Chapter 9 Part 1 Cellular Respiration AP Biology
Chapter 9 (Part 1): Cellular Respiration AP Biology Ms. Day
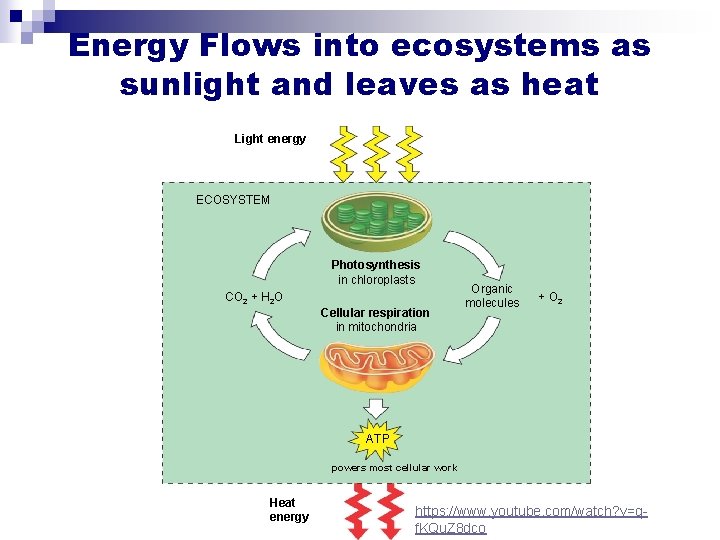
Energy Flows into ecosystems as sunlight and leaves as heat Light energy ECOSYSTEM Photosynthesis in chloroplasts CO 2 + H 2 O Cellular respiration in mitochondria Organic molecules + O 2 ATP powers most cellular work Heat energy https: //www. youtube. com/watch? v=qf. KQu. Z 8 dco
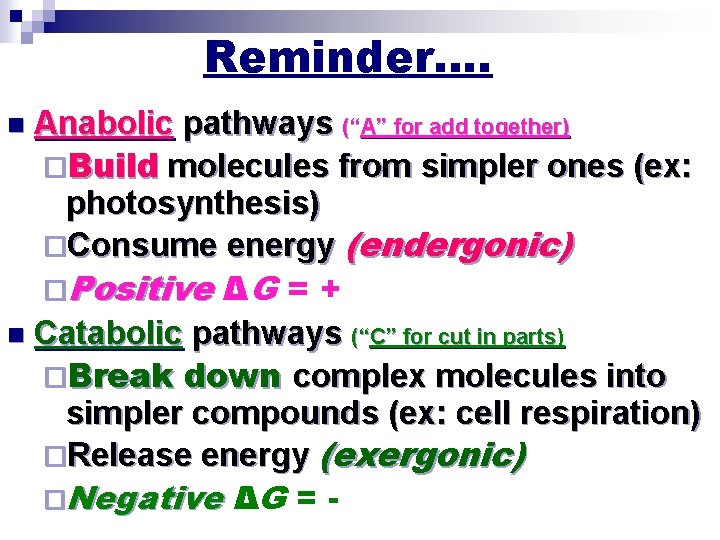
Reminder…. n Anabolic pathways (“A” for add together) ¨Build molecules from simpler ones (ex: photosynthesis) ¨Consume energy (endergonic) ¨Positive ΔG = + n Catabolic pathways (“C” for cut in parts) ¨Break down complex molecules into simpler compounds (ex: cell respiration) ¨Release energy (exergonic) ¨Negative ΔG = -
Cellular respiration ¨Most efficient catabolic pathway ¨Consumes O 2 and organic molecules (ex: glucose) ¨Yields ATP To keep working cells must regenerate ATP
Catabolic pathways n The breakdown of organic molecules is exergonic n One catabolic process, fermentation ¨Is a partial degradation of sugars that occurs without oxygen n Another example is cellular respiration
Cellular respiration n Occurs in mitochondria ¨Like using gas in an engine after O 2 is mixed with hydrocarbon fuel n Food = fuel for respiration. n The exhaust =CO 2 and H 2 O. The overall process is: organic compounds + O 2 CO 2 + H 2 O + energy (ATP + heat) ¨Carbohydrates, fats, and proteins n most useful is glucose
Mitochondria • Has own DNA (circular) • Has own ribosomes (small) REMEMBER… • according to the endosymbiotic theory mitochondria (and chloroplasts) are descended from free-living prokaryotes.
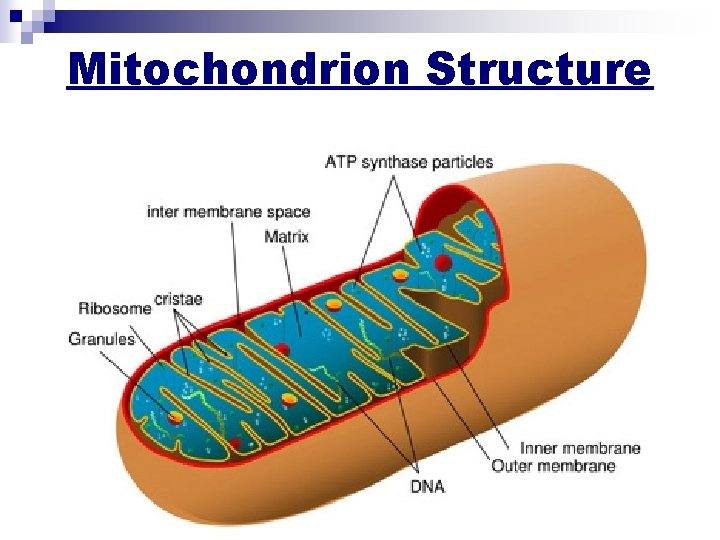
