Chapter 9 Phase Diagrams ISSUES TO ADDRESS When



- Slides: 34

Chapter 9: Phase Diagrams ISSUES TO ADDRESS. . . • When we combine two elements. . . what is the resulting equilibrium state? • In particular, if we specify. . . -- the composition (e. g. , wt% Cu - wt% Ni), and -- the temperature (T ) then. . . How many phases form? What is the composition of each phase? What is the amount of each phase? What is the structure of each phase Phase A Phase Behavior Laughlin p. 67 Phase B Nickel atom Copper atom

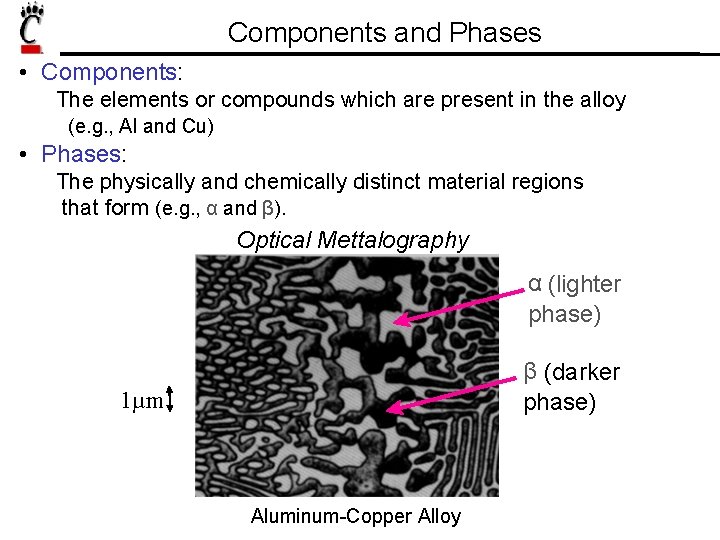
Components and Phases • Components: The elements or compounds which are present in the alloy (e. g. , Al and Cu) • Phases: The physically and chemically distinct material regions that form (e. g. , α and β). Optical Mettalography α (lighter phase) β (darker phase) 1µm Aluminum-Copper Alloy

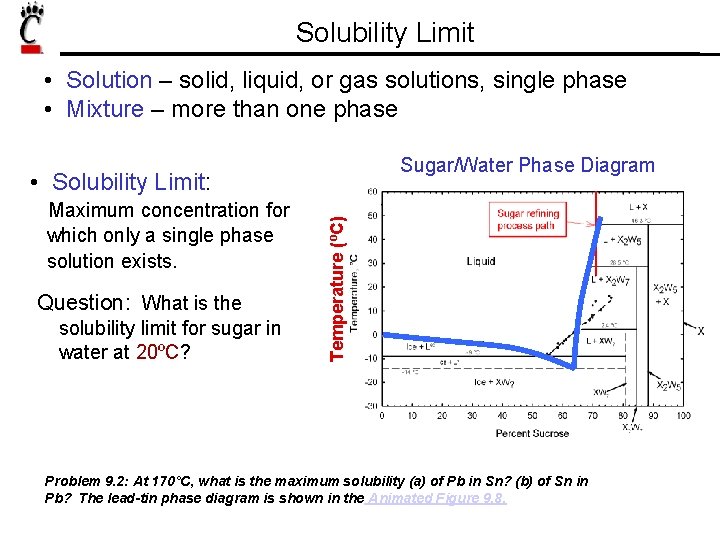
Solubility Limit • Solution – solid, liquid, or gas solutions, single phase • Mixture – more than one phase Sugar/Water Phase Diagram • Solubility Limit: Question: What is the solubility limit for sugar in water at 20ºC? Temperature (ºC) Maximum concentration for which only a single phase solution exists. 100 Solubility Limit 80 60 40 L (syrup) 20 0 20 L (liquid) + S (solid sugar) 40 6065 80 100 Composition (wt% sugar) Problem 9. 2: At 170°C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown in the Animated Figure 9. 8.

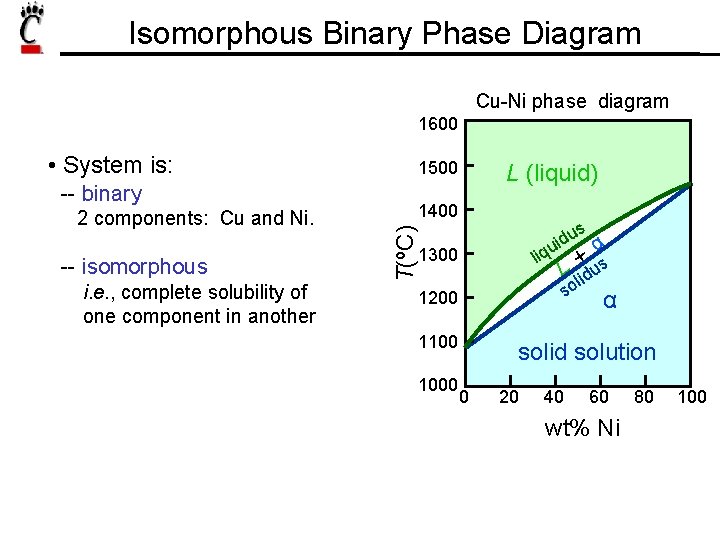
Isomorphous Binary Phase Diagram Cu-Ni phase diagram 1600 -- binary 2 components: Cu and Ni. -- isomorphous i. e. , complete solubility of one component in another 1500 L (liquid) 1400 s u d α ui liq L +lidus so T(ºC) • System is: 1300 α 1200 1100 1000 solid solution 0 20 40 60 wt% Ni 80 100

