Chapter 9 Phase Diagrams 1 Phase Diagram Vocabulary

Chapter 9 Phase Diagrams 1
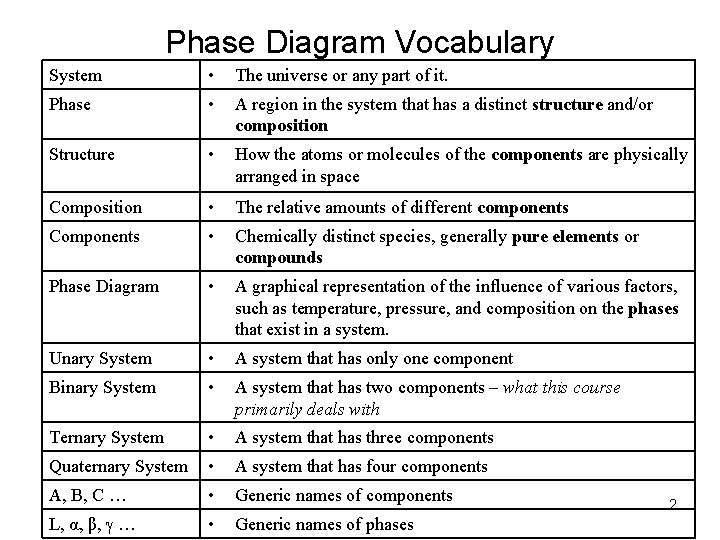
Phase Diagram Vocabulary System • The universe or any part of it. Phase • A region in the system that has a distinct structure and/or composition Structure • How the atoms or molecules of the components are physically arranged in space Composition • The relative amounts of different components Components • Chemically distinct species, generally pure elements or compounds Phase Diagram • A graphical representation of the influence of various factors, such as temperature, pressure, and composition on the phases that exist in a system. Unary System • A system that has only one component Binary System • A system that has two components – what this course primarily deals with Ternary System • A system that has three components Quaternary System • A system that has four components A, B, C … • Generic names of components L, α, β, … • Generic names of phases 2
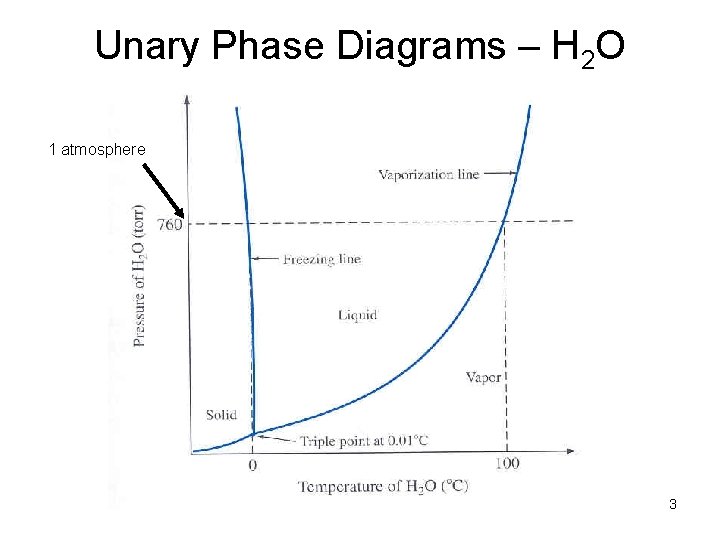
