Chapter 9 Organic Chemistry Some Definitions Hydrocarbon Saturated
Chapter 9 Organic Chemistry

Some Definitions • • Hydrocarbon Saturated hydrocarbon Unsaturated hydrocarbon Cyclic hydrocarbon Structural formula Condensed structural formula Isomer Functional Group
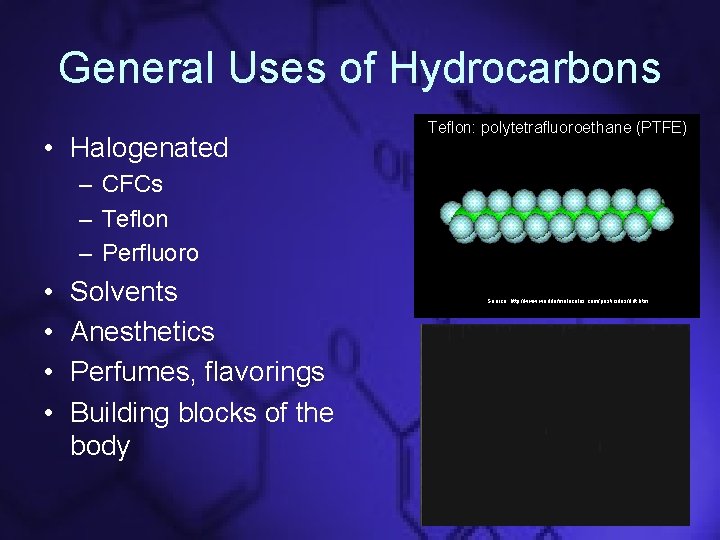
General Uses of Hydrocarbons • Halogenated Teflon: polytetrafluoroethane (PTFE) – CFCs – Teflon – Perfluoro • • Solvents Anesthetics Perfumes, flavorings Building blocks of the body Source: http: //www. worldofmolecules. com/pesticides/ddt. htm

Alkanes • Hydrocarbons that contain only single bonds • General formula Cn. H 2 n+2 • Named from the number of carbons • Can have isomers • Can be cyclic (different formula)

Properties of Alkanes • State of matter – Less than 5 carbons = gas – Between 5 and 16 carbons = liquid – Greater than 16 carbons = solid • Non-polar molecules (insoluble in water) • Very flammable: used for combustion • Physiological effects: dissolve fats, can act as emollients