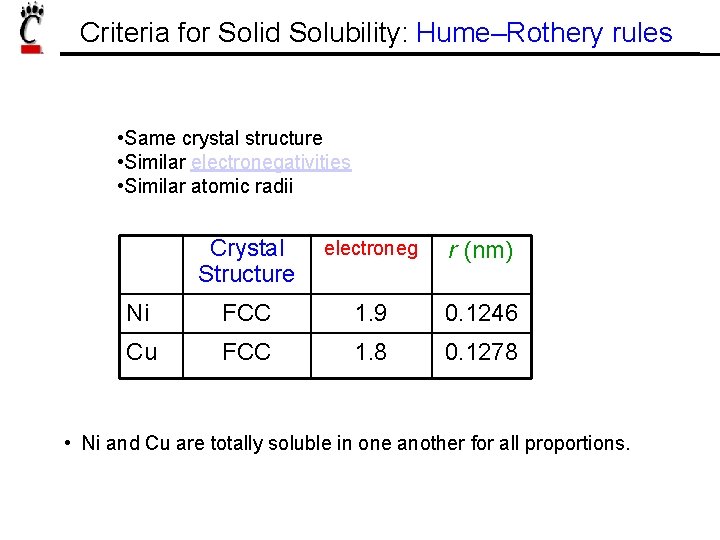
Criteria for Solid Solubility: Hume–Rothery rules • Same crystal structure • Similar electronegativities • Similar atomic radii Crystal Structure electroneg r (nm) Ni FCC 1. 9 0. 1246 Cu FCC 1. 8 0. 1278 • Ni and Cu are totally soluble in one another for all proportions.

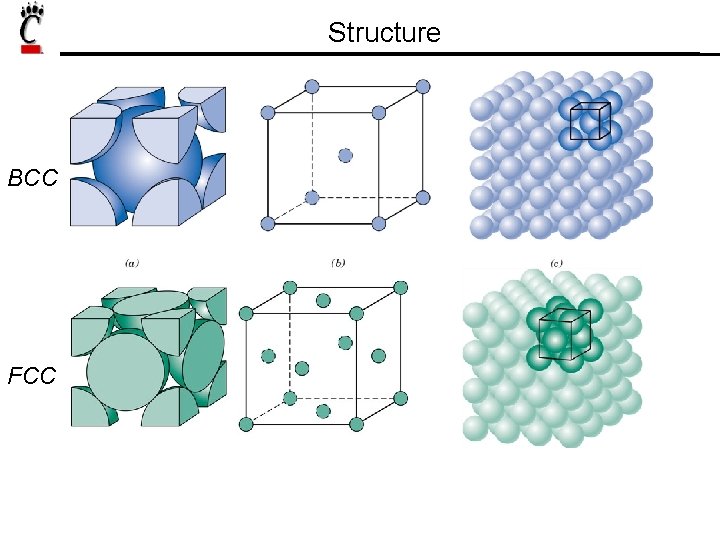
Structure BCC FCC

Periodic Table

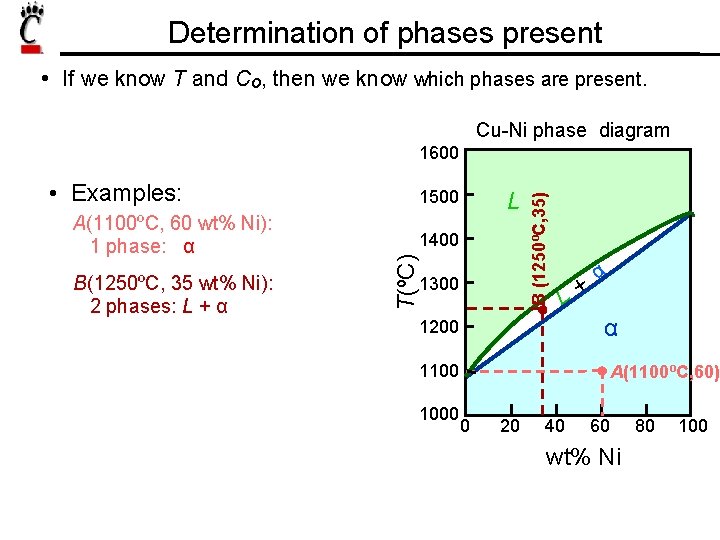
Determination of phases present • If we know T and Co, then we know which phases are present. Cu-Ni phase diagram A(1100ºC, 60 wt% Ni): 1 phase: α B(1250ºC, 35 wt% Ni): 2 phases: L + α 1500 L 1400 T(ºC) • Examples: 1300 B (1250ºC, 35) 1600 L+ α α 1200 1100 1000 A(1100ºC, 60) 0 20 40 60 wt% Ni 80 100

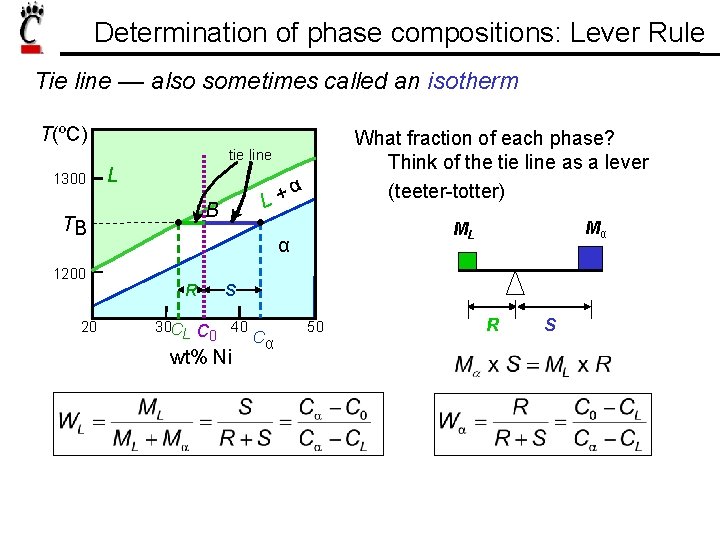
Determination of phase compositions: Lever Rule Tie line –– also sometimes called an isotherm T(ºC) What fraction of each phase? Think of the tie line as a lever (teeter-totter) tie line 1300 L B TB 1200 20 α + L α R 30 CL Mα ML S C 0 40 C α wt% Ni 50 R S