Unary Phase Diagrams – H 2 O 1 atmosphere 3
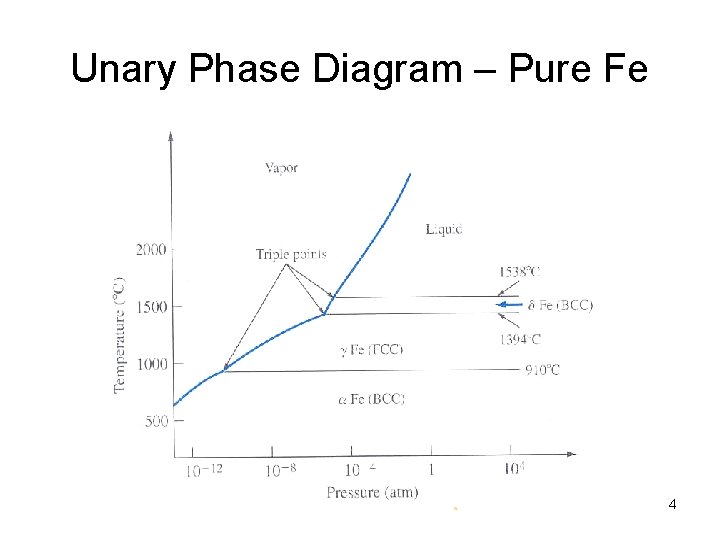
Unary Phase Diagram – Pure Fe 4
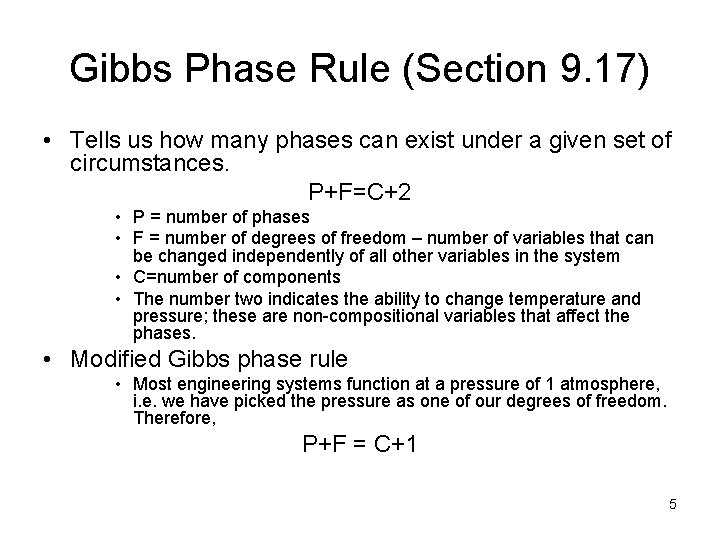
Gibbs Phase Rule (Section 9. 17) • Tells us how many phases can exist under a given set of circumstances. P+F=C+2 • P = number of phases • F = number of degrees of freedom – number of variables that can be changed independently of all other variables in the system • C=number of components • The number two indicates the ability to change temperature and pressure; these are non-compositional variables that affect the phases. • Modified Gibbs phase rule • Most engineering systems function at a pressure of 1 atmosphere, i. e. we have picked the pressure as one of our degrees of freedom. Therefore, P+F = C+1 5
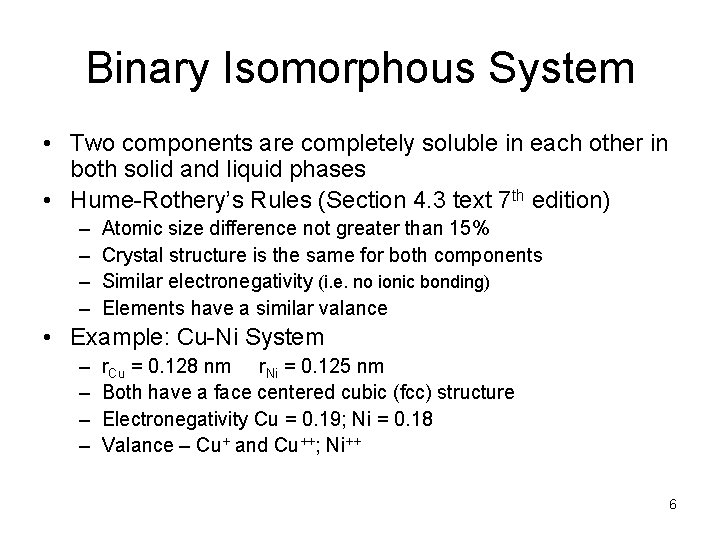
Binary Isomorphous System • Two components are completely soluble in each other in both solid and liquid phases • Hume-Rothery’s Rules (Section 4. 