Mitochondrion Structure
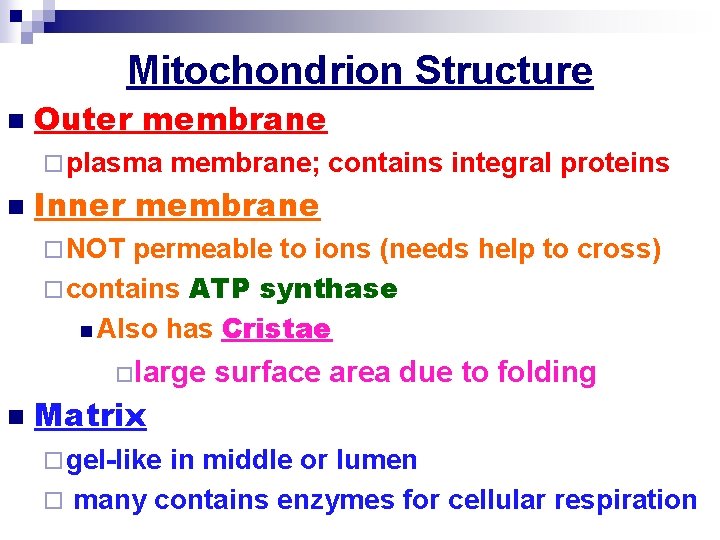
Mitochondrion Structure n Outer membrane ¨ plasma n membrane; contains integral proteins Inner membrane ¨ NOT permeable to ions (needs help to cross) ¨ contains ATP synthase n Also has Cristae ¨large n surface area due to folding Matrix ¨ gel-like in middle or lumen ¨ many contains enzymes for cellular respiration
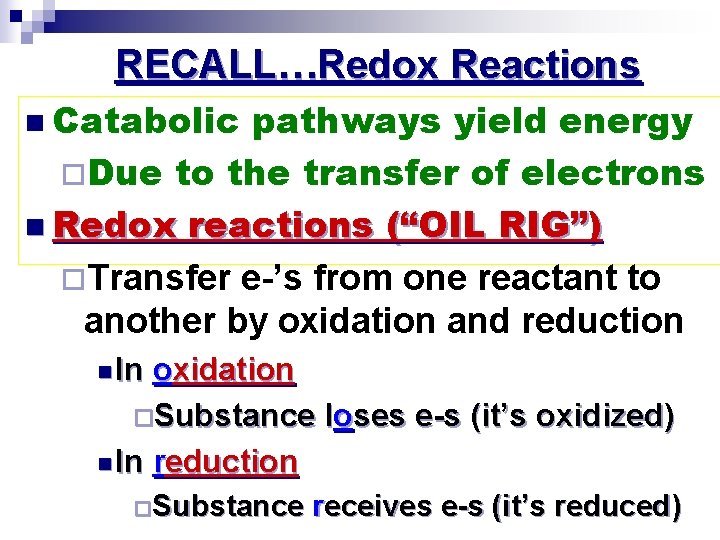
RECALL…Redox Reactions n Catabolic pathways yield energy ¨Due to the transfer of electrons n Redox reactions (“OIL RIG”) ¨Transfer e-’s from one reactant to another by oxidation and reduction n In oxidation ¨Substance loses e-s (it’s oxidized) n In reduction ¨Substance receives e-s (it’s reduced)
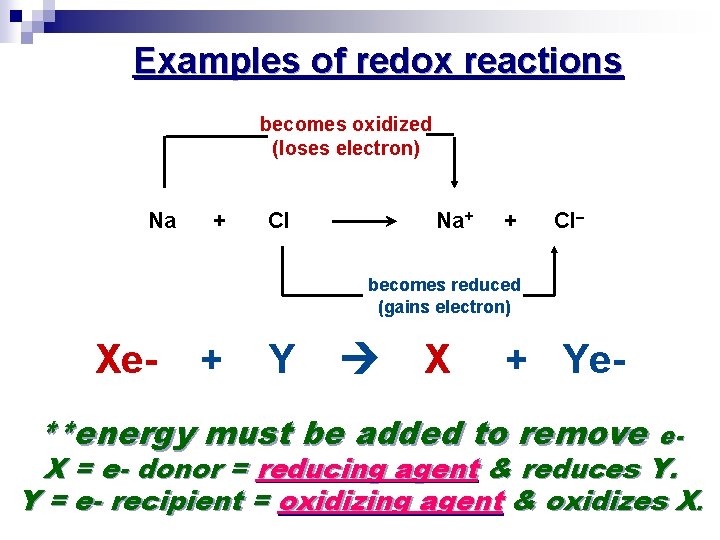
Examples of redox reactions becomes oxidized (loses electron) Na + Cl Na + + Cl– becomes reduced (gains electron) Xe- + Y X + Ye- **energy must be added to remove e- X = e- donor = reducing agent & reduces Y. Y = e- recipient = oxidizing agent & oxidizes X.
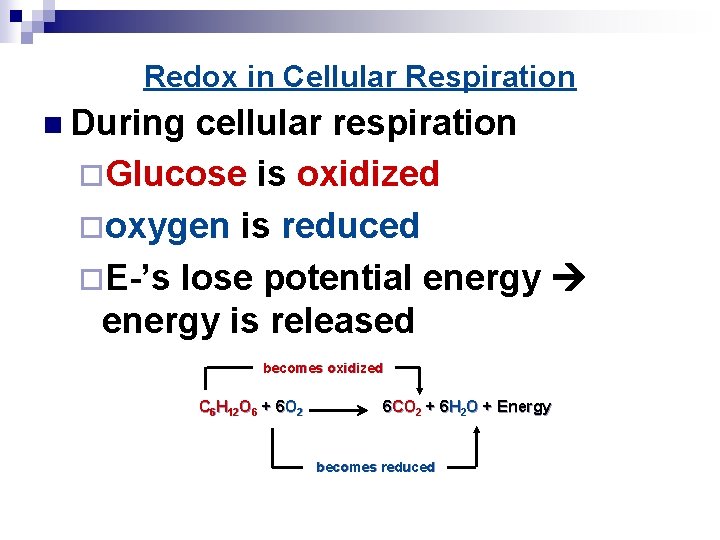
Redox in Cellular Respiration n During cellular respiration ¨Glucose is oxidized ¨oxygen is reduced ¨E-’s lose potential energy is released becomes oxidized C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + Energy becomes reduced
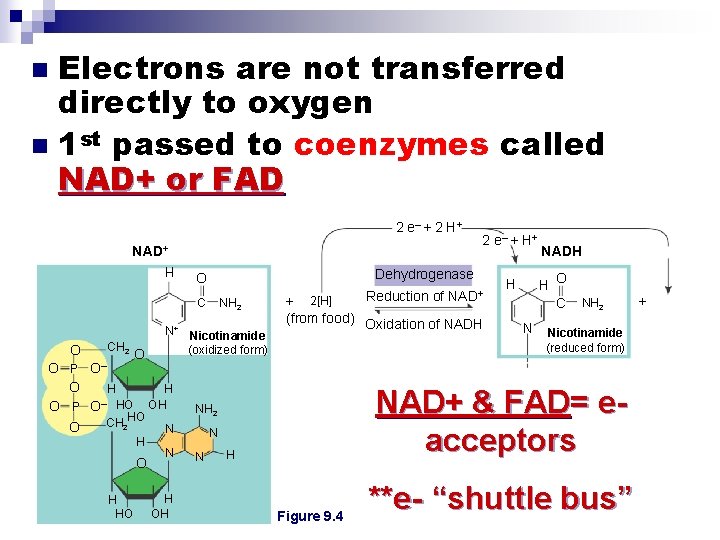
Electrons are not transferred directly to oxygen n 1 st passed to coenzymes called NAD+ or FAD n 2 e– + 2 H + NAD+ Dehydrogenase O NH 2 H C CH 2 O O– O N+ Nicotinamide (oxidized form) O P O H H – O P O HO OH HO CH 2 O N H O H HO N H OH Reduction of NAD+ + 2[H] (from food) Oxidation of NADH H O C H N NH 2 Nicotinamide (reduced form) NAD+ & FAD= eacceptors NH 2 N N 2 e– + H Figure 9. 4 **e- “shuttle bus” +