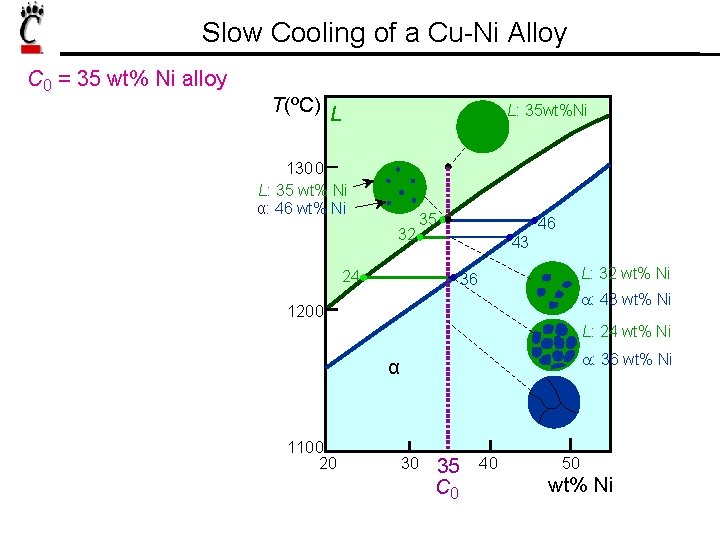
Slow Cooling of a Cu-Ni Alloy C 0 = 35 wt% Ni alloy T(ºC) L L: 35 wt%Ni 1300 L: 35 wt% Ni α: 46 wt% Ni 32 35 24 46 43 L: 32 wt% Ni 36 a: 43 wt% Ni 1200 L: 24 wt% Ni a: 36 wt% Ni α 1100 20 30 35 C 0 40 50 wt% Ni

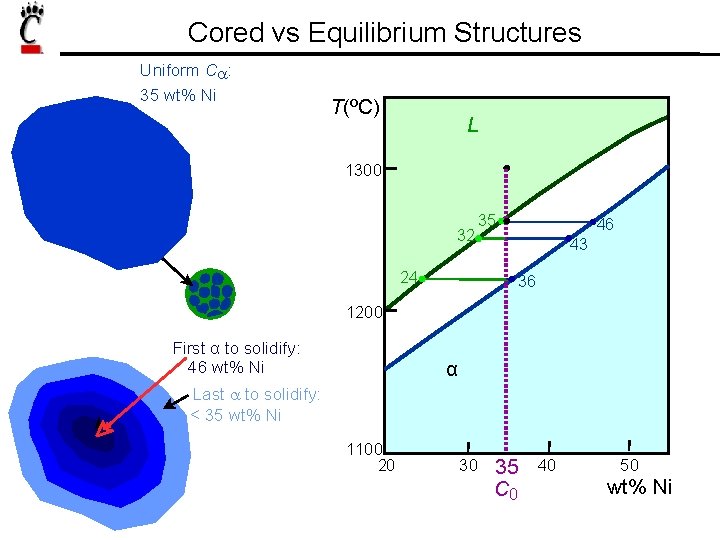
Cored vs Equilibrium Structures Uniform Ca: 35 wt% Ni T(ºC) L 1300 32 35 24 46 43 36 1200 First α to solidify: 46 wt% Ni α Last a to solidify: < 35 wt% Ni 1100 20 30 35 C 0 40 50 wt% Ni

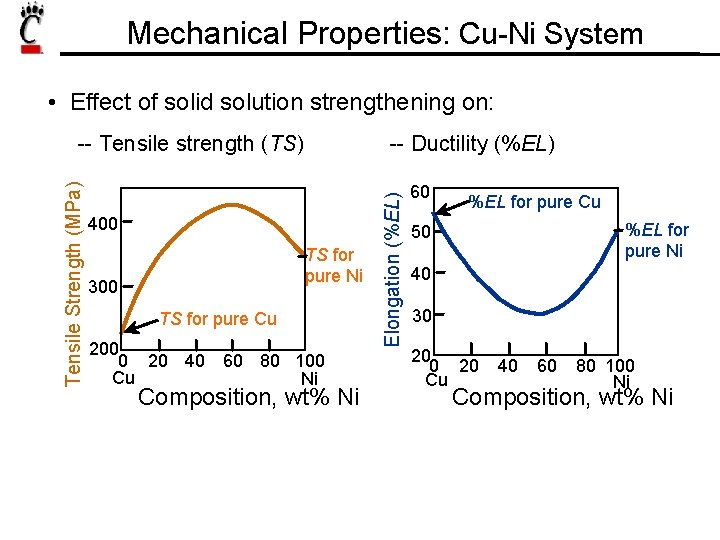
Mechanical Properties: Cu-Ni System • Effect of solid solution strengthening on: -- Ductility (%EL) 400 TS for pure Ni 300 TS for pure Cu 200 0 20 40 60 80 100 Cu Ni Composition, wt% Ni Elongation (%EL) Tensile Strength (MPa) -- Tensile strength (TS) 60 %EL for pure Cu %EL for pure Ni 50 40 30 20 Cu 40 60 80 100 Ni Composition, wt% Ni

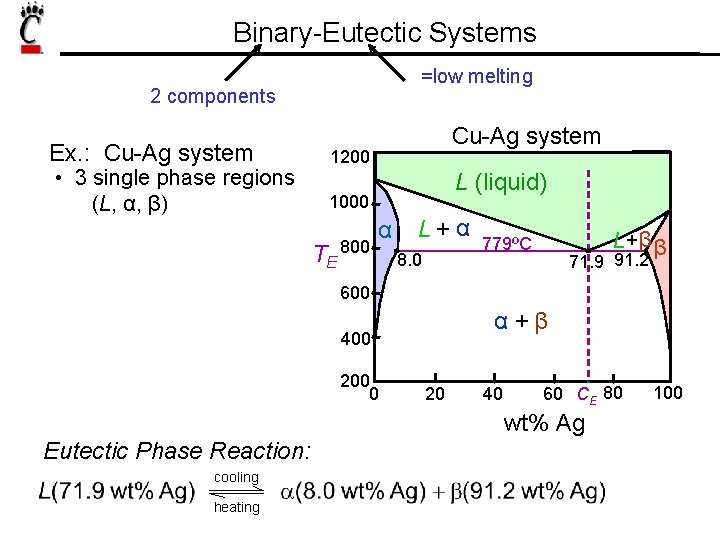
Binary-Eutectic Systems =low melting 2 components Ex. : Cu-Ag system • 3 single phase regions (L, α, β) Cu-Ag system 1200 L (liquid) 1000 TE 800 α L+α 8. 0 L+β β 779ºC 71. 9 91. 2 600 α +β 400 200 0 20 40 60 CE 80 wt% Ag Eutectic Phase Reaction: cooling heating 100