Functional Groups • Any variation on the single carbon chain • Hydrocarbon stem called an alkyl group • Additions of bonds, halogens, oxygen or nitrogen – Alkene – Alkyne – Alcohol – Carboxylic Acid – Ester – Amine – Amide
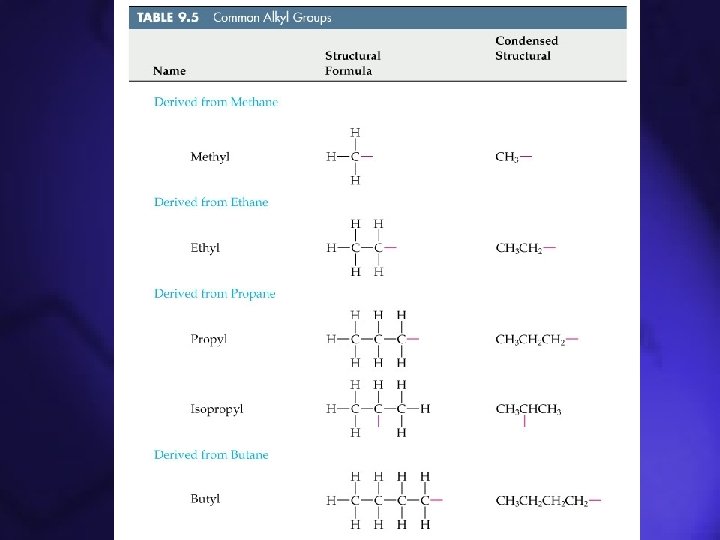
Functional Groups
Alkenes • Hydrocarbons that contain at least one double bond • General formula Cn. H 2 n • Named from the number of carbons • Can be cyclic – Aromatic hydrocarbons: benzene
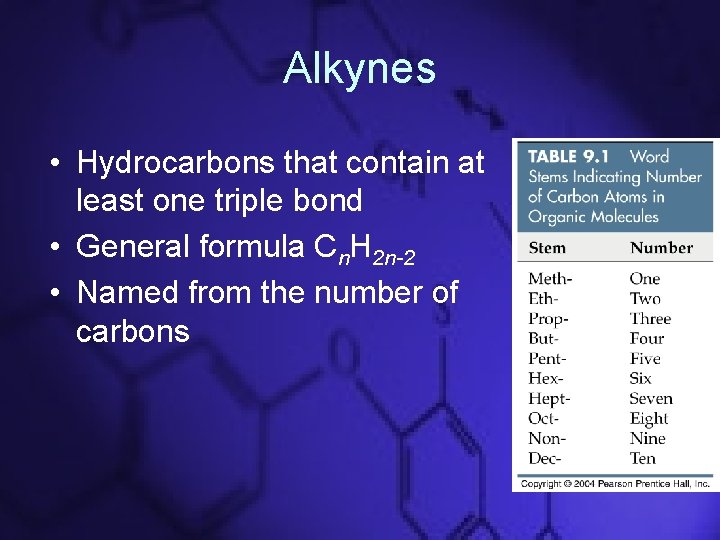
Alkynes • Hydrocarbons that contain at least one triple bond • General formula Cn. H 2 n-2 • Named from the number of carbons
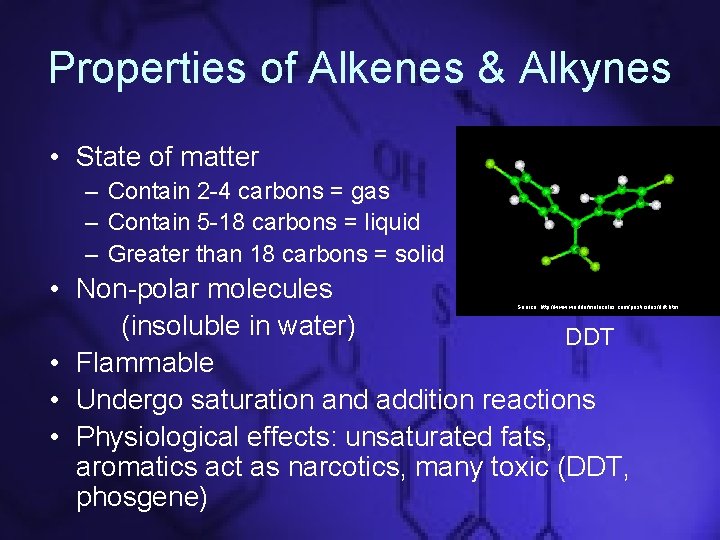
Properties of Alkenes & Alkynes • State of matter – Contain 2 -4 carbons = gas – Contain 5 -18 carbons = liquid – Greater than 18 carbons = solid • Non-polar molecules (insoluble in water) DDT • Flammable • Undergo saturation and addition reactions • Physiological effects: unsaturated fats, aromatics act as narcotics, many toxic (DDT, phosgene) Source: http: //www. worldofmolecules. com/pesticides/ddt. htm

Alcohols • Alkanes that contain an –OH substituted for any hydrogen – Called a hydroxyl functional group • Named according to number of carbons in alkyl group • -e on alkane name changes to -ol

Properties of Alcohols • State of matter – Liquid • Soluble in water • Formed by fermentation or catalyzed chemical reactions • Many are toxic! • Other common alcohols: Source: http: //www. worldofmolecules. com/pesticides/ddt. htm – Propylene glycol: antifreeze – Glycerol: moisturizer, food additive for moisture Source: http: //www. sonarestaurant. com/wine. php
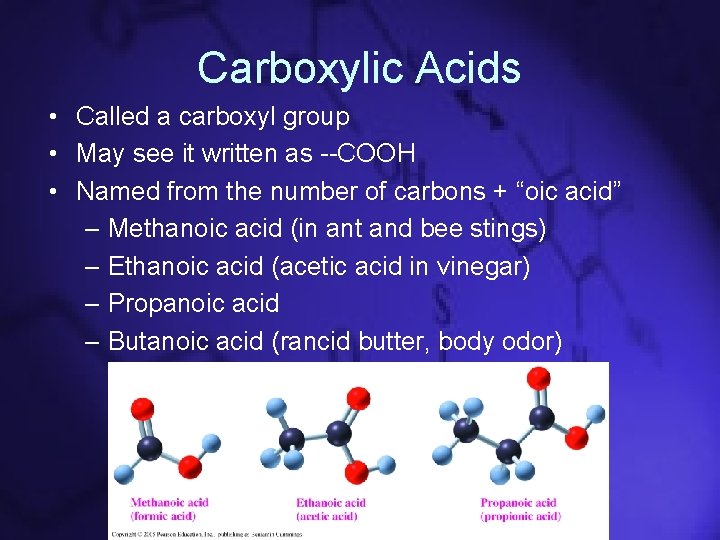
Carboxylic Acids • Called a carboxyl group • May see it written as --COOH • Named from the number of carbons + “oic acid” – Methanoic acid (in ant and bee stings) – Ethanoic acid (acetic acid in vinegar) – Propanoic acid – Butanoic acid (rancid butter, body odor)