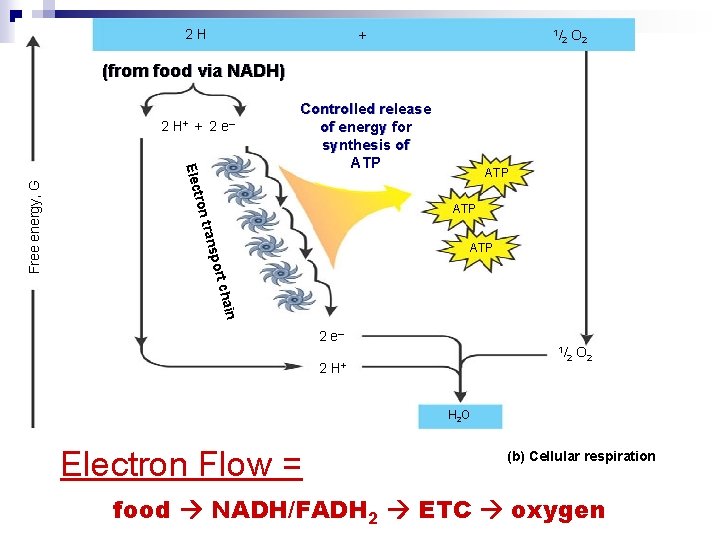
2 H + 1/ 2 O 2 (from food via NADH) + 2 e– Elec Controlled release of energy for synthesis of ATP tron ATP trans ATP port Free energy, G 2 H+ n chai 2 e– 1/ 2 H+ 2 O 2 H 2 O Electron Flow = (b) Cellular respiration food NADH/FADH 2 ETC oxygen
The Stages of Cellular Respiration n Respiration is a cumulative process of 3 metabolic stages 1. Glycolysis 2. The citric acid cycle 3. Oxidative phosphorylation http: //www. sumanasinc. com/webcontent/animations/content/cellularrespiration. html n Watch the “BIG PICTURE”
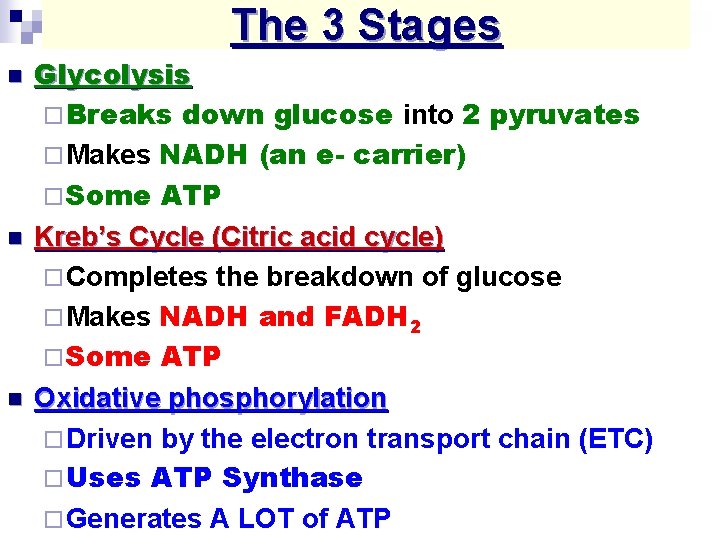
The 3 Stages n n n Glycolysis ¨ Breaks down glucose into 2 pyruvates ¨ Makes NADH (an e- carrier) ¨ Some ATP Kreb’s Cycle (Citric acid cycle) ¨ Completes the breakdown of glucose ¨ Makes NADH and FADH 2 ¨ Some ATP Oxidative phosphorylation ¨ Driven by the electron transport chain (ETC) ¨ Uses ATP Synthase ¨ Generates A LOT of ATP
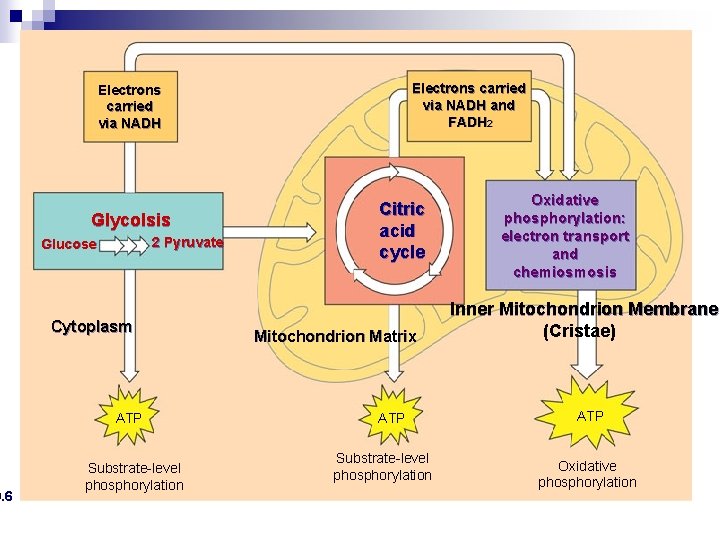
9. 6 Electrons carried via NADH and FADH 2 Electrons carried via NADH n An overview of cellular respiration Oxidative Glycolsis 2 Pyruvate Glucose Cytoplasm ATP Substrate-level phosphorylation Citric acid cycle Mitochondrion Matrix ATP Substrate-level phosphorylation: electron transport and chemiosmosis Inner Mitochondrion Membrane (Cristae) ATP Oxidative phosphorylation
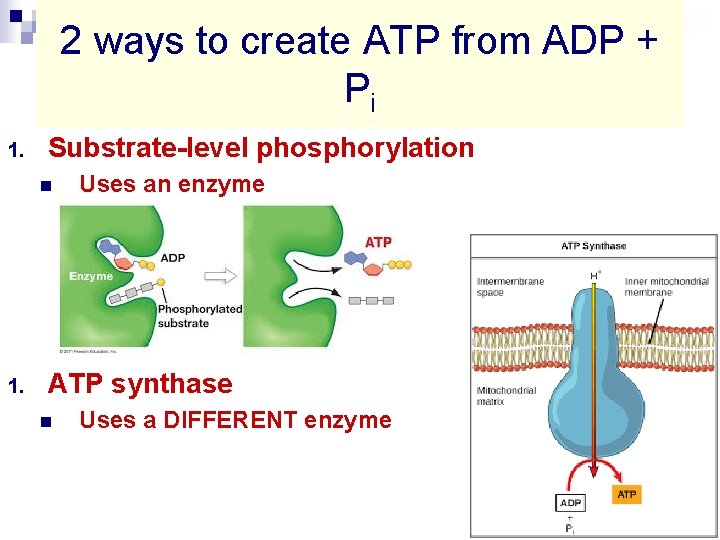
2 ways to create ATP from ADP + Pi 1. Substrate-level phosphorylation n 1. Uses an enzyme ATP synthase n Uses a DIFFERENT enzyme
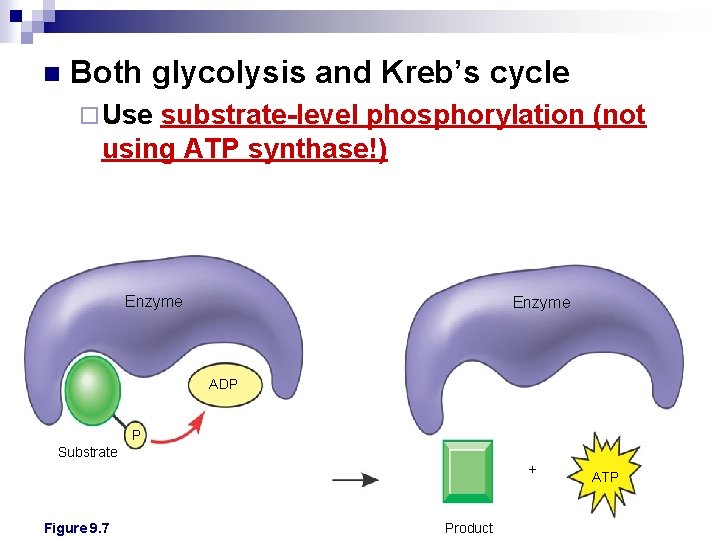
n Both glycolysis and Kreb’s cycle ¨ Use substrate-level phosphorylation (not using ATP synthase!) Enzyme ADP P Substrate + Figure 9. 7 Product ATP