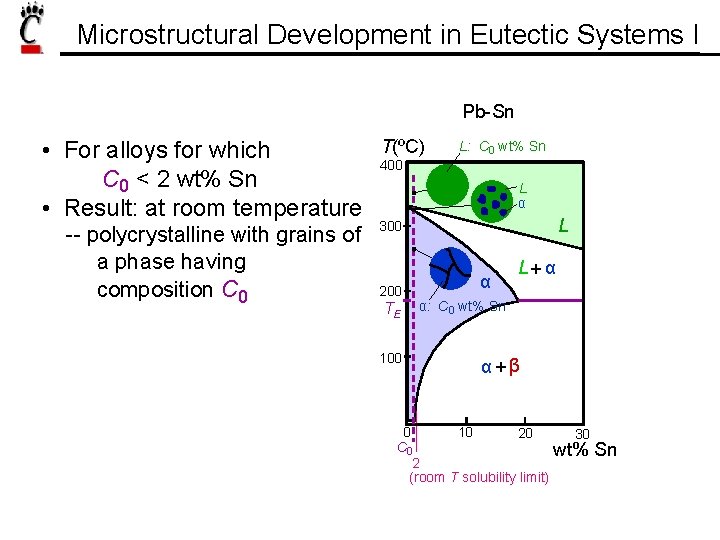
Microstructural Development in Eutectic Systems I Pb-Sn • For alloys for which C 0 < 2 wt% Sn • Result: at room temperature -- polycrystalline with grains of a phase having composition C 0 T(ºC) L: C 0 wt% Sn 400 L α L 300 200 TE 100 α L+ α α: C 0 wt% Sn α+β 0 10 20 30 C 0 wt% Sn 2 (room T solubility limit)

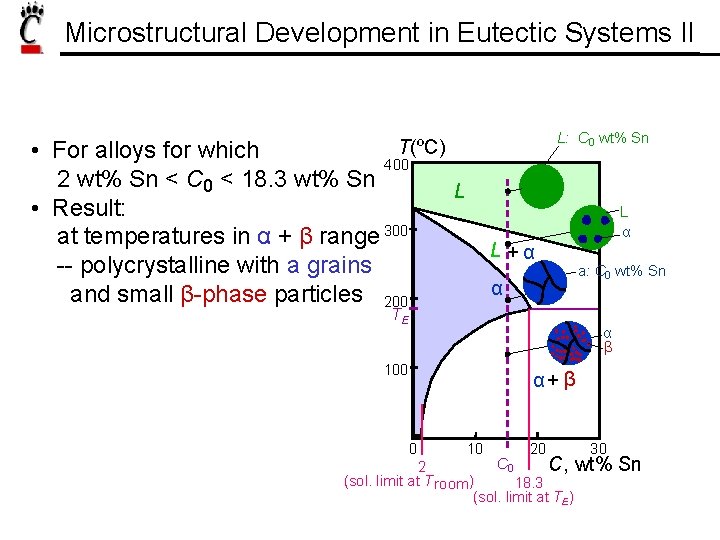
Microstructural Development in Eutectic Systems II L: C 0 wt% Sn T(ºC) • For alloys for which 400 2 wt% Sn < C 0 < 18. 3 wt% Sn • Result: at temperatures in α + β range 300 -- polycrystalline with a grains and small β-phase particles 200 L L +α α TE L α a: C 0 wt% Sn α β 100 α+ β 0 10 20 30 C 0 C, wt% 2 (sol. limit at T room ) 18. 3 (sol. limit at TE) Sn

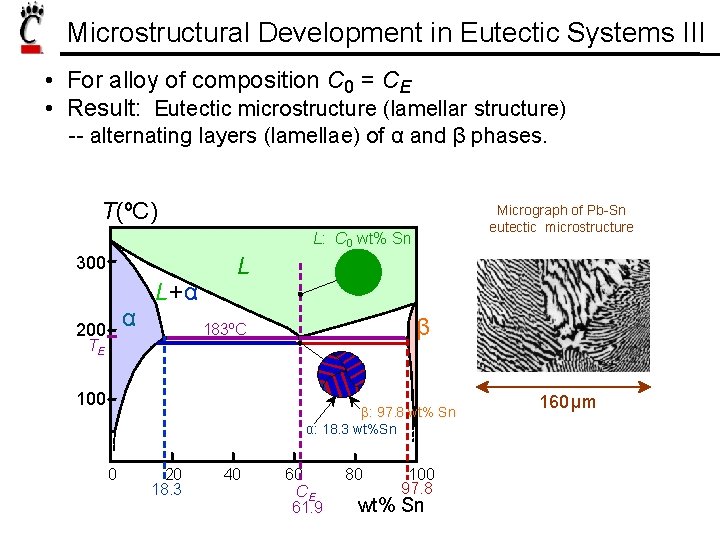
Microstructural Development in Eutectic Systems III • For alloy of composition C 0 = CE • Result: Eutectic microstructure (lamellar structure) -- alternating layers (lamellae) of α and β phases. T(ºC) Micrograph of Pb-Sn eutectic microstructure L: C 0 wt% Sn 300 α 200 L+ α L β 183ºC TE 100 β: 97. 8 wt% Sn α: 18. 3 wt%Sn 0 20 18. 3 40 60 CE 61. 9 80 100 97. 8 wt% Sn 160 μm