Properties of Carboxylic Acids • Weak acids • Sour taste, neutralize base • Produce H+ ions in water • Carboxylic acid salts = used as anti-mold food additives
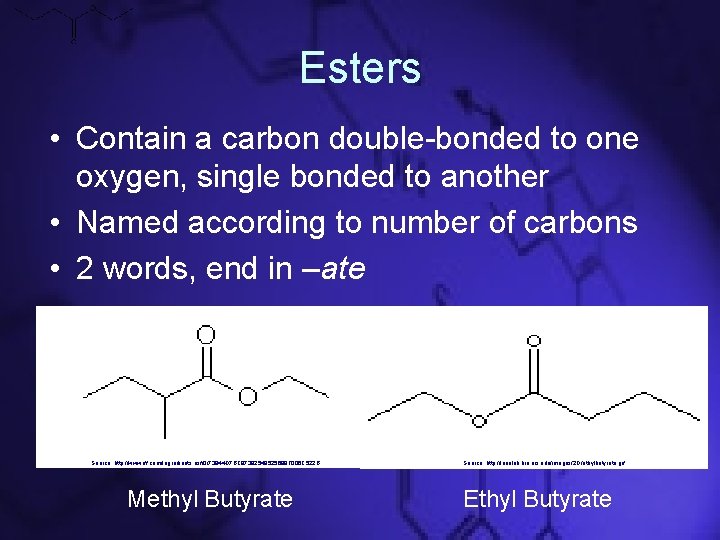
Esters • Contain a carbon double-bonded to one oxygen, single bonded to another • Named according to number of carbons • 2 words, end in –ate Source: http: //www. iff. com/Ingredients. nsf/0/73944 D 7 BC 97392548525699 F 006 C 522 B Methyl Butyrate Source: http: //leonlab. bio. uci. edu/images/2 D/ethylbutyrate. gif Ethyl Butyrate