Stage #1: Glycolysis GOAL: STARTS oxidizing (stripping e-’s from) glucose n Makes pyruvate n Makes some ATP (sub. Level phosphorylation) n Glycolysis ¨Means “splitting of sugar” n Glucose pyruvate ¨Occurs in the cytoplasm 1 glucose breaks down 4 ATP made + 2 pyruvate molecules (net gain of 2 ATP…NOT 4 ATP)
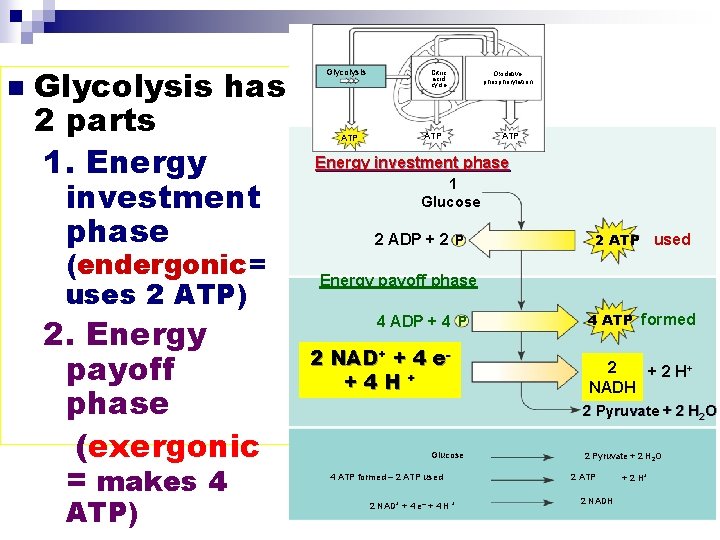
n Glycolysis has 2 parts 1. Energy investment phase (endergonic= uses 2 ATP) 2. Energy payoff phase (exergonic = makes 4 ATP) Glycolysis ATP Citric acid cycle ATP Oxidative phosphorylation ATP Energy investment phase 1 Glucose 2 ADP + 2 P 2 ATP used Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e+4 H+ 4 ATP formed 2 + 2 H+ NADH 2 Pyruvate + 2 H 2 O Glucose 4 ATP formed – 2 ATP used 2 NAD+ + 4 e– + 4 H + 2 Pyruvate + 2 H 2 O 2 ATP 2 NADH + 2 H+
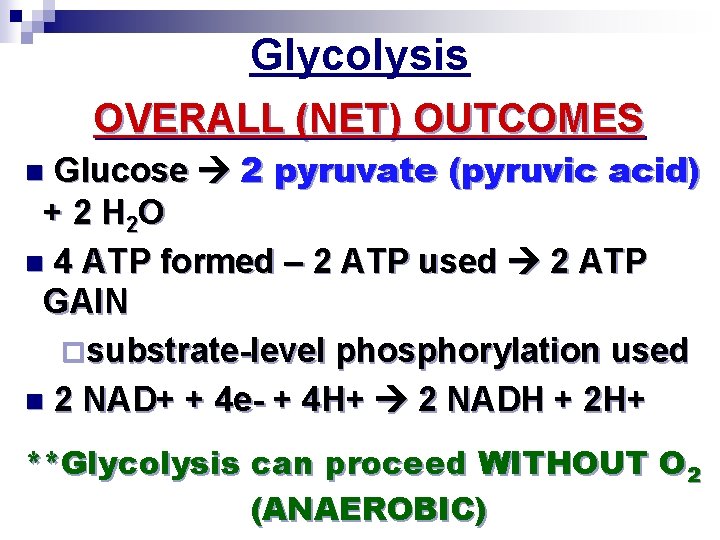
Glycolysis OVERALL (NET) OUTCOMES Glucose 2 pyruvate (pyruvic acid) + 2 H 2 O n 4 ATP formed – 2 ATP used 2 ATP GAIN ¨substrate-level phosphorylation used n 2 NAD+ + 4 e- + 4 H+ 2 NADH + 2 H+ n **Glycolysis can proceed WITHOUT O 2 (ANAEROBIC)
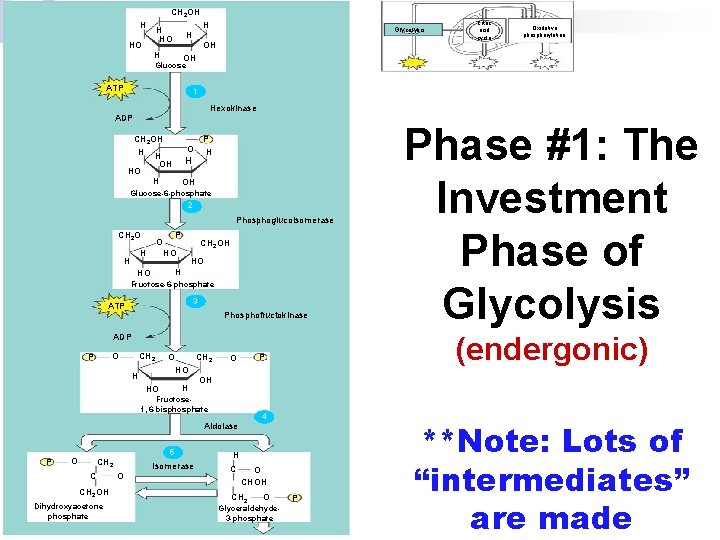
CH 2 OH H HO H H OH HO Glycolysis OH Citric acid cycle Oxidative phosphorylation Glucose ATP 1 Hexokinase ADP CH 2 OH H H OH HO H P H OH Glucose-6 -phosphate 2 Phosphoglucoisomerase CH 2 O H H P O CH 2 OH HO HO H HO Fructose-6 -phosphate 3 ATP Phosphofructokinase (endergonic) ADP O P CH 2 O CH 2 HO H H HO P O OH Fructose 1, 6 -bisphosphate 4 Aldolase P O 5 CH 2 C CH 2 OH Dihydroxyacetone phosphate Isomerase O Phase #1: The Investment Phase of Glycolysis H C O CHOH CH 2 O Glyceraldehyde 3 -phosphate P **Note: Lots of “intermediates” are made
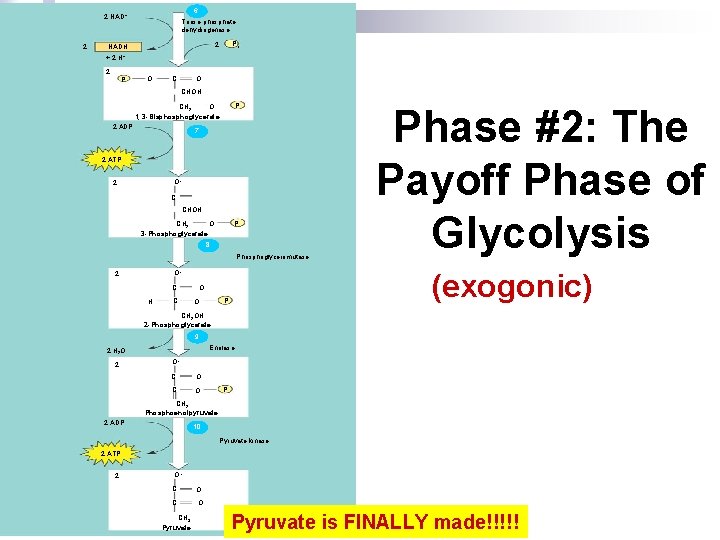
6 2 NAD+ 2 Triose phosphate dehydrogenase 2 Pi CH 2 O 1, 3 -Bisphoglycerate P NADH + 2 H+ 2 P O CHOH 2 ADP 7 2 ATP O– 2 C CHOH O CH 2 3 -Phosphoglycerate P 8 Phosphoglyceromutase (exogonic) O– 2 C H C O Phase #2: The Payoff Phase of Glycolysis CH 2 OH 2 -Phosphoglycerate 9 Enolase 2 H 2 O 2 O– C O P CH 2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 O– C O CH 3 Pyruvate is FINALLY made!!!!!