3 text 7 th edition) – – Atomic size difference not greater than 15% Crystal structure is the same for both components Similar electronegativity (i. e. no ionic bonding) Elements have a similar valance • Example: Cu-Ni System – – r. Cu = 0. 128 nm r. Ni = 0. 125 nm Both have a face centered cubic (fcc) structure Electronegativity Cu = 0. 19; Ni = 0. 18 Valance – Cu+ and Cu++; Ni++ 6
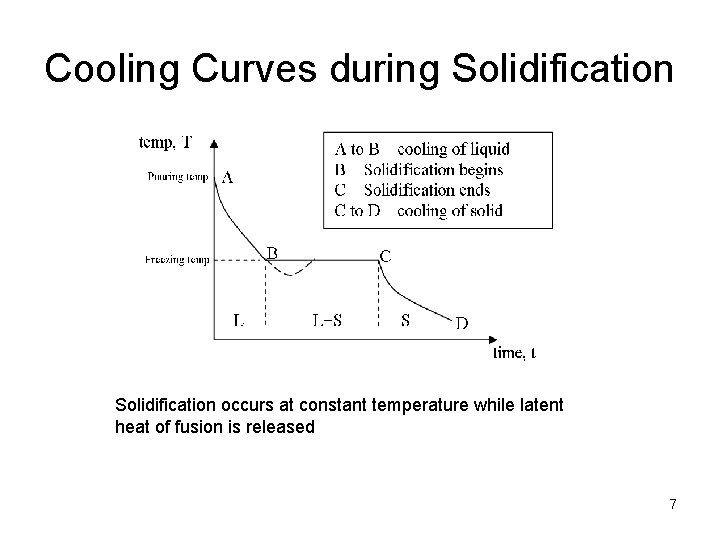
Cooling Curves during Solidification occurs at constant temperature while latent heat of fusion is released 7
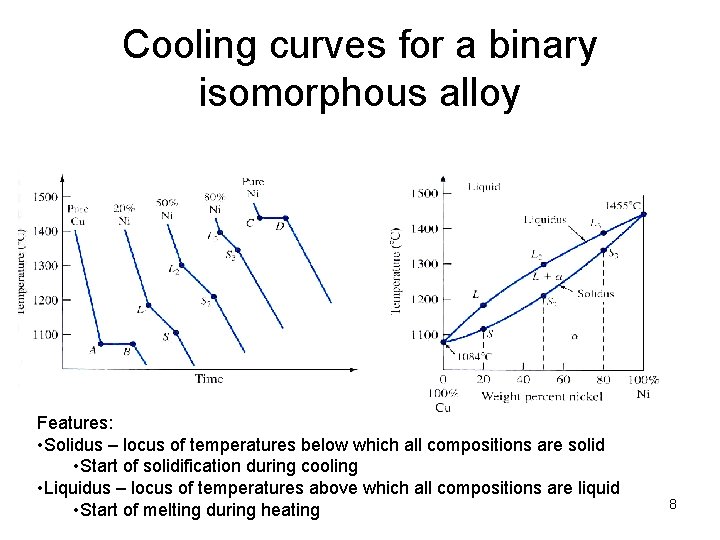
Cooling curves for a binary isomorphous alloy Features: • Solidus – locus of temperatures below which all compositions are solid • Start of solidification during cooling • Liquidus – locus of temperatures above which all compositions are liquid • Start of melting during heating 8
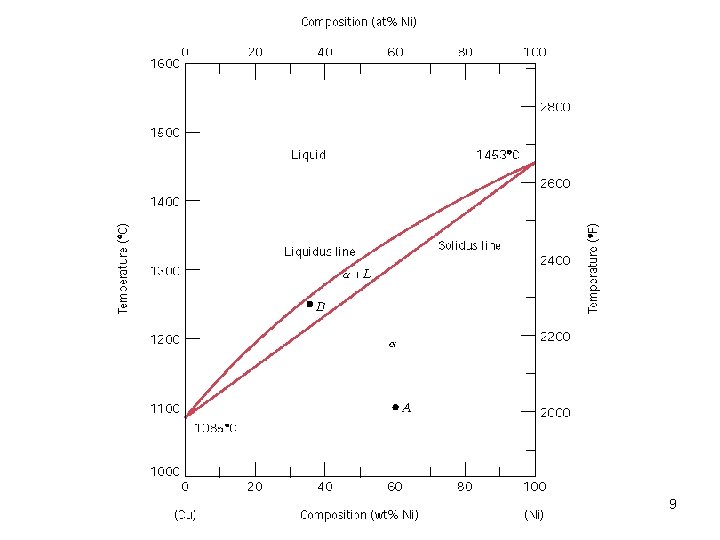
9
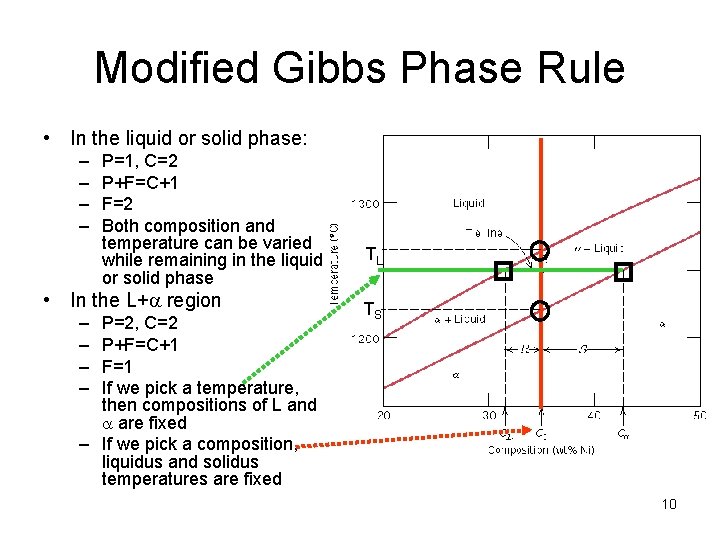