Properties of Esters • Formed by combination of an alcohol and an acid • Some soluble in water, some insoluble • Fragrant: used in foods and perfumes – Many fruity • Other important esters: – Salicylic acid • Antipyretic • Analgesic • Blood thinner Source: http: //www. worldofmolecules. com/pesticides/ddt. htm
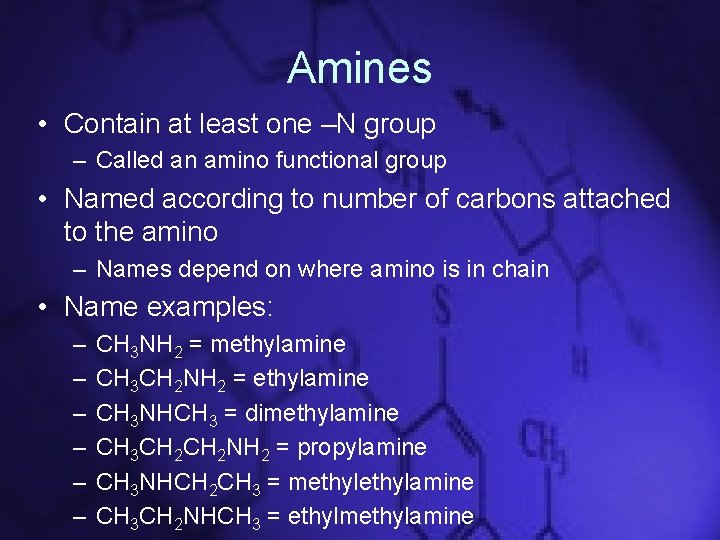
Amines • Contain at least one –N group – Called an amino functional group • Named according to number of carbons attached to the amino – Names depend on where amino is in chain • Name examples: – – – CH 3 NH 2 = methylamine CH 3 CH 2 NH 2 = ethylamine CH 3 NHCH 3 = dimethylamine CH 3 CH 2 NH 2 = propylamine CH 3 NHCH 2 CH 3 = methylamine CH 3 CH 2 NHCH 3 = ethylmethylamine
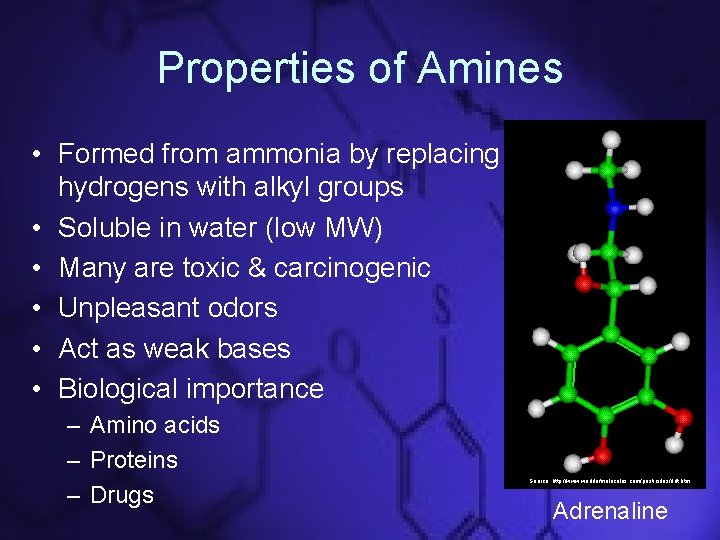
Properties of Amines • Formed from ammonia by replacing hydrogens with alkyl groups • Soluble in water (low MW) • Many are toxic & carcinogenic • Unpleasant odors • Act as weak bases • Biological importance – Amino acids – Proteins – Drugs Source: http: //www. worldofmolecules. com/pesticides/ddt. htm Adrenaline
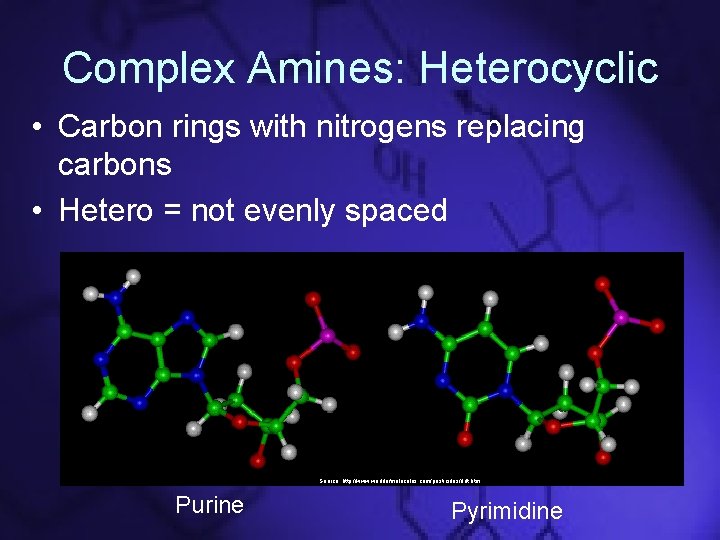
Complex Amines: Heterocyclic • Carbon rings with nitrogens replacing carbons • Hetero = not evenly spaced Source: http: //www. worldofmolecules. com/pesticides/ddt. htm Purine Pyrimidine

Amides • Contain a carbon double-bonded to oxygen, single bonded to nitrogen • Soluble in water (low MW) • High boiling points and melting points • Most (more than 1 carbon) are solid at room temperature • Most important amides are complex – Proteins – Nylon, wool, silk Source: http: //www. worldofmolecules. com/pesticides/ddt. htm Nylon = hydrocarbons with amide linkages
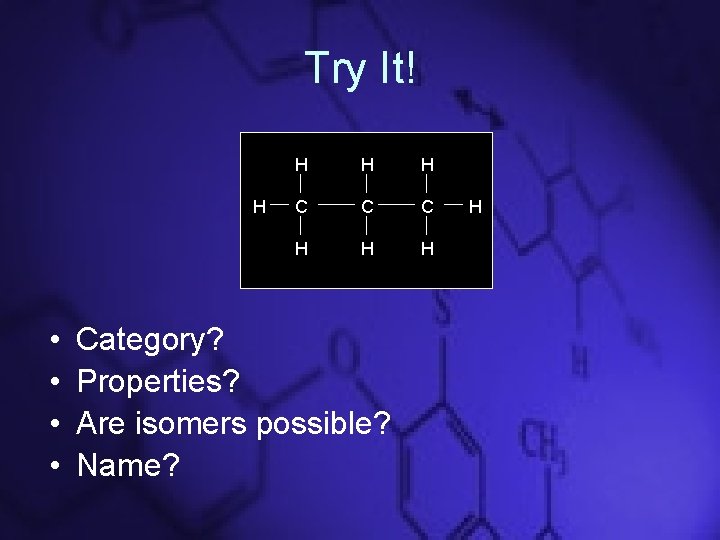
Try It! H • • H H H C C C H H H Category? Properties? Are isomers possible? Name? H
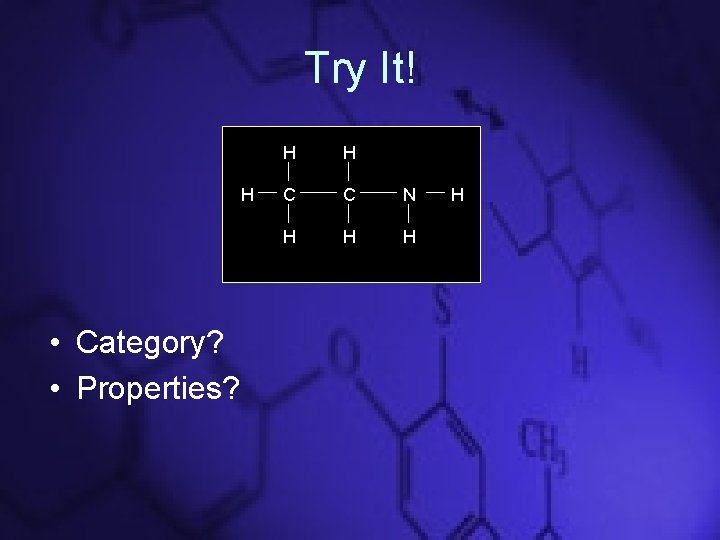
Try It! H • Category? • Properties? H H C C N H H
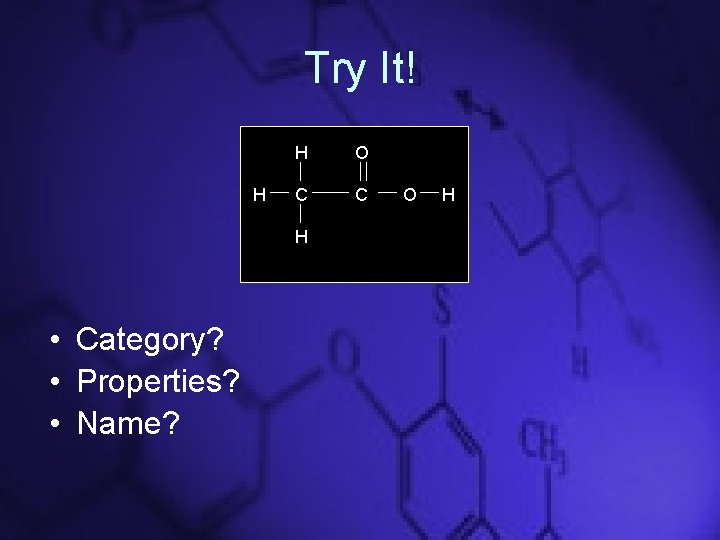
Try It! H H O C C H • Category? • Properties? • Name? O H

Of our 8 molecule types, can you… • Name the 3 types of hydrocarbons? • Name the hydrocarbon with a triple bond? • Name the molecules that include any double bonds? • Name the molecules that include any nitrogens? • Name the molecules that include any oxygens? • Name the molecules that are soluble in water? • Name the molecules that are insoluble in water?
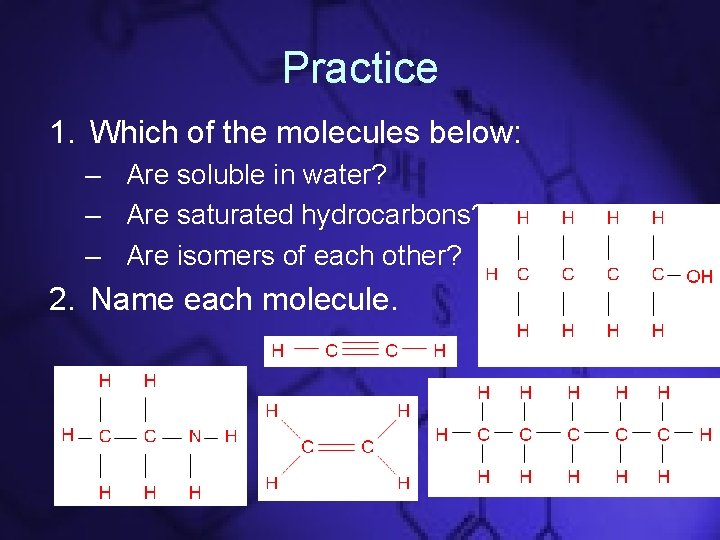
Practice 1. Which of the molecules below: – Are soluble in water? – Are saturated hydrocarbons? – Are isomers of each other? 2. Name each molecule.
Practice 3. Draw a molecule that contains 4 carbons single bonded to each other, and one amine group. Hydrogens make up the rest of the bonds. – Name 2 physical properties of this molecule. 4. Draw a molecule of propene. – Name 3 physical properties of this molecule.
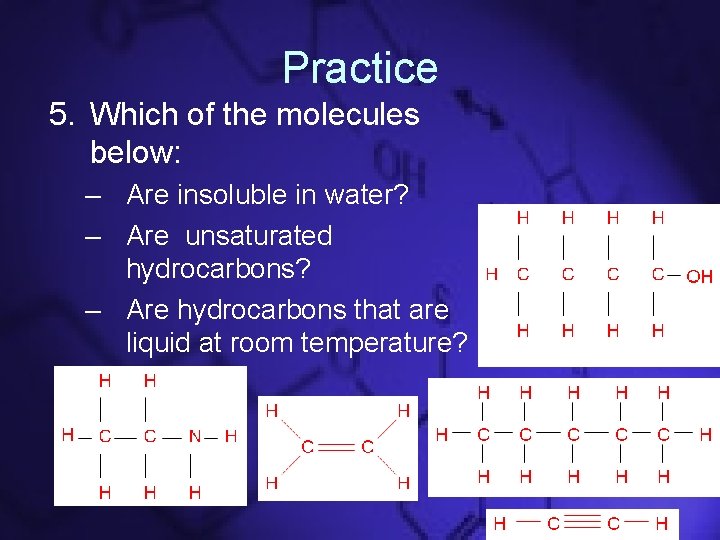
Practice 5. Which of the molecules below: – Are insoluble in water? – Are unsaturated hydrocarbons? – Are hydrocarbons that are liquid at room temperature?
Practice 6. Draw a molecule that contains 5 carbons single-bonded to each other and one hydroxyl group. Hydrogens make up the rest of the bonds. – Name this molecule – Name 2 physical properties of this molecule. 7. Draw a molecule of pentyne. – Name 3 physical properties of this molecule.

Practice 6. Draw a molecule that contains 5 carbons and one ester group. Hydrogens make up the rest of the bonds. – Name 2 physical properties of this molecule. 7. Draw a molecule of pentyne. – Name 3 physical properties of this molecule. 8. Write the molecular formula and name of an alkene that contains 18 hydrogens.
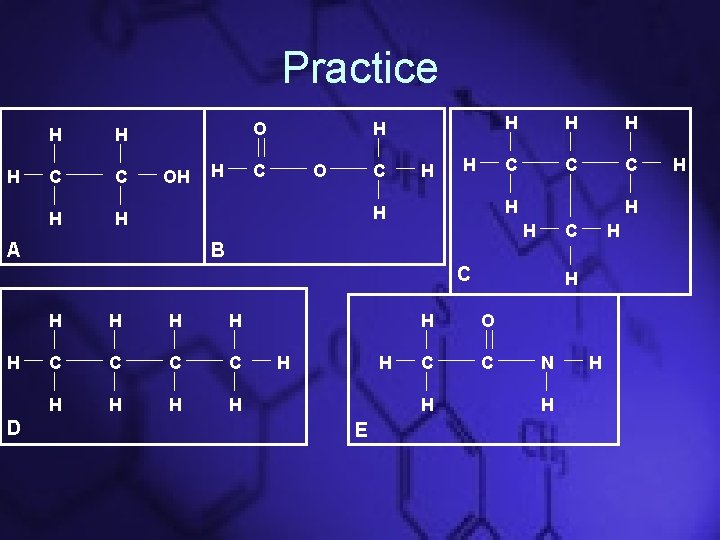
Practice H H H C C H H O OH H H C O C H H C C C H H A H H H B C C H D H H C C H H H E H H O C C H H N H H H
- Slides: 30