Glycolysis n http: //www. sumanasinc. com/webcontent/animations/content/cellularr espiration. html n http: //highered. mheducation. com/sites/0072507470/student_view 0/c hapter 25/animation__how_glycolysis_works. html n http: //www. northland. cc. mn. us/biology/Biology 1111/animations/glyc olysis. html n http: //www. science. smith. edu/departments/Biology/Bio 231/glycolysis. html

Stage #2: The Kreb’s Cycle place in the matrix of the mitochondrion ¨Takes **NEEDS O 2 TO PROCEED (unlike glycolysis) so it is an AEROBIC process
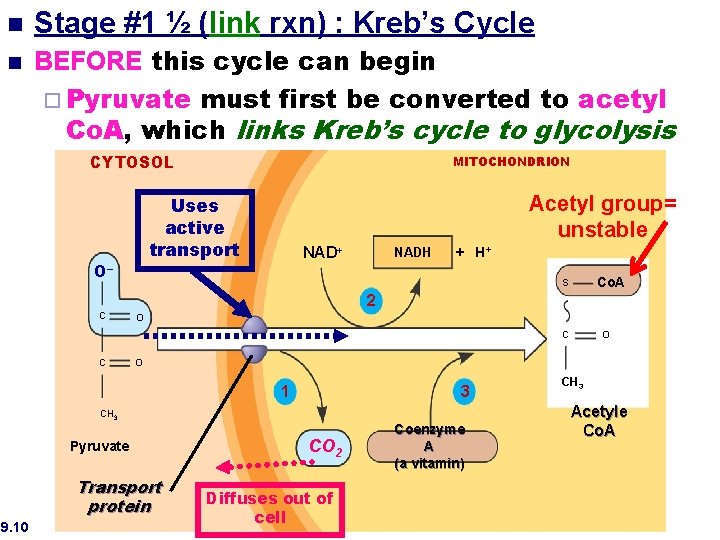
n n Stage #1 ½ (link rxn) : Kreb’s Cycle BEFORE this cycle can begin ¨ Pyruvate must first be converted to acetyl Co. A, which links Kreb’s cycle to glycolysis CYTOSOL Acetyl group= unstable Uses active transport O– C MITOCHONDRION NAD+ NADH + H+ Co. A S 2 O C C O 1 3 CH 3 Pyruvate Transport protein 9. 10 O CO 2 Diffuses out of cell Coenzyme A (a vitamin) CH 3 Acetyle Co. A
The Kreb’s Cycle n Aka: ¨ Tricarboxylic Acid Cycle or ¨ Citric Acid cycle GOAL: Finish oxidizing (stripping e-’s from) glucose Makes: NADH AND FADH 2 using pyruvate intermediates n Makes some ATP (sub. Level phosphorylation) n
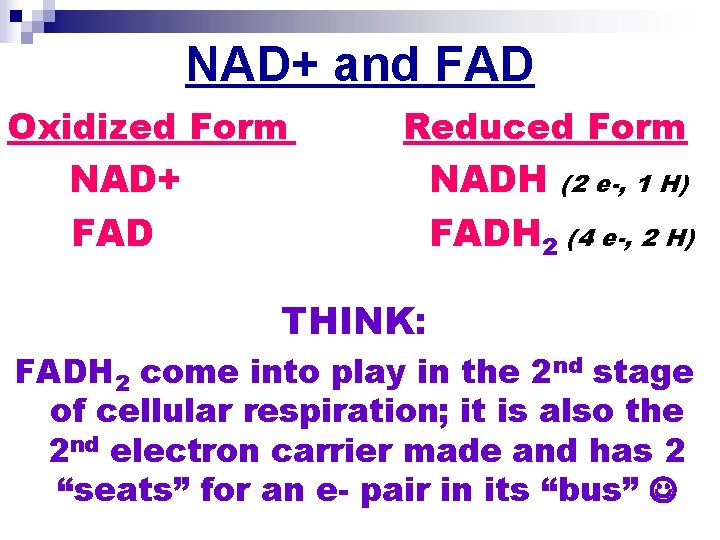
NAD+ and FAD Oxidized Form NAD+ FAD Reduced Form NADH (2 e-, 1 H) FADH 2 (4 e-, 2 H) THINK: FADH 2 come into play in the 2 nd stage of cellular respiration; it is also the 2 nd electron carrier made and has 2 “seats” for an e- pair in its “bus”
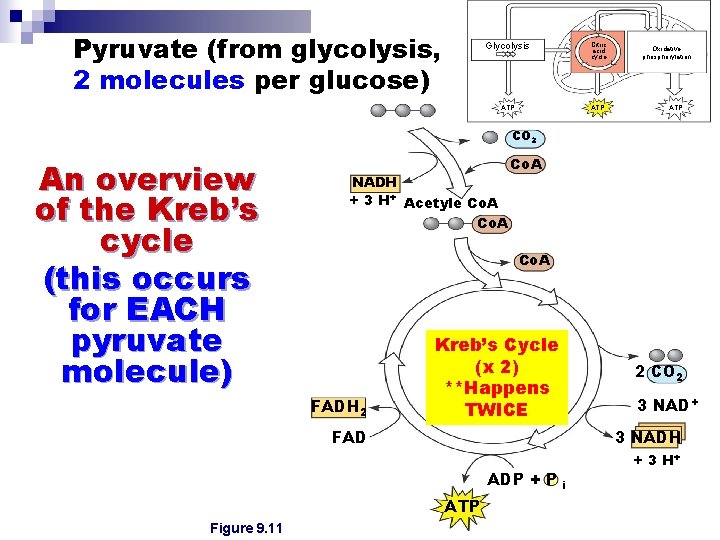
Pyruvate (from glycolysis, 2 molecules per glucose) Glycolysis Citric acid cycle ATP Oxidative phosphorylation ATP CO 2 An overview of the Kreb’s cycle (this occurs for EACH pyruvate molecule) NADH + 3 H+ Acetyle Co. A FADH 2 Kreb’s Cycle (x 2) **Happens TWICE 2 CO 2 3 NAD+ FAD 3 NADH ADP + P ATP Figure 9. 11 + 3 H+ i
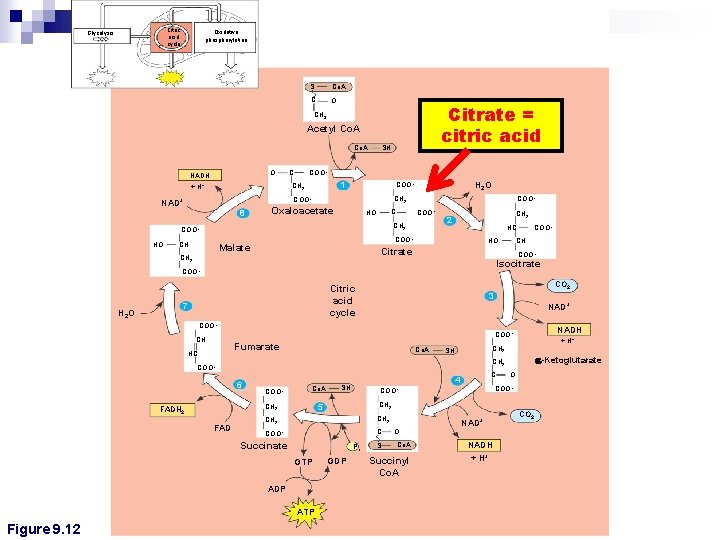
Citric acid cycle Glycolysis Oxidative phosphorylation S Co. A C O Citrate = citric acid CH 3 Acetyl Co. A O NADH + C COO– 1 CH 2 H+ SH 8 Oxaloacetate C HO CH CH 2 2 HC COO– Malate HO COO– Isocitrate Figure 9. 12 COO– CO 2 Citric acid cycle 7 COO– CH Citrate CH 2 H 2 O COO– CH 2 COO– HO COO– CH 2 COO– NAD+ H 2 O COO– 3 NAD+ COO– Fumarate HC Co. A COO– Co. A CH 2 C COO– Succinate Pi ADP ATP O COO– CH 2 GTP C 4 SH 5 CH 2 FAD a-Ketoglutarate CH 2 6 FADH 2 + H+ CH 2 SH COO– Figure 9. 12 NADH COO– CH GDP S O Co. A Succinyl Co. A NAD+ NADH + H+ CO 2
Kreb’s Cycle Summary n Pyruvate Acetyl-Co. A + 1 NADH + 1 CO 2 ¨Occurs in the “Link Reaction” turn of cycle yields more NADH, 1 ATP, and some FADH 2 and 2 CO 2 (as waste product) n 1 Remember to multiply by 2…why? n http: //www. sumanasinc. com/webcontent/animations/content/cellularrespiration. html n Http: //highered. mheducation. com/sites/0072507470/student_view 0/chapter 25/animation__how_the_krebs _cycle_works__quiz_1_. html
Stage #3: Oxidative Phosphorylation (Electron Transport Chain (ETC) + Chemiosmosis) n Chemiosmosis couples electron transport to ATP synthesis n NADH and FADH 2 ¨Donate e-s to ETC, which powers ATP synthesis using oxidative phosphorylation **OCUURS IN CRISTAE (folds of inner membrane)