Modified Gibbs Phase Rule • In the liquid or solid phase: – – P=1, C=2 P+F=C+1 F=2 Both composition and temperature can be varied while remaining in the liquid or solid phase • In the L+a region – – P=2, C=2 P+F=C+1 F=1 If we pick a temperature, then compositions of L and a are fixed – If we pick a composition, liquidus and solidus temperatures are fixed TL TS 10
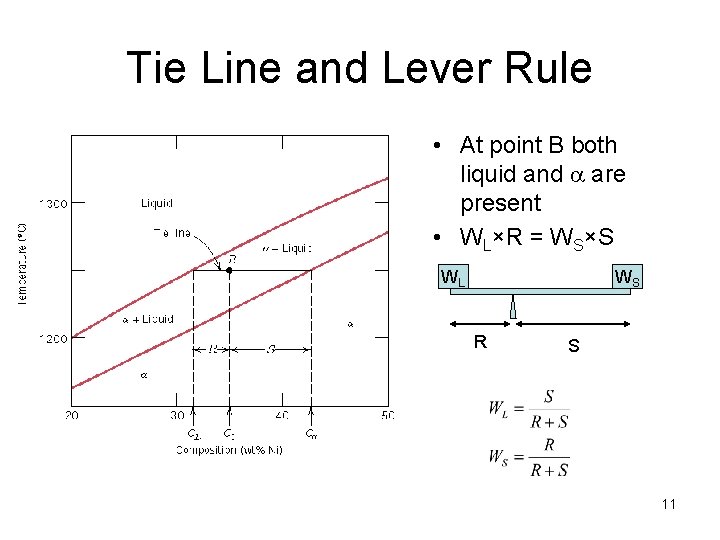
Tie Line and Lever Rule • At point B both liquid and a are present • WL×R = WS×S WL WS R S 11
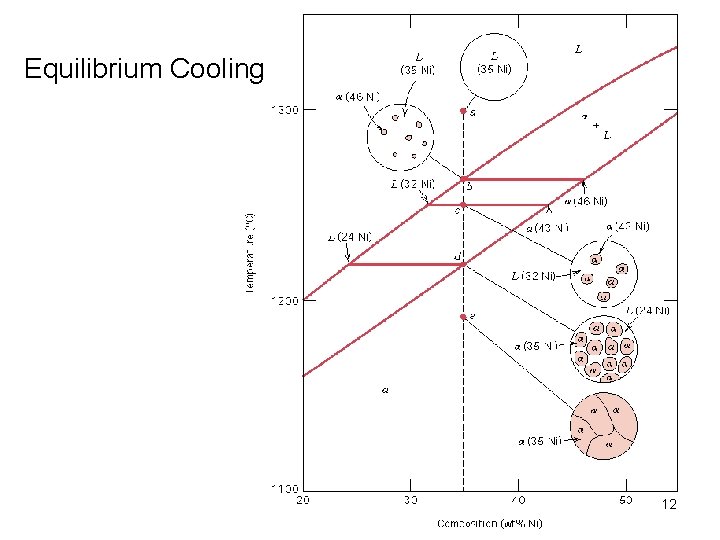
Equilibrium Cooling 12
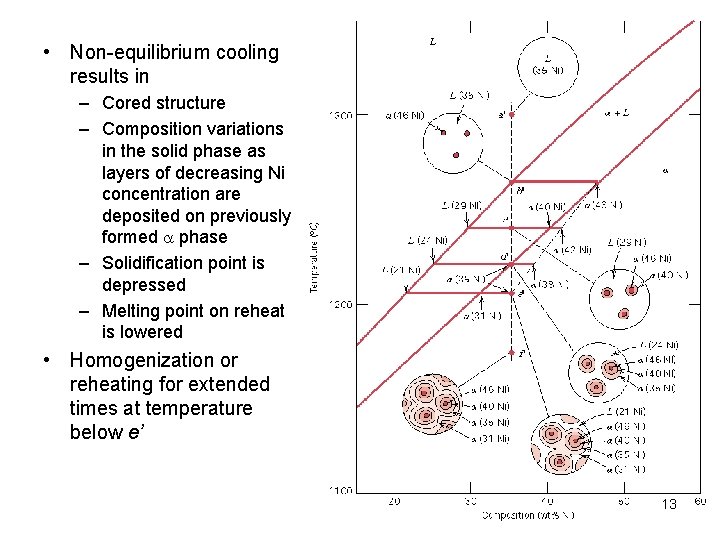
• Non-equilibrium cooling results in – Cored structure – Composition variations in the solid phase as layers of decreasing Ni concentration are deposited on previously formed a phase – Solidification point is depressed – Melting point on reheat is lowered • Homogenization or reheating for extended times at temperature below e’ 13
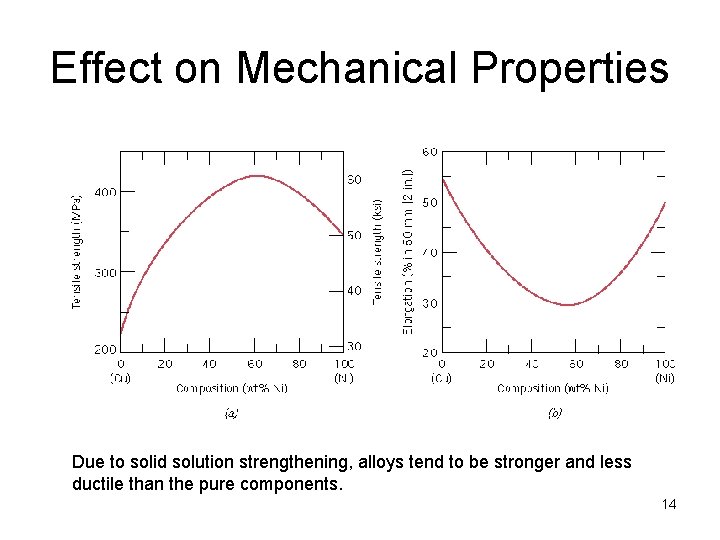
Effect on Mechanical Properties Due to solid solution strengthening, alloys tend to be stronger and less ductile than the pure components. 14
