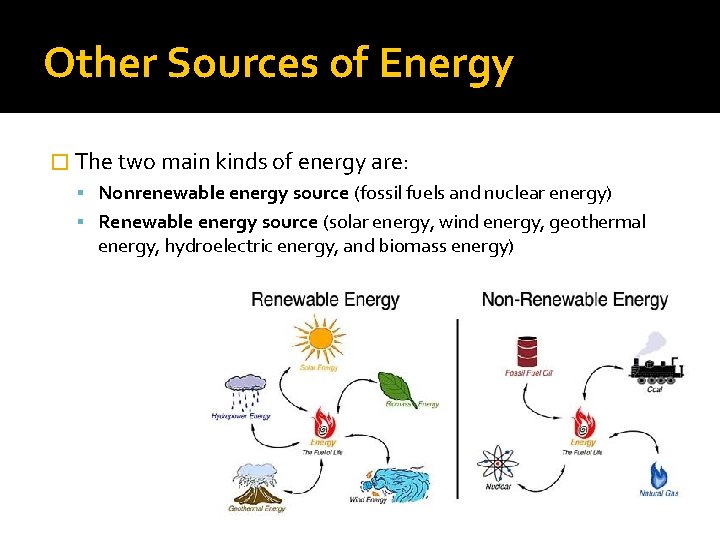
Other Sources of Energy � The two main kinds of energy are: Nonrenewable energy source (fossil fuels and nuclear energy) Renewable energy source (solar energy, wind energy, geothermal energy, hydroelectric energy, and biomass energy)

Reference Lecture: Theodore E. B. , Eugene, H. L. H. , Bruce R. B. , Catherine M. , Patrick W. , (2011). Chemistry: The Center Science (12 Ed). Prentice Hall. USA. 2. Laboratory: Theodore E. B. , John H. N. , Kenneth C. K. , Matthew S. (2011). Laboratory Experiments for Chemistry: The Central Science (12 Ed). Prentice Hall. USA. 3. Theodore E. B. , (2011). Solutions to Exercises for Chemistry: The Central Science. Prentice Hall. USA. 4. John M. , Robert C. F. (2010). Chemistry (4 Ed): Prentice Hall Companion Website. http: //wps. prenhall. com/esm_mcmurry_chemistry_4/9/2408/616516. cw/index. h tml 5. Chemistry Online at http: //preparatorychemistry. com/Bishop_Chemistry_First. html 6. Chemistry and You at http: //www. saskschools. ca/curr_content/science 9/chemistry/index. html 7. Teachers Notes. 8. Work http: //ch 301. cm. utexas. edu/section 2. php? target=thermo/firstlaw/work. html 9. http: //slideplayer. com/slide/272991/ 10. http: //slideplayer. com/slide/6949420/ 1.

END
- Slides: 31