What is “oxidative phosphorylation”? n. Recall… ¨Take H+/e-s away, molecule = “oxidized” ¨Give H+/e-s, molecule = “reduced” ¨Give phosphate, molecule = “phosphorylated” n So…oxidative phosphorylation = removal of e-’s (and H+’s) from one molecule then… 2. gives phosphates to another molecule 1.

Oh my little e-’s…where are you going? ? ? n E-’s are dumped off in the ETC ¨e-s from NADH and FADH 2 **NEEDS O 2 TO PROCEED (unlike glycolysis)
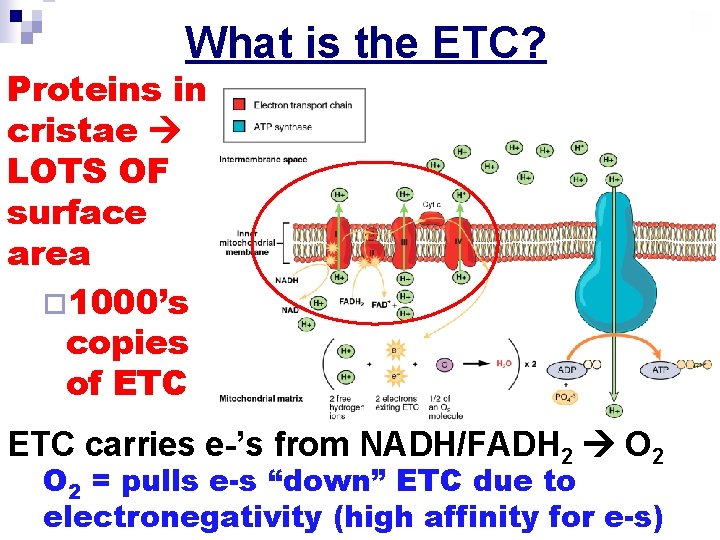
What is the ETC? Proteins in cristae LOTS OF surface area ¨ 1000’s copies of ETC carries e-’s from NADH/FADH 2 O 2 = pulls e-s “down” ETC due to electronegativity (high affinity for e-s)
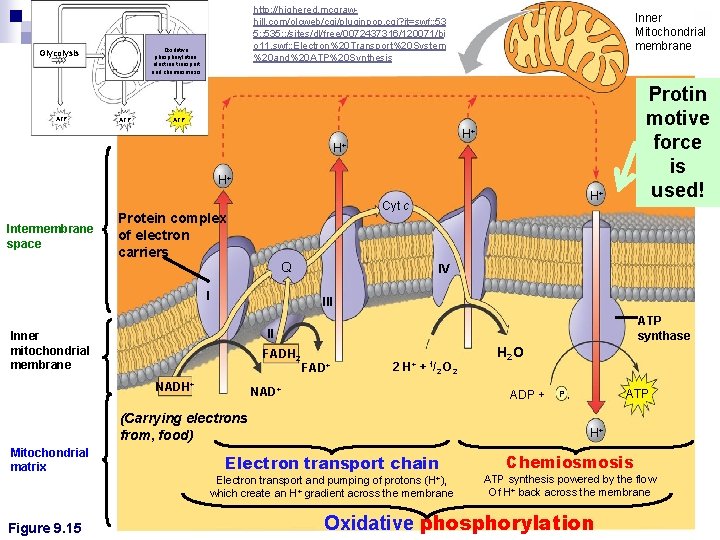
http: //highered. mcgrawhill. com/olcweb/cgi/pluginpop. cgi? it=swf: : 53 5: : 535: : /sites/dl/free/0072437316/120071/bi o 11. swf: : Electron%20 Transport%20 System %20 and%20 ATP%20 Synthesis Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP Inner Mitochondrial membrane Protin motive force is used! ATP H+ H+ n Chemiosmosis and the electron transport Protein complex chain of electron H+ H+ Cyt c Intermembrane space carriers Q I IV III ATP synthase II Inner mitochondrial membrane FADH 2 NADH+ FAD+ 2 H+ + 1/2 O 2 NAD+ ADP + (Carrying electrons from, food) Mitochondrial matrix ATP Pi H+ Electron transport chain Electron transport and pumping of protons (H+), which create an H+ gradient across the membrane Figure 9. 15 H 2 O Chemiosmosis ATP synthesis powered by the flow Of H+ back across the membrane Oxidative phosphorylation
Stage 3 - Overview (2 parts) PART 1: ETC ¨Sets “stage” for chemiosmosis ¨Creates a H+ gradient across the inner membrane n PART 2: Chemiosmosis 1. H+ gradient is USED to make a proton motive force 2. H+ ions diffuse THROUGH ATP synthase 3. ATP Synthase spins to make ATP from ADP and P n

What happens at the end of the ETC chain? n Electrons are passed to oxygen, forming water ¨So…. O 2 = final e- acceptor delivers e- higher than FAD NADH provides more electrons to make more ATP n NAD
What happens in the ETC? 1. 2. 3. 4. NADH/FADH 2 drops off e-’s into ETC proteins e-s “fall” from protein to protein Energy is released This energy pumps H+’s from matrix to inter membrane space n A H+ (proton) gradient forms inside mitochondria does NOT make ATP directly but provides stage for CHEMIOSOMOSIS to occur n ETC
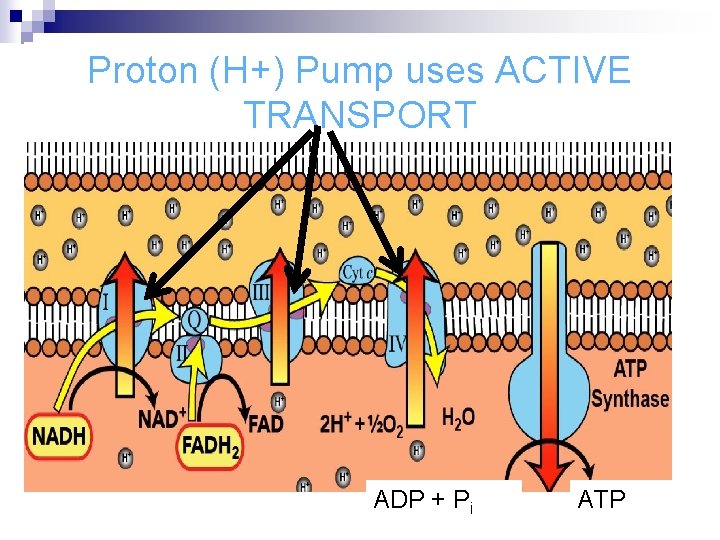
Proton (H+) Pump uses ACTIVE TRANSPORT ADP + Pi ATP
RECALL…Chemiosmosis n Uses energy from H+ gradient across a membrane ¨Called n H+ a PROTON MOTIVE FORCE use facilitated diffusion n Uses ATP synthase n Makes ~90% of ATP (32 ATP)
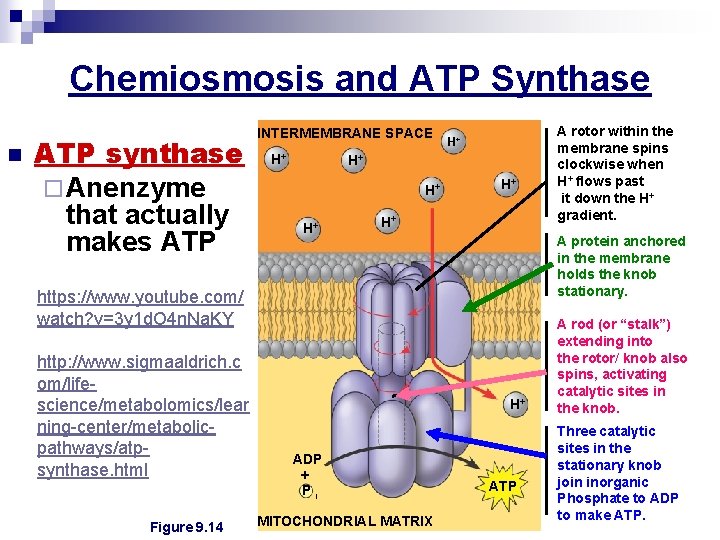
Chemiosmosis and ATP Synthase n ATP synthase ¨ Anenzyme that actually makes ATP INTERMEMBRANE SPACE H+ H+ A protein anchored in the membrane holds the knob stationary. https: //www. youtube. com/ watch? v=3 y 1 d. O 4 n. Na. KY http: //www. sigmaaldrich. c om/lifescience/metabolomics/lear ning-center/metabolicpathways/atpsynthase. html Figure 9. 14 H+ ADP + Pi MITOCHONDRIAL MATRIX A rotor within the membrane spins clockwise when H+ flows past it down the H+ gradient. ATP A rod (or “stalk”) extending into the rotor/ knob also spins, activating catalytic sites in the knob. Three catalytic sites in the stationary knob join inorganic Phosphate to ADP to make ATP.
Chemiosmosis in Chloroplasts and Mitochondria n Chloroplasts and mitochondria ATP by the SAME basic mechanism: chemiosmosis ¨But use different sources of energy to accomplish this ¨Generate
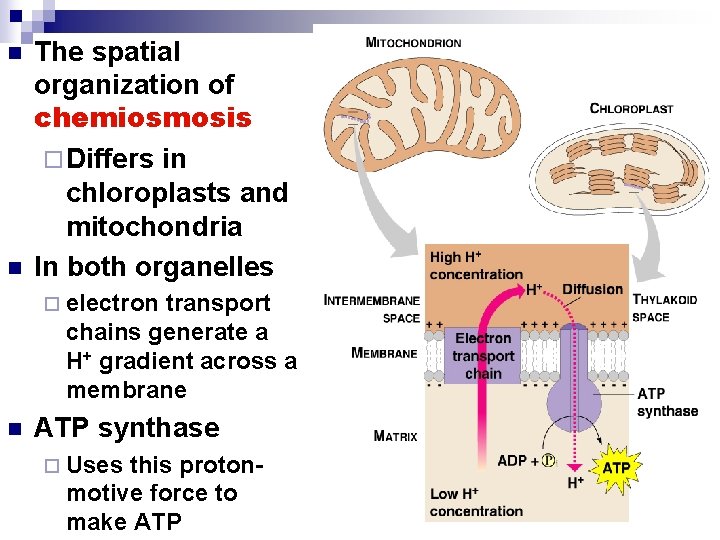
n n The spatial organization of chemiosmosis ¨ Differs in chloroplasts and mitochondria In both organelles ¨ electron transport chains generate a H+ gradient across a membrane n ATP synthase ¨ Uses this protonmotive force to make ATP
n At certain steps along the ETC ¨Electron transfer causes protein complexes to pump H+ from the mitochondrial matrix intermembrane space n. Inside (matrix) = low [H+] n. Outside = high [H+]
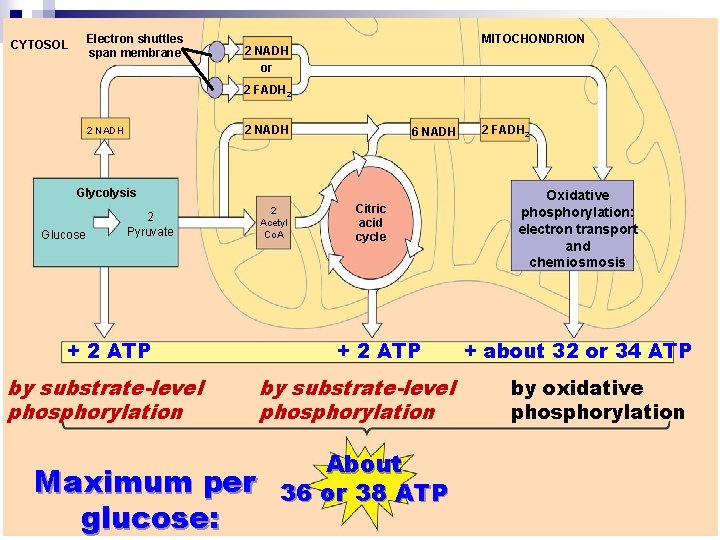
Electron shuttles span membrane CYTOSOL MITOCHONDRION 2 NADH or 2 FADH 2 2 NADH n 6 NADH 2 FADH 2 There are three main processes in. Oxidative this phosphorylation: metabolic enterprise electron transport Glycolysis Glucose 2 Pyruvate + 2 ATP by substrate-level phosphorylation Maximum per glucose: 2 Acetyl Co. A Citric acid cycle + 2 ATP by substrate-level phosphorylation About 36 or 38 ATP and chemiosmosis + about 32 or 34 ATP by oxidative phosphorylation