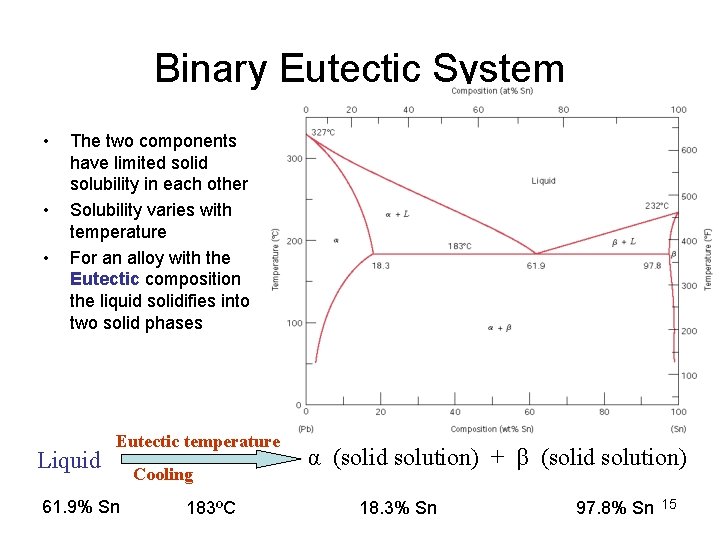
Binary Eutectic System • • • The two components have limited solid solubility in each other Solubility varies with temperature For an alloy with the Eutectic composition the liquid solidifies into two solid phases Liquid Eutectic temperature 61. 9% Sn Cooling 183ºC α (solid solution) + β (solid solution) 18. 3% Sn 97. 8% Sn 15

Binary Eutectic System • Apply Modified Gibbs Phase Rule Phases present: L, a and b (P=3) Components: Pb and Sn (C=2) P+F=C+1 F=0 no degrees of freedom Therefore, three phases can coexist in a binary system only at a unique temperature and for unique compositions of the three phases – Upon cooling, there is a temperature arrest during the solidification process (eutectic reaction) – – – 16
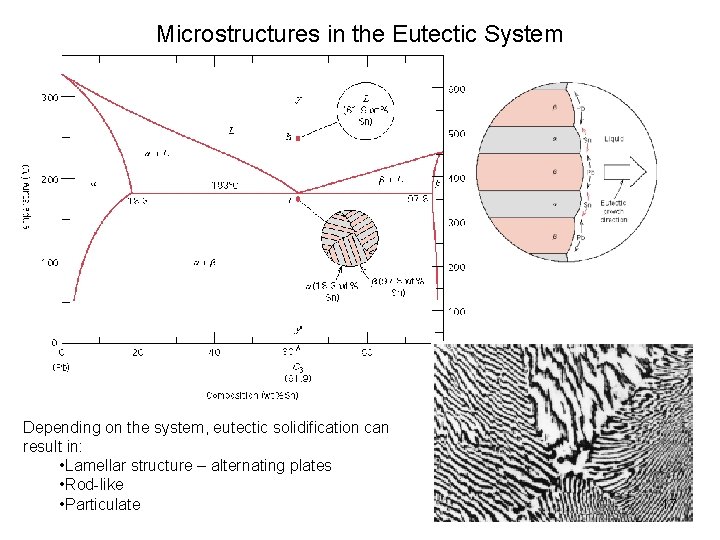
Microstructures in the Eutectic System Depending on the system, eutectic solidification can result in: • Lamellar structure – alternating plates • Rod-like • Particulate 17