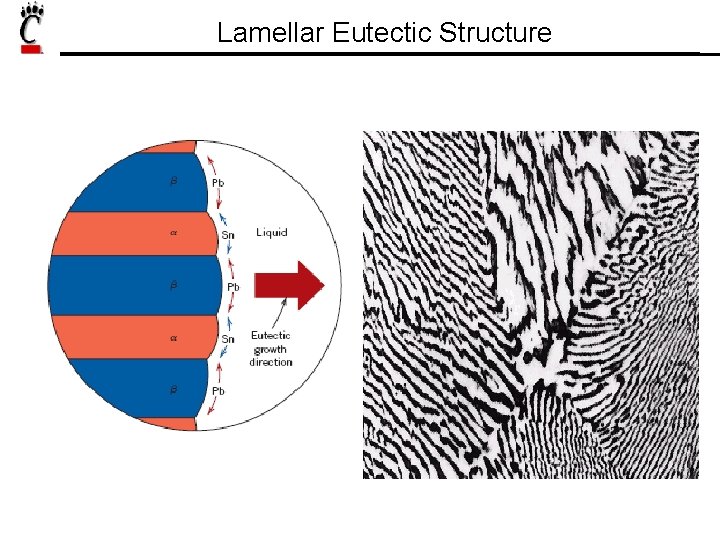
Lamellar Eutectic Structure

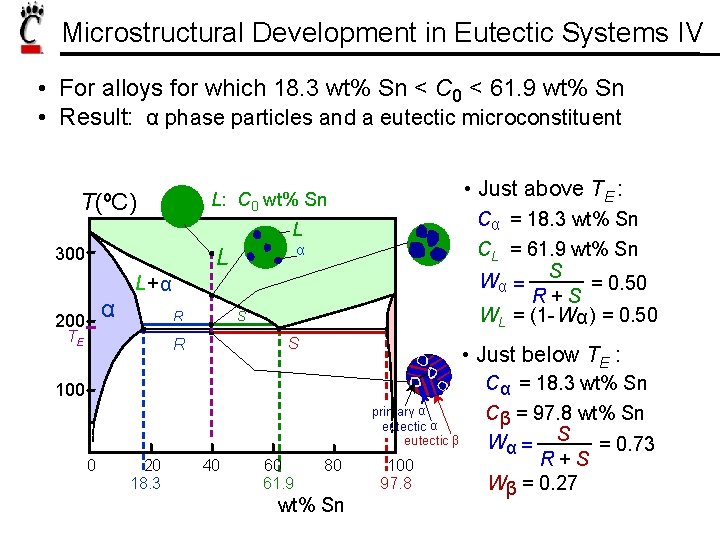
Microstructural Development in Eutectic Systems IV • For alloys for which 18. 3 wt% Sn < C 0 < 61. 9 wt% Sn • Result: α phase particles and a eutectic microconstituent T(ºC) • Just above TE : L: C 0 wt% Sn Cα = 18. 3 wt% Sn CL = 61. 9 wt% Sn Wα = S = 0. 50 R+S WL = (1 - Wα ) = 0. 50 L 300 α L L+ α α 200 R TE S S R • Just below TE : Cα = 18. 3 wt% Sn 100 primary α eutectic β 0 20 18. 3 40 60 61. 9 80 wt% Sn 100 97. 8 Cβ = 97. 8 wt% Sn Wα = S = 0. 73 R+S Wβ = 0. 27

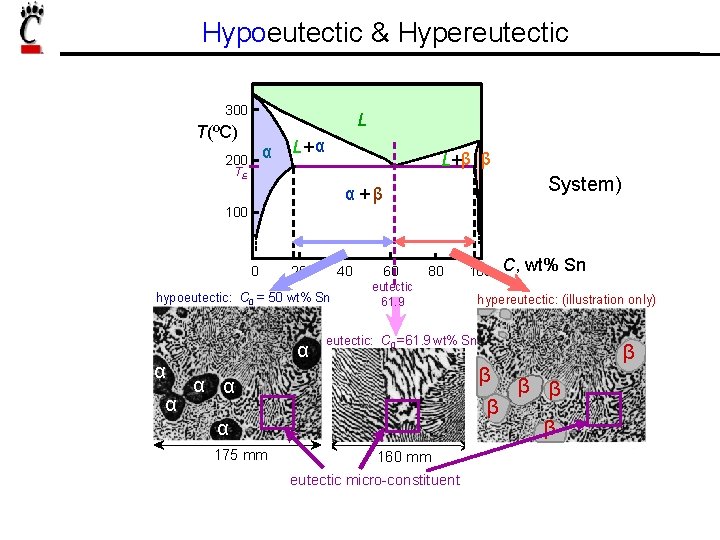
Hypoeutectic & Hypereutectic 300 L T(ºC) α 200 L+ α L+β β TE System) α+β 100 0 20 40 hypoeutectic: C 0 = 50 wt% Sn α α α 60 80 eutectic 61. 9 C, wt% Sn hypereutectic: (illustration only) eutectic: C 0 = 61. 9 wt% Sn β β α α β α 175 mm 100 160 mm eutectic micro-constituent β β β

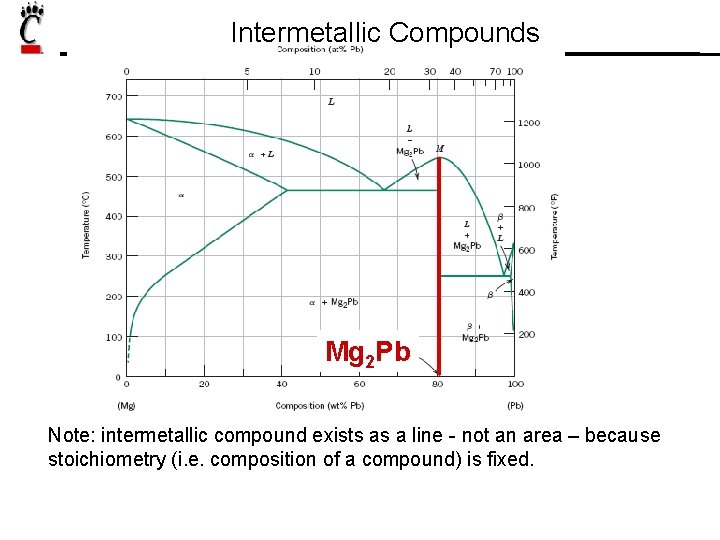
Intermetallic Compounds Mg 2 Pb Note: intermetallic compound exists as a line - not an area – because stoichiometry (i. e. composition of a compound) is fixed.