n About 40% of the energy in a glucose molecule ¨Is transferred to ATP during cellular respiration, making ~36 - 38 ATP http: //www. sumanasinc. com/webcontent/animations/content/cell ularrespiration. html ¨ https: //www. youtube. com/watch? v=Fcu_8 URp 4 Ac ¨
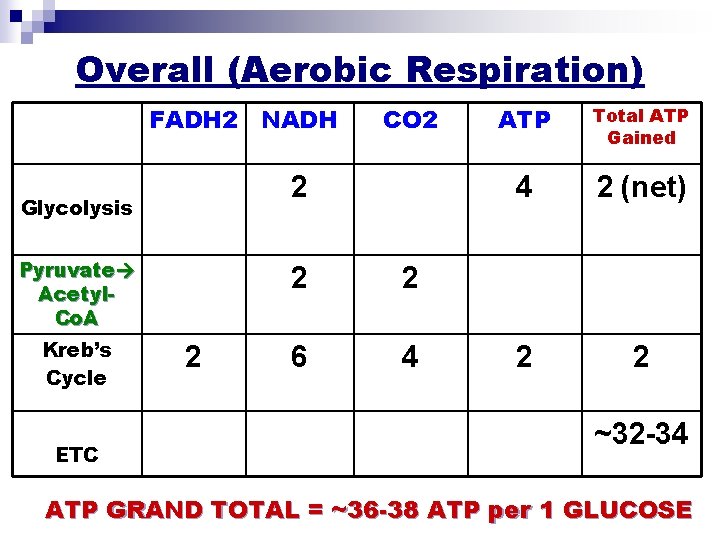
Overall (Aerobic Respiration) FADH 2 NADH 2 Glycolysis Pyruvate Acetyl. Co. A Kreb’s Cycle ETC CO 2 2 6 4 ATP Total ATP Gained 4 2 (net) 2 2 ~32 -34 ATP GRAND TOTAL = ~36 -38 ATP per 1 GLUCOSE
Chapter 9 (Part 2): Fermentation AP Biology Ms. Gaynor
Fermentation n Fermentation enables some cells to produce ATP without the use of oxygen (O 2) n Cellular respiration ¨Relies on oxygen to produce ATP n In the absence of oxygen ¨Cells can still produce ATP through fermentation

n. Glycolysis ¨Can produce ATP with or without oxygen, in aerobic or anaerobic conditions ¨Couples with fermentation to produce ATP

Types of Fermentation n Fermentation consists of ¨Glycolysis plus reactions that regenerate NAD+, which can be reused by glyocolysis

Alcohol Fermentation n. Pyruvate is converted to ethanol (ethyl alcohol) in two steps, one of which releases CO 2 n. Ex: bacteria and yeast

alcohol fermentation ¨Pyruvate is converted to ethanol (ethyl alcohol) in two steps, one of which releases CO 2 n. In GLUCOSE Pyruvate Ethanol and CO 2 n. Ex: bacteria and yeast do this
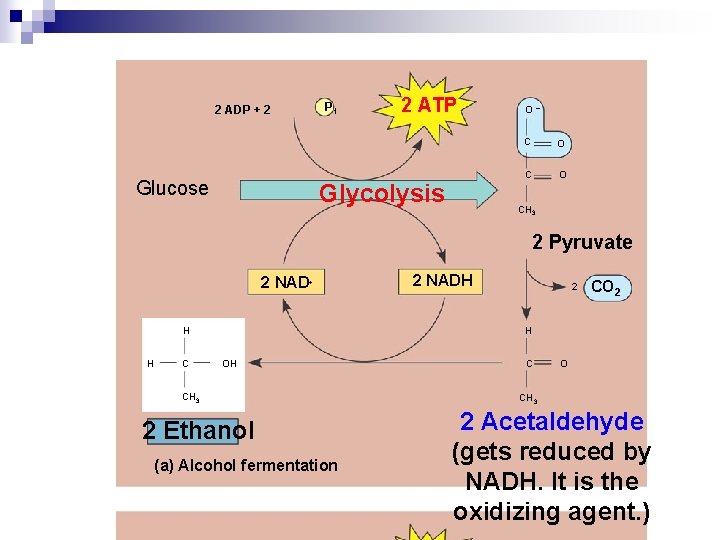
2 ADP + 2 Glucose P 1 2 ATP Glycolysis O– C O CH 3 2 Pyruvate 2 NAD+ H H C 2 NADH 2 CO 2 H OH CH 3 2 Ethanol (a) Alcohol fermentation C O CH 3 2 Acetaldehyde (gets reduced by NADH. It is the oxidizing agent. )