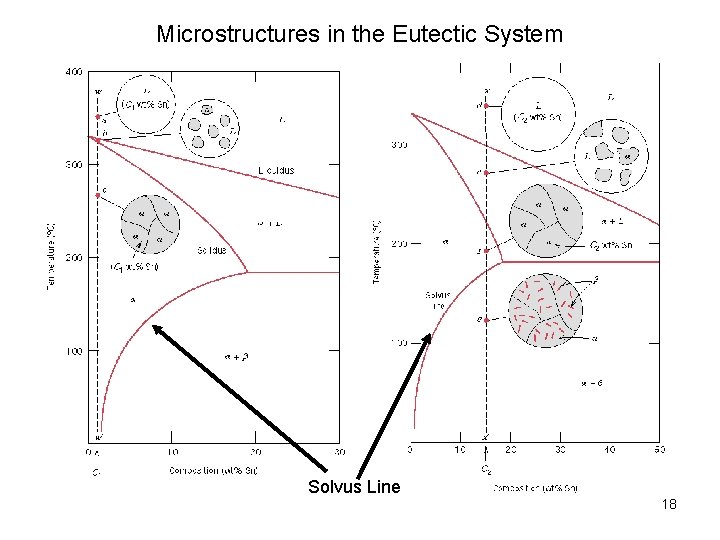
Microstructures in the Eutectic System Solvus Line 18
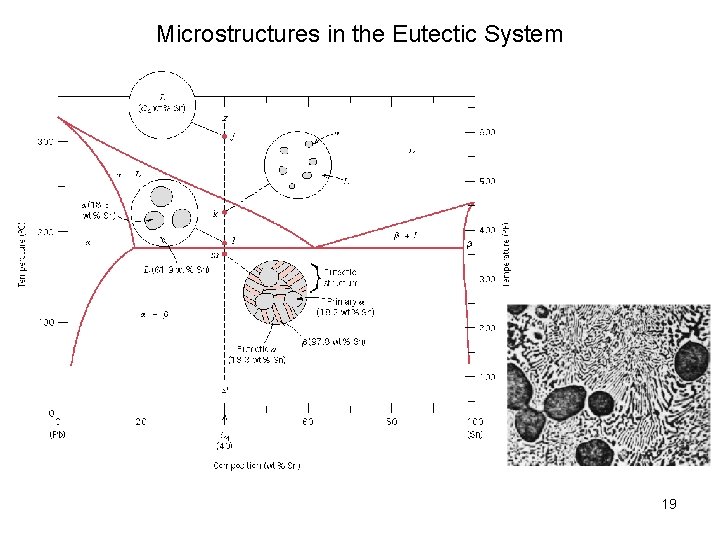
Microstructures in the Eutectic System 19
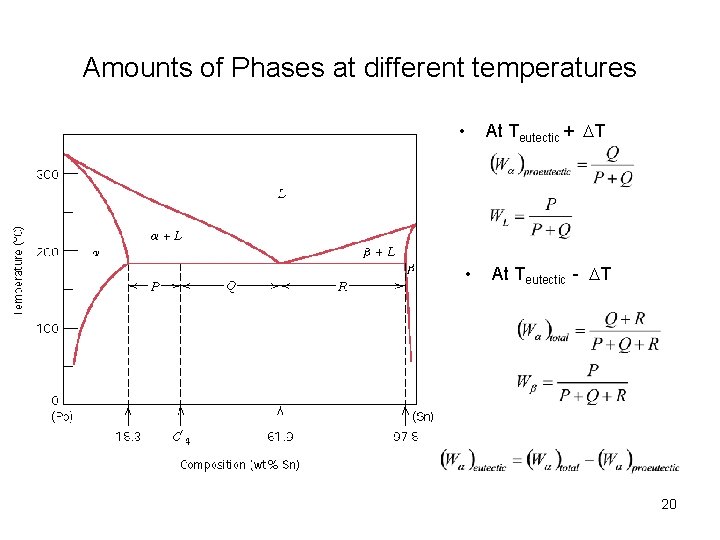
Amounts of Phases at different temperatures At Teutectic + DT • • At Teutectic - DT 20
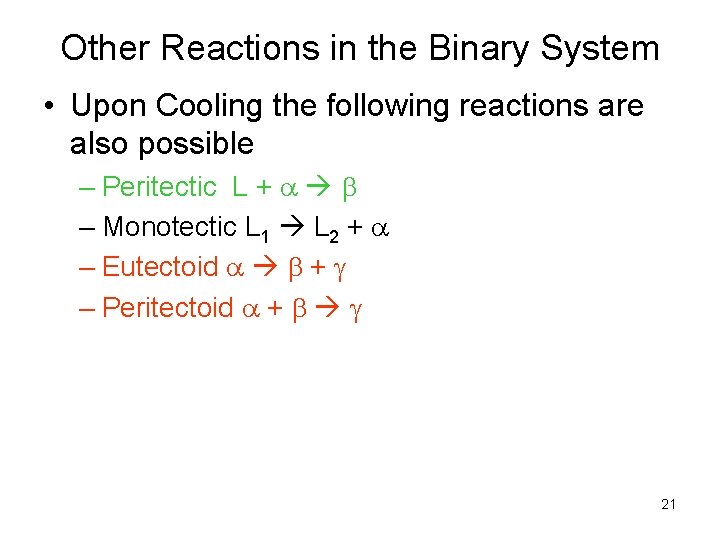
Other Reactions in the Binary System • Upon Cooling the following reactions are also possible – Peritectic L + a b – Monotectic L 1 L 2 + a – Eutectoid a b + – Peritectoid a + b 21
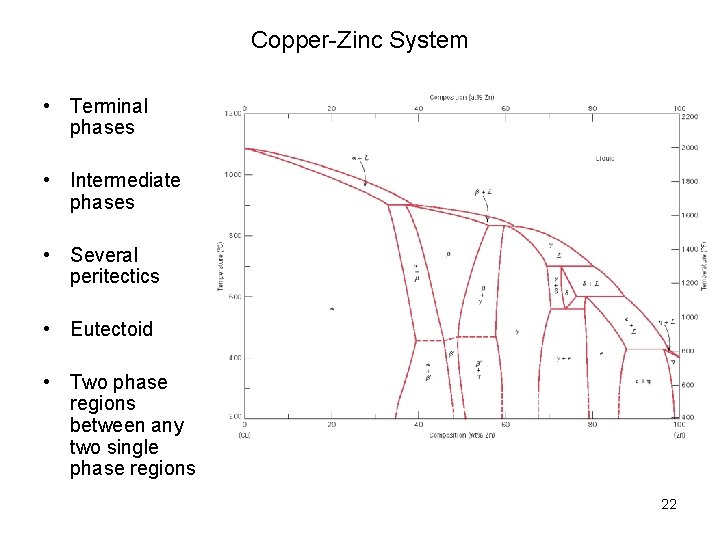
Copper-Zinc System • Terminal phases • Intermediate phases • Several peritectics • Eutectoid • Two phase regions between any two single phase regions 22
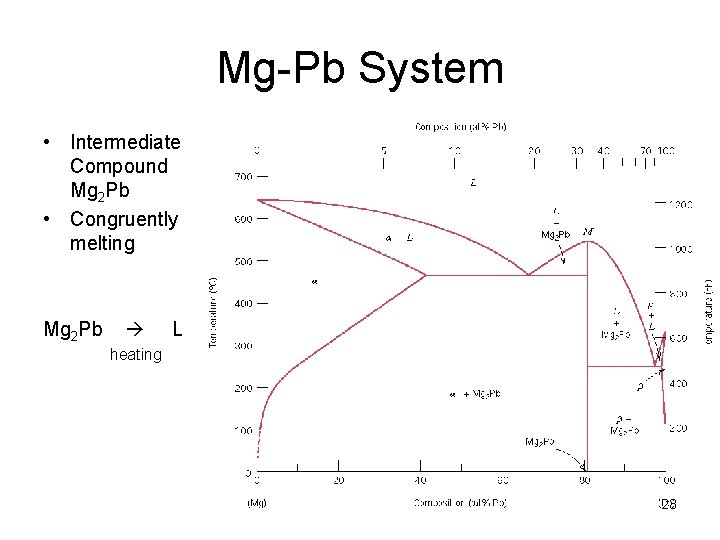
Mg-Pb System • Intermediate Compound Mg 2 Pb • Congruently melting Mg 2 Pb L heating 23
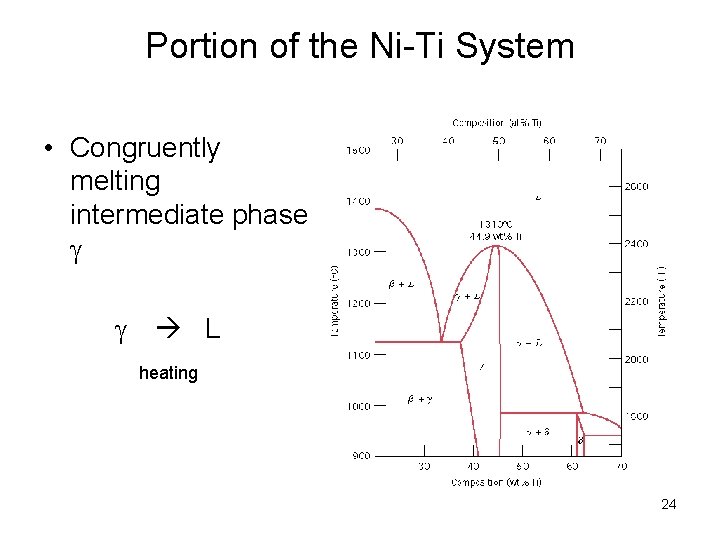
Portion of the Ni-Ti System • Congruently melting intermediate phase L heating 24
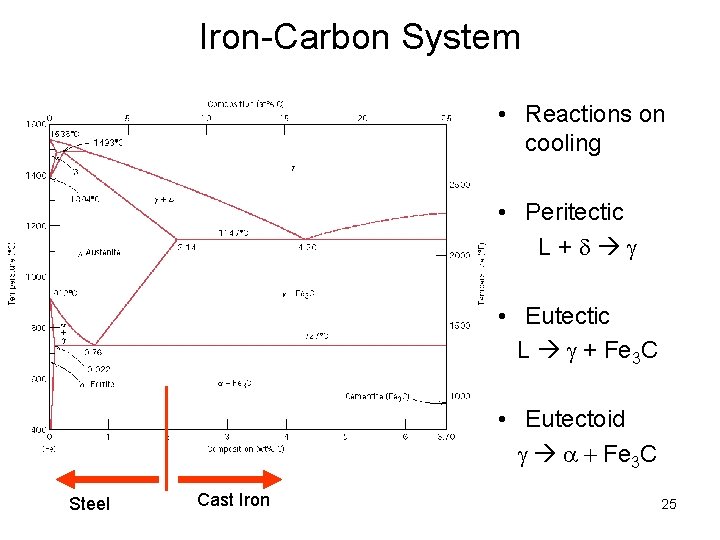
Iron-Carbon System • Reactions on cooling • Peritectic L+d • Eutectic L + Fe 3 C • Eutectoid a + Fe 3 C Steel Cast Iron 25
Iron-Carbon or Iron-Fe 3 C • In principle, the components of the phase diagram should be iron (Fe) and carbon/graphite (C). – Fe and C form an intermediate compound Fe 3 C, which is very stable – There isn’t anything of interest at carbon contents greater than 25 at. % or 6. 7 wt. % C. – Fe 3 C is considered to be a component, and the binary phase diagram is drawn using Fe and Fe 3 C. • Names of phases: – – Ferrite - a iron – bcc structure Austenite – iron – fcc structure High temperature d iron – bcc structure Cementite – Fe 3 C • Steels have carbon contents <2%, usually <1. 2% • Cast irons have carbon contents >2% 26
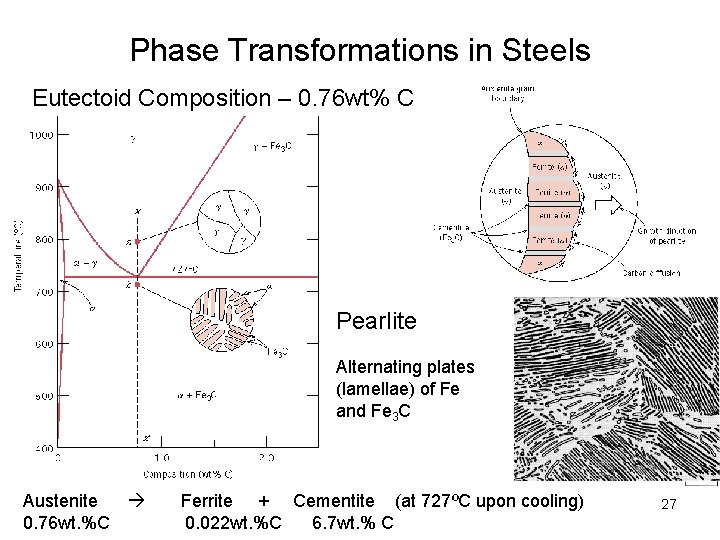
Phase Transformations in Steels Eutectoid Composition – 0. 76 wt% C Pearlite Alternating plates (lamellae) of Fe and Fe 3 C Austenite 0. 76 wt. %C Ferrite + Cementite (at 727ºC upon cooling) 0. 022 wt. %C 6. 7 wt. % C 27
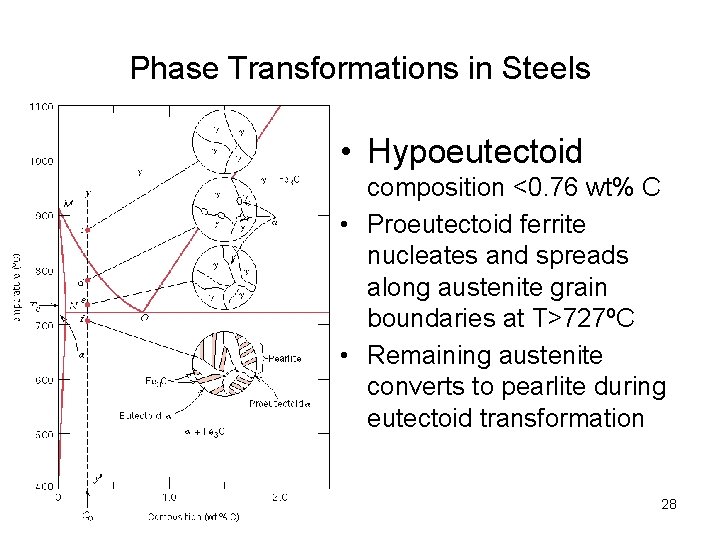
Phase Transformations in Steels • Hypoeutectoid composition <0. 76 wt% C • Proeutectoid ferrite nucleates and spreads along austenite grain boundaries at T>727ºC • Remaining austenite converts to pearlite during eutectoid transformation 28
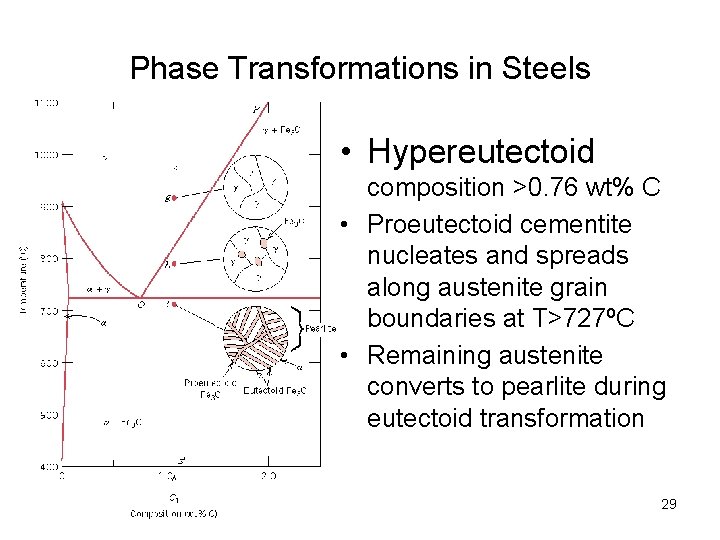
Phase Transformations in Steels • Hypereutectoid composition >0. 76 wt% C • Proeutectoid cementite nucleates and spreads along austenite grain boundaries at T>727ºC • Remaining austenite converts to pearlite during eutectoid transformation 29
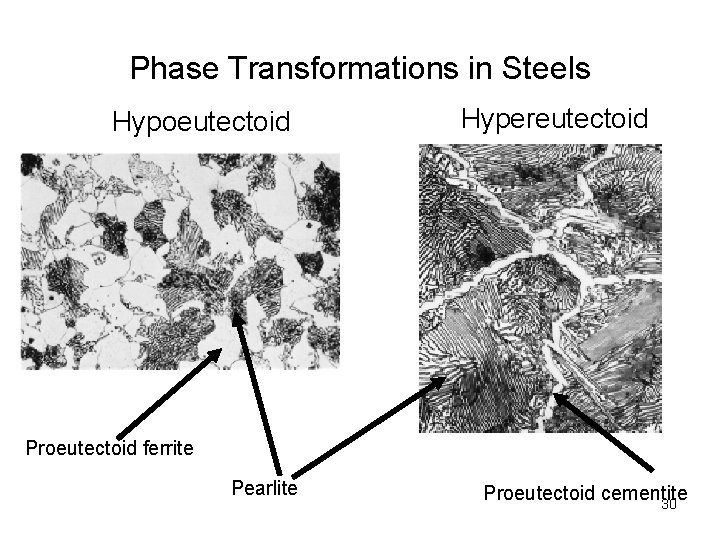
Phase Transformations in Steels Hypoeutectoid Hypereutectoid Proeutectoid ferrite Pearlite Proeutectoid cementite 30
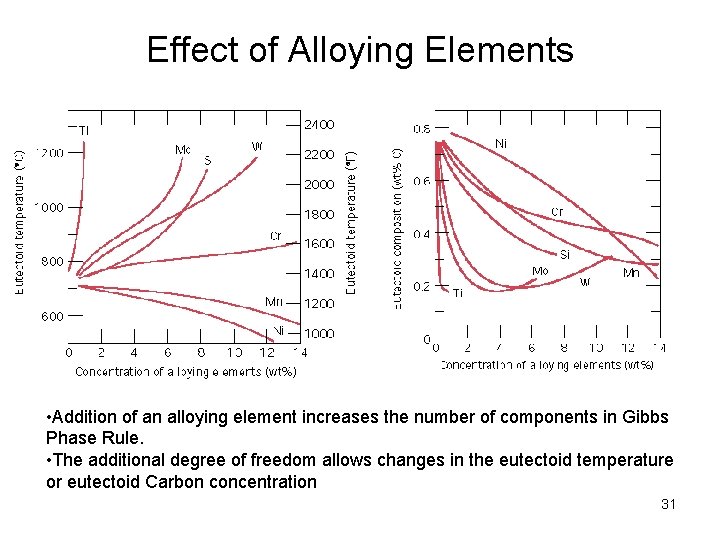
Effect of Alloying Elements • Addition of an alloying element increases the number of components in Gibbs Phase Rule. • The additional degree of freedom allows changes in the eutectoid temperature or eutectoid Carbon concentration 31
- Slides: 31