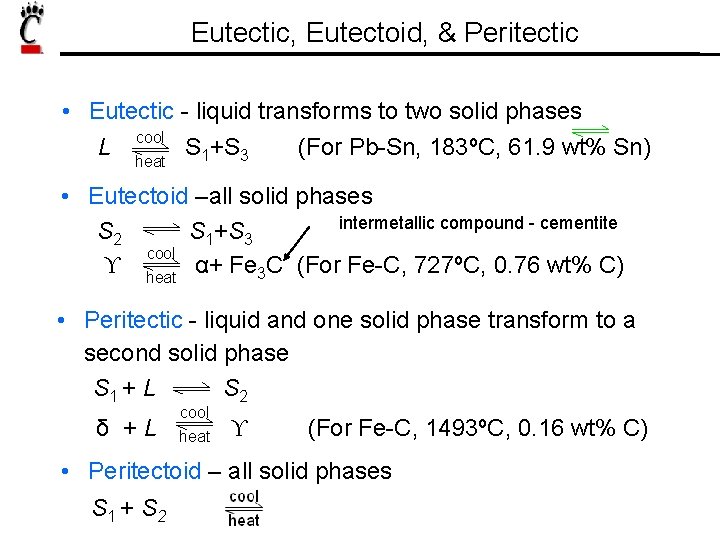
Eutectic, Eutectoid, & Peritectic • Eutectic - liquid transforms to two solid phases cool L heat S 1+S 3 (For Pb-Sn, 183ºC, 61. 9 wt% Sn) • Eutectoid –all solid phases intermetallic compound - cementite S 2 S 1+S 3 cool ϒ heat α+ Fe 3 C (For Fe-C, 727ºC, 0. 76 wt% C) • Peritectic - liquid and one solid phase transform to a second solid phase S 1 + L S 2 δ +L cool heat ϒ (For Fe-C, 1493ºC, 0. 16 wt% C) • Peritectoid – all solid phases S 1 + S 2 S 3

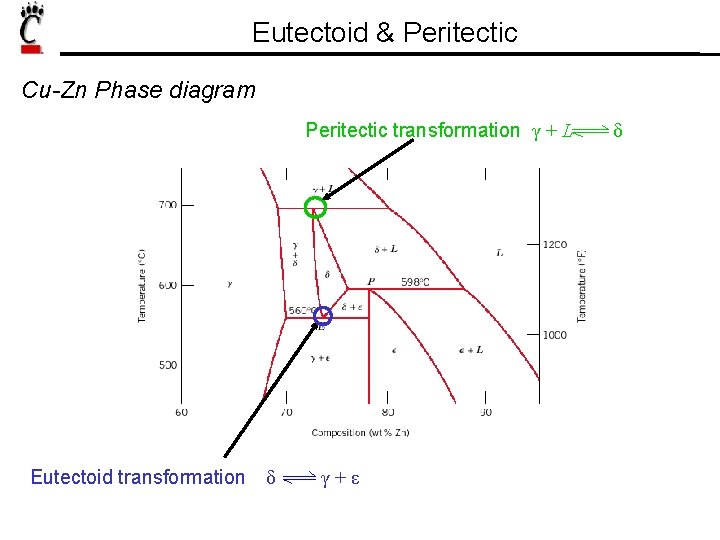
Eutectoid & Peritectic Cu-Zn Phase diagram Peritectic transformation γ + L Eutectoid transformation δ γ+ε δ

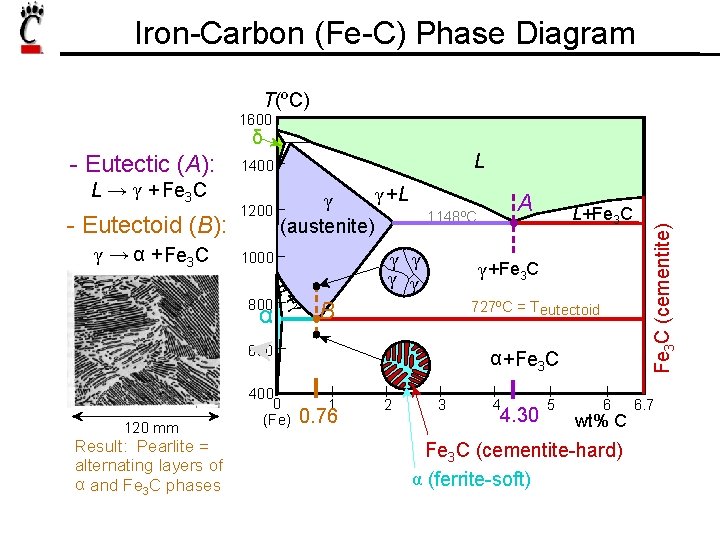
Iron-Carbon (Fe-C) Phase Diagram T(ºC) 1600 δ L → γ + Fe 3 C - Eutectoid (B): γ → α + Fe 3 C L 1400 1200 γ +L γ (austenite) γ γ 1000 γ α+ 800 α 120 mm Result: Pearlite = alternating layers of α and Fe 3 C phases L+Fe 3 C γ +Fe 3 C B 727ºC = T eutectoid 600 400 0 (Fe) A 1148ºC α +Fe 3 C 1 0. 76 2 3 4 4. 30 5 6 wt% C Fe 3 C (cementite-hard) α (ferrite-soft) Fe 3 C (cementite) - Eutectic (A): 6. 7

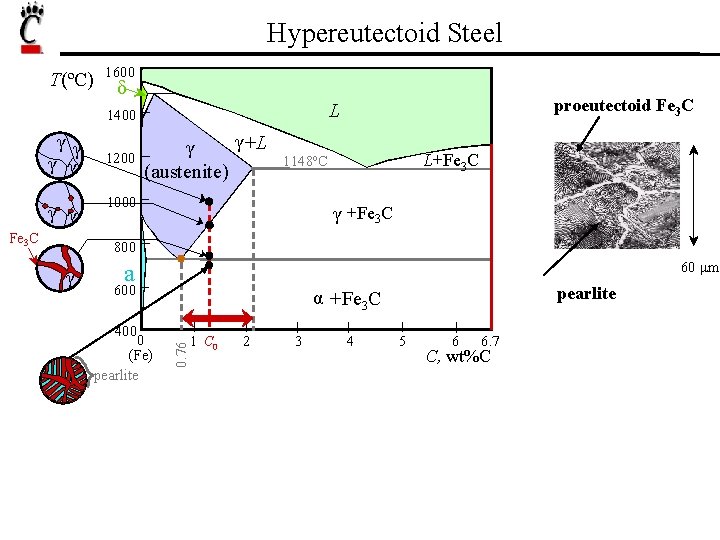
Hypereutectoid Steel T(ºC) 1600 δ γγ γ γ Fe 3 C 1200 proeutectoid Fe 3 C L 1400 γ +L γ (austenite) L+Fe 3 C 1148ºC 1000 γ +Fe 3 C 800 60 μm a 600 400 0 (Fe) pearlite α +Fe 3 C 0. 76 γ 1 C 0 2 3 4 5 6 6. 7 C, wt%C

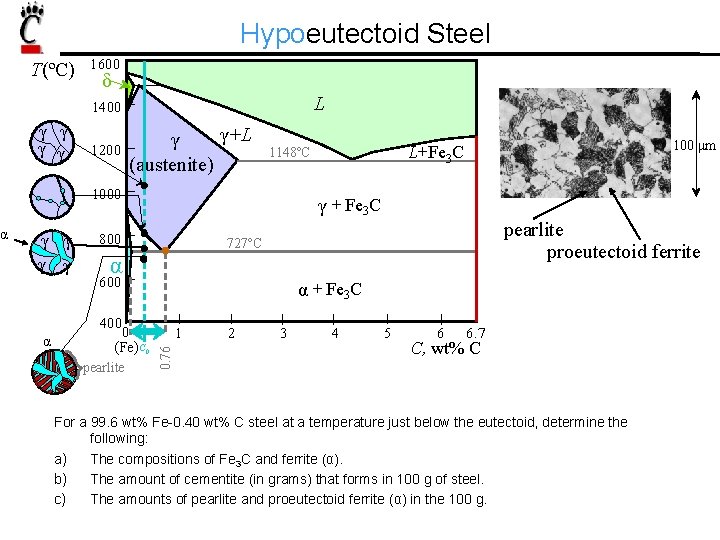
T(ºC) 1600 δ L 1400 γ γ 1200 γ +L γ (austenite) γ γ γ + Fe 3 C 800 pearlite proeutectoid ferrite 727ºC α 600 α + Fe 3 C 400 α 0 (Fe) C 0 pearlite 100 μm L+Fe 3 C 1148ºC 1000 1 0. 76 α Hypoeutectoid Steel 2 3 4 5 6 6. 7 C, wt% C For a 99. 6 wt% Fe-0. 40 wt% C steel at a temperature just below the eutectoid, determine the following: a) The compositions of Fe 3 C and ferrite (α). b) The amount of cementite (in grams) that forms in 100 g of steel. c) The amounts of pearlite and proeutectoid ferrite (α) in the 100 g.

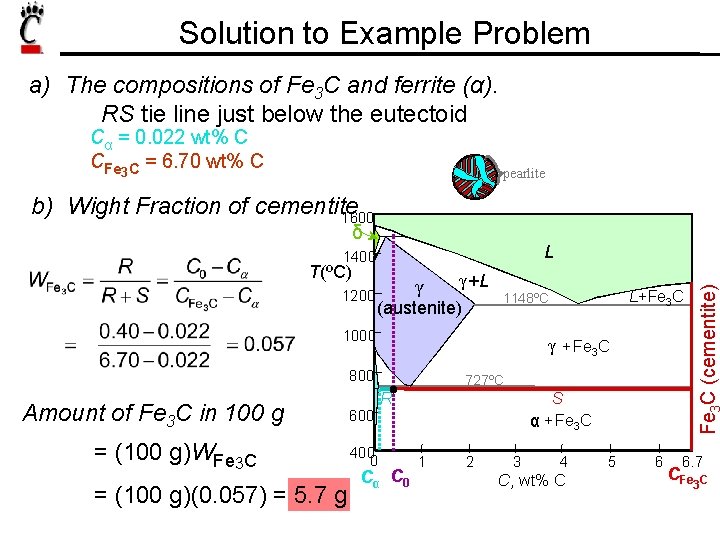
Solution to Example Problem a) The compositions of Fe 3 C and ferrite (α). RS tie line just below the eutectoid Cα = 0. 022 wt% C CFe 3 C = 6. 70 wt% C pearlite b) Wight Fraction of cementite 1600 δ T(ºC) 1200 γ γ +L (austenite) γ + Fe 3 C 800 Amount of Fe 3 C in 100 g = (100 g)WFe 3 C = (100 g)(0. 057) = 5. 7 g 727ºC R S α + Fe 3 C 600 400 0 L+Fe 3 C 1148ºC 1000 Fe C (cementite) L 1400 Cα C 0 1 2 3 4 C , wt% C 5 6 6. 7 CFe 3 C

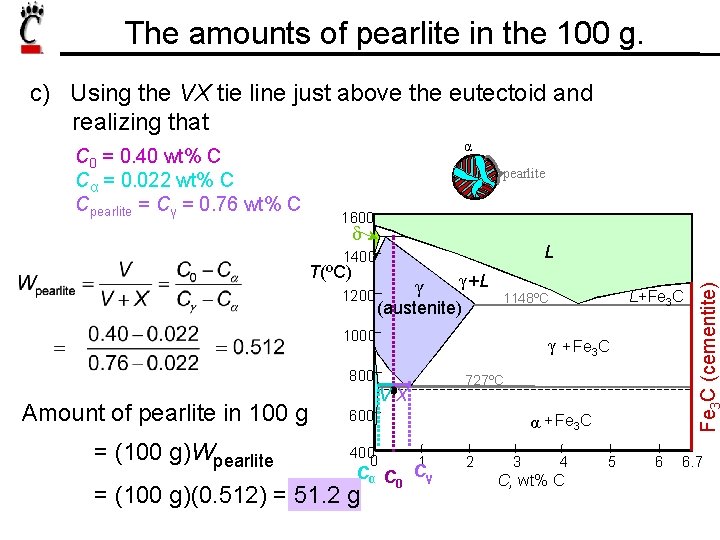
The amounts of pearlite in the 100 g. c) Using the VX tie line just above the eutectoid and realizing that α pearlite 1600 δ L 1400 T(ºC) 1200 γ γ +L (austenite) 1000 γ + Fe 3 C 800 Amount of pearlite in 100 g = (100 g)Wpearlite 727ºC VX 600 400 0 α + Fe 3 C 1 Cα C 0 Cγ = (100 g)(0. 512) = 51. 2 g L+Fe 3 C 1148ºC 2 3 4 C, wt% C 5 6 Fe C (cementite) C 0 = 0. 40 wt% C Cα = 0. 022 wt% C Cpearlite = Cγ = 0. 76 wt% C 6. 7

Alloying with Other Elements Ti Mo Si W Cr Mn Ni wt. % of alloying elements • Ceutectoid changes: Ceutectoid (wt% C) T Eutectoid (ºC) • Teutectoid changes: Ni Cr Si Ti Mo W Mn wt. % of alloying elements

VMSE: Interactive Phase Diagrams Microstructure, phase compositions, and phase fractions respond interactively Change alloy composition

Summary • Phase diagrams are useful tools to determine: -- the number and types of phases present, -- the composition of each phase, -- and the weight fraction of each phase given the temperature and composition of the system. • The microstructure of an alloy depends on -- its composition, and -- whether or not cooling rate allows for maintenance of equilibrium. • Important phase diagram phase transformations include eutectic, eutectoid, and peritectic.


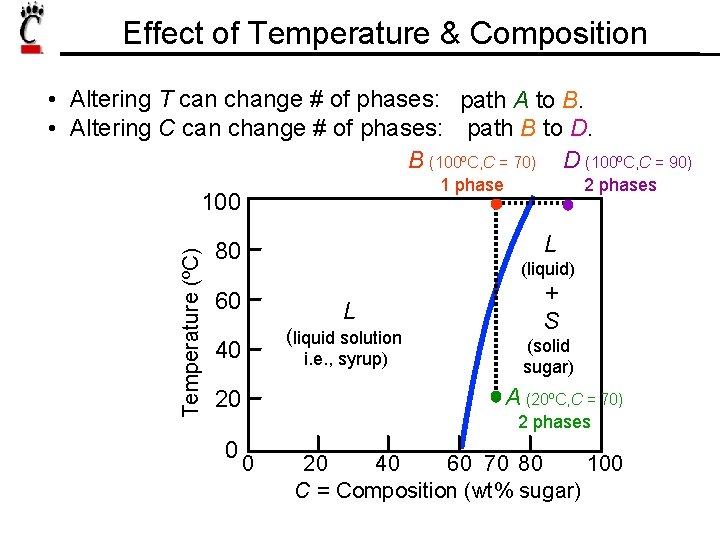
Effect of Temperature & Composition • Altering T can change # of phases: path A to B. • Altering C can change # of phases: path B to D. B (100ºC, C = 70) D (100ºC, C = 90) 1 phase Temperature (ºC) 100 L 80 (liquid) 60 L (liquid solution 40 i. e. , syrup) + S (solid sugar) A (20ºC, C = 70) 20 0 2 phases 0 20 40 60 70 80 100 C = Composition (wt% sugar)

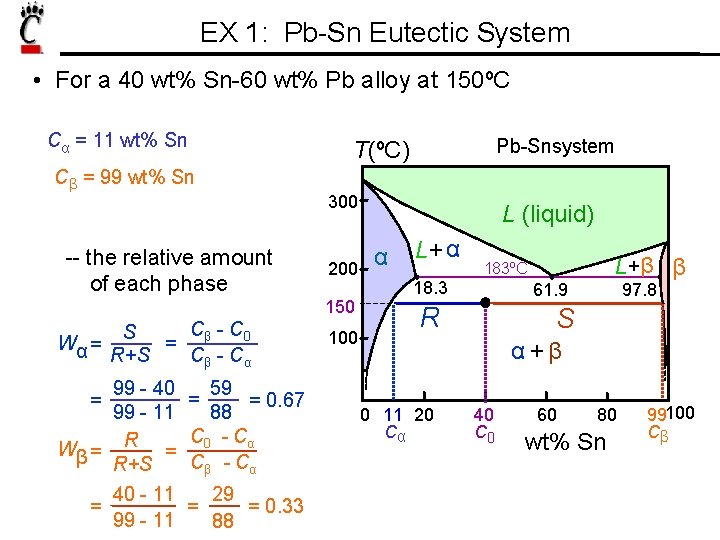
EX 1: Pb-Sn Eutectic System • For a 40 wt% Sn-60 wt% Pb alloy at 150ºC Cα = 11 wt% Sn Cβ = 99 wt% Sn 300 -- the relative amount of each phase 200 150 Cβ - C 0 S = Wα = R+S Cβ - Cα 99 - 40 99 - 11 Wβ = R+S 40 - 11 = 99 - 11 = Pb-Snsystem T(ºC) 59 = 0. 67 88 C 0 - Cα Cβ - Cα = = 29 = 0. 33 88 100 L (liquid) α L+ α 18. 3 L+β β 183ºC 61. 9 R 97. 8 S α+β 0 11 20 Cα 40 C 0 60 80 wt% Sn 99100 Cβ

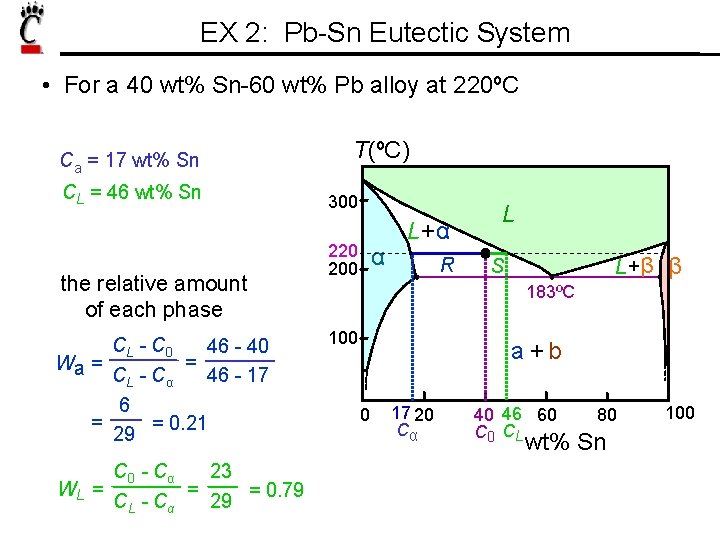
EX 2: Pb-Sn Eutectic System • For a 40 wt% Sn-60 wt% Pb alloy at 220ºC Ca = 17 wt% Sn CL = 46 wt% Sn the relative amount of each phase CL - C 0 46 - 40 = Wa = CL - Cα 46 - 17 6 = = 0. 21 29 C 0 - Cα 23 = WL = = 0. 79 CL - Cα 29 T(ºC) 300 220 200 α L+ α R L L+β β S 183ºC 100 a +b 0 17 20 Cα 40 46 60 C 0 CL 80 wt% Sn 100