Lactic Acid Fermentation lactic acid fermentation ¨Pyruvate is reduced directly to NADH to form lactate as a waste product ¨NO CO 2 is released n During n Ex #1: fungus and bacteria in dairy industry to make cheese/ yogurt n Ex #2: Human muscle cells
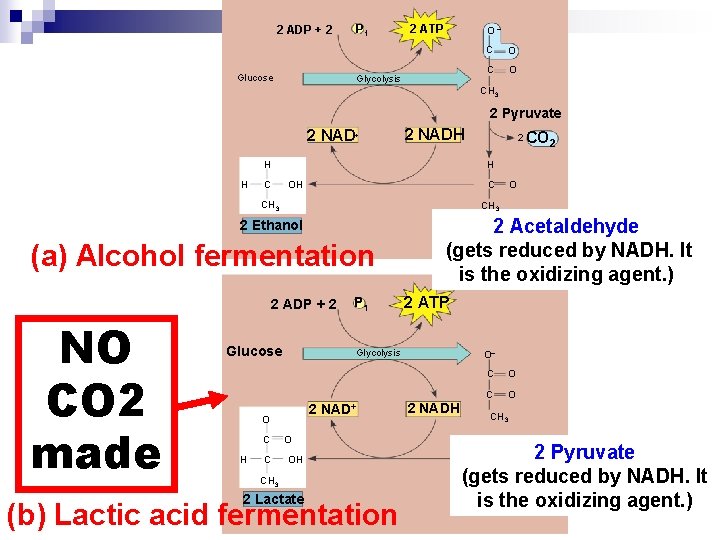
2 ADP + 2 Glucose P 1 2 ATP O– Glycolysis C O CH 3 2 Pyruvate 2 NAD+ 2 NADH H H C C OH (a) Alcohol fermentation 2 ADP + 2 Glucose C P 1 2 Acetaldehyde (gets reduced by NADH. It is the oxidizing agent. ) 2 ATP Glycolysis 2 O CH 3 2 Ethanol H CO 2 H CH 3 NO CO 2 made 2 NAD+ O OH CH 3 2 Lactate (b) Lactic acid fermentation O– 2 NADH C O CH 3 2 Pyruvate (gets reduced by NADH. It is the oxidizing agent. )

Fermentation and Cellular Respiration Compared n Both fermentation and cellular respiration ¨Use glycolysis to oxidize glucose and other organic fuels to pyruvate
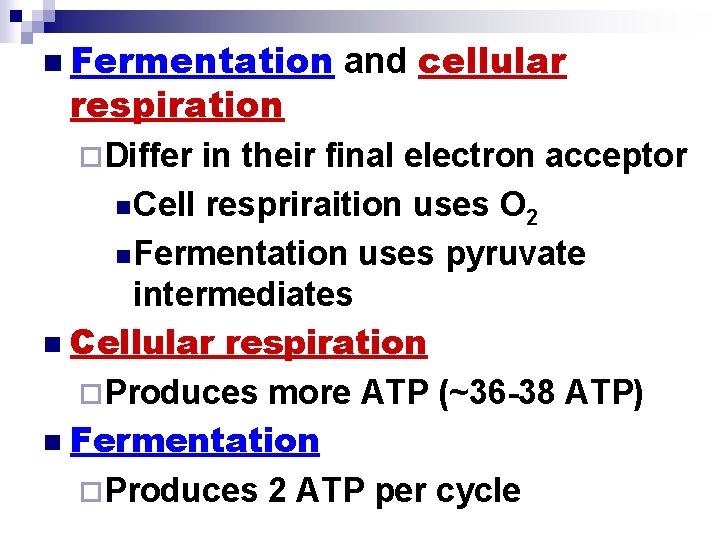
n Fermentation respiration ¨Differ and cellular in their final electron acceptor n Cell respriraition uses O 2 n Fermentation uses pyruvate intermediates n Cellular respiration ¨Produces more ATP (~36 -38 ATP) n Fermentation ¨Produces 2 ATP per cycle
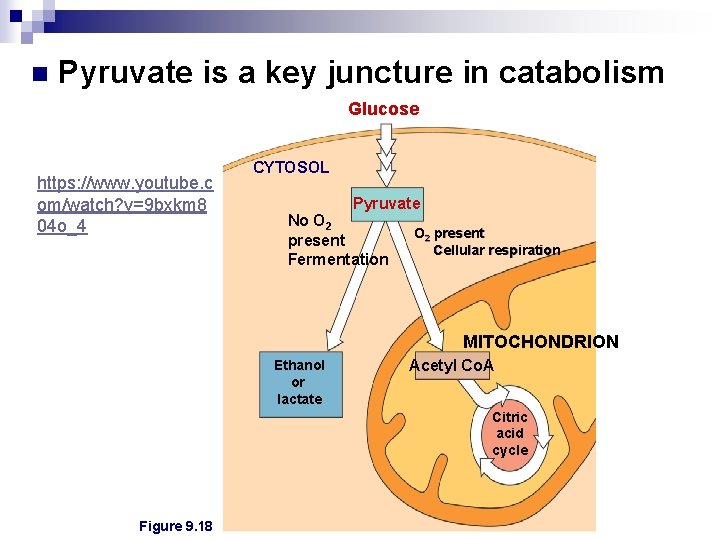
n Pyruvate is a key juncture in catabolism Glucose https: //www. youtube. c om/watch? v=9 bxkm 8 04 o_4 CYTOSOL Pyruvate No O 2 present Fermentation O 2 present Cellular respiration MITOCHONDRION Ethanol or lactate Acetyl Co. A Citric acid cycle Figure 9. 18

The Evolutionary Significance of Glycolysis n Glycolysis ¨Occurs in nearly all organisms ¨Probably evolved in ancient prokaryotes before there was oxygen in the atmosphere n O 2 in air ~2. 7 bya; oldest prokaryotes ~3. 5 bya n Also does not require organelles
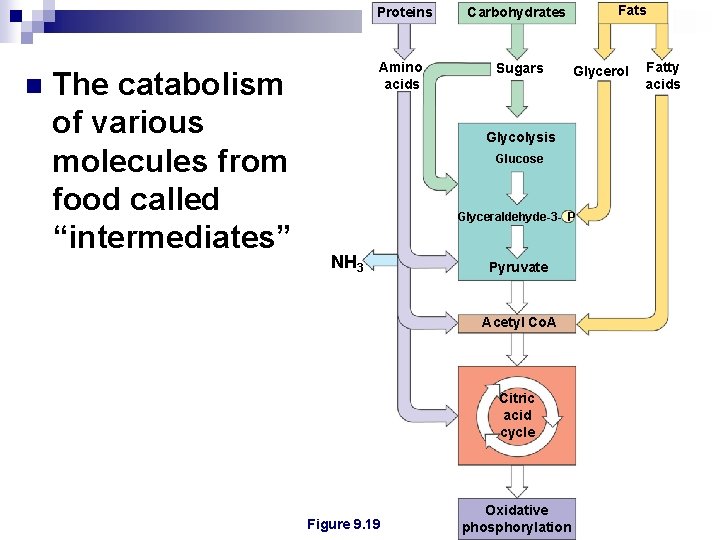
Proteins n The catabolism of various molecules from food called “intermediates” Amino acids Fats Carbohydrates Sugars Glycerol Glycolysis Glucose Glyceraldehyde-3 - P NH 3 Pyruvate Acetyl Co. A Citric acid cycle Figure 9. 19 Oxidative phosphorylation Fatty acids

Excellent Overall Tutorial of Cell Respiration n http: //www. wiley. com/college/pratt/04713938 78/student/animations/citric_acid_cycle/index. html
- Slides: 64