Chapter 9 Nuclear Radiation 9 3 Radiation Measurement




- Slides: 21

Chapter 9 Nuclear Radiation 9. 3 Radiation Measurement 9. 4 Half-Life of a Radioisotope 9. 5 Medical Applications Using Radioactivity Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

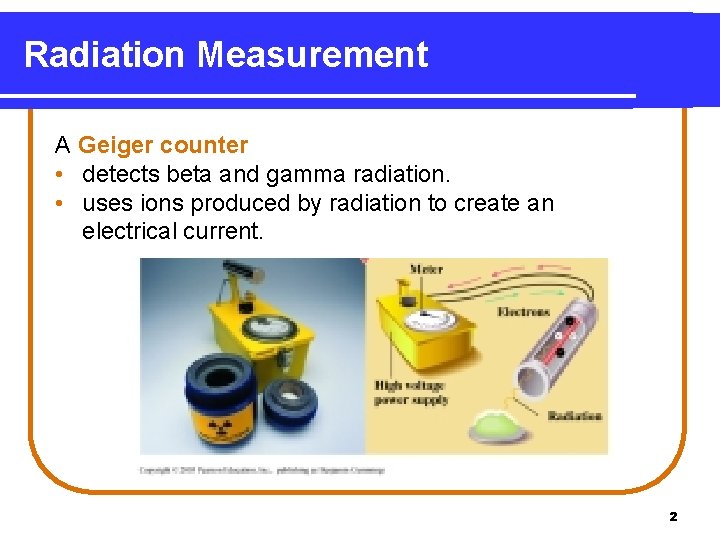
Radiation Measurement A Geiger counter • detects beta and gamma radiation. • uses ions produced by radiation to create an electrical current. 2

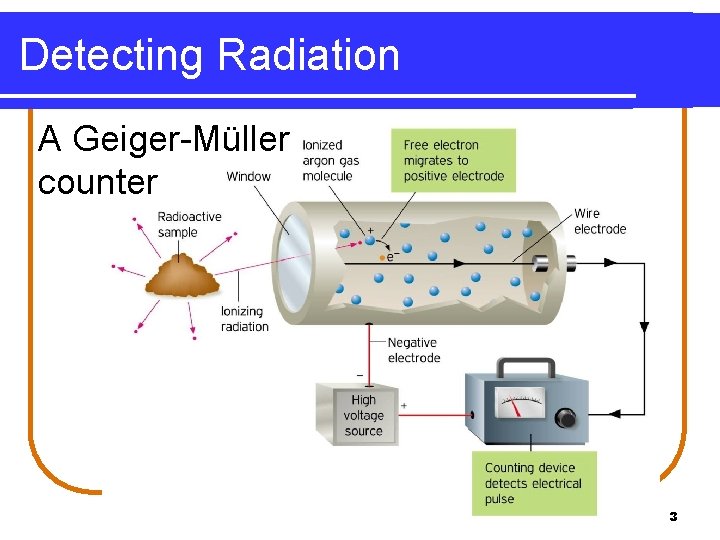
Detecting Radiation A Geiger-Müller counter 3

Geiger-Müller counter with radioactive antique orange plate 4

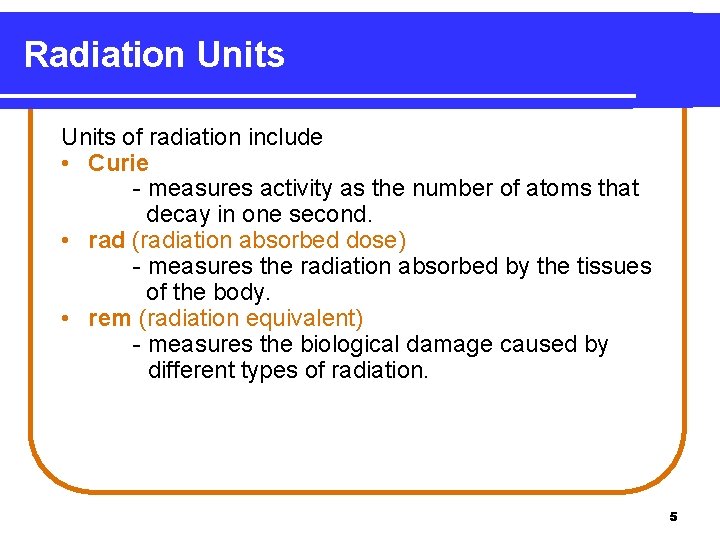
Radiation Units of radiation include • Curie - measures activity as the number of atoms that decay in one second. • rad (radiation absorbed dose) - measures the radiation absorbed by the tissues of the body. • rem (radiation equivalent) - measures the biological damage caused by different types of radiation. 5

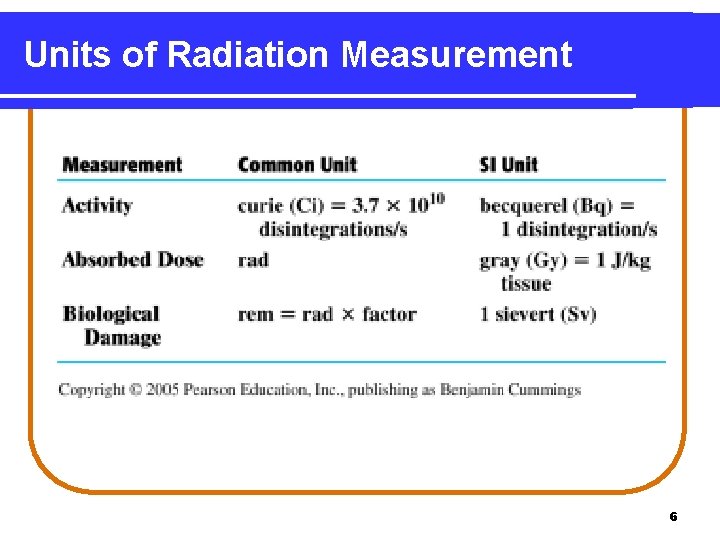
Units of Radiation Measurement 6

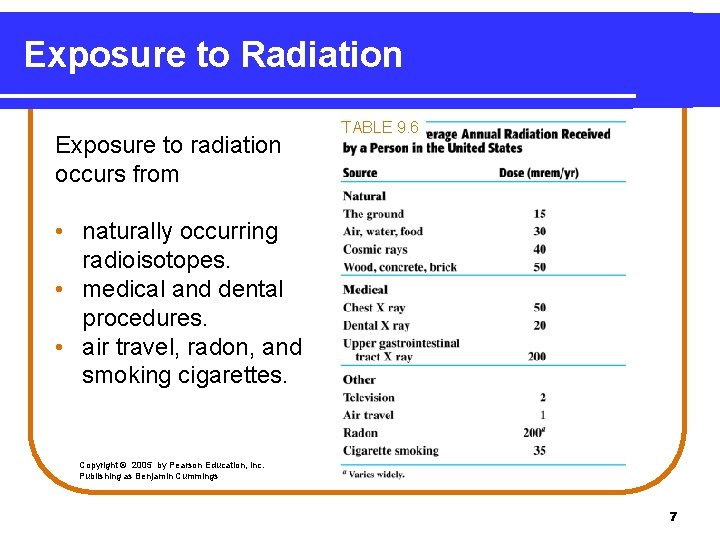
Exposure to Radiation Exposure to radiation occurs from TABLE 9. 6 • naturally occurring radioisotopes. • medical and dental procedures. • air travel, radon, and smoking cigarettes. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 7

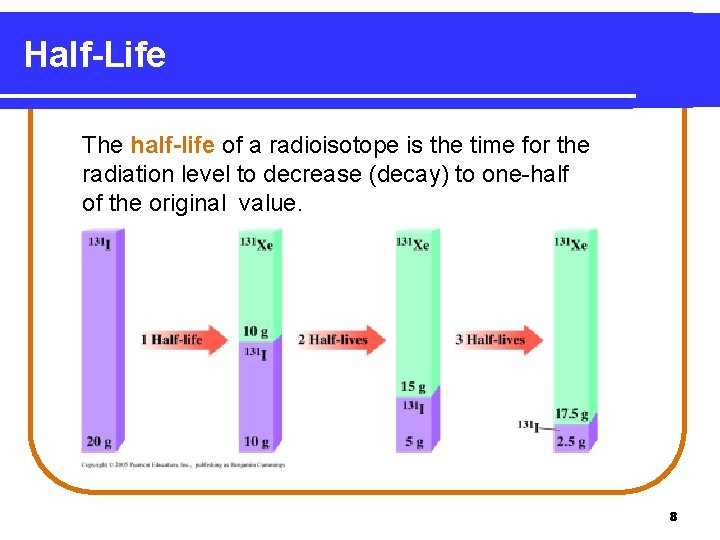
Half-Life The half-life of a radioisotope is the time for the radiation level to decrease (decay) to one-half of the original value. 8

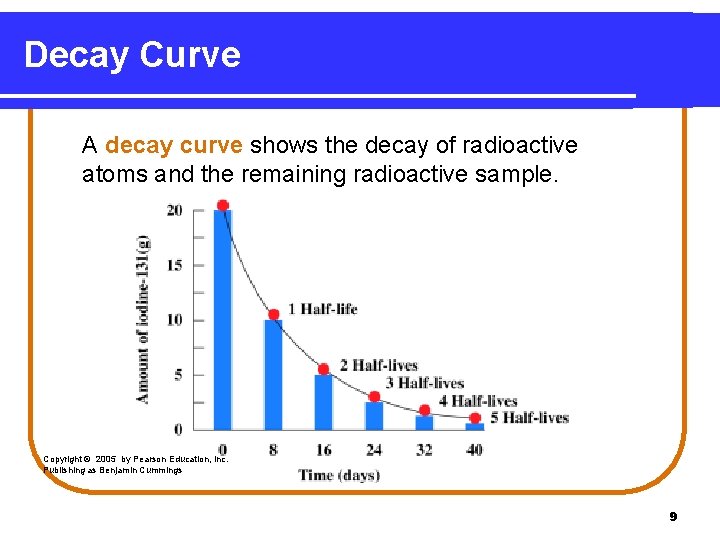
Decay Curve A decay curve shows the decay of radioactive atoms and the remaining radioactive sample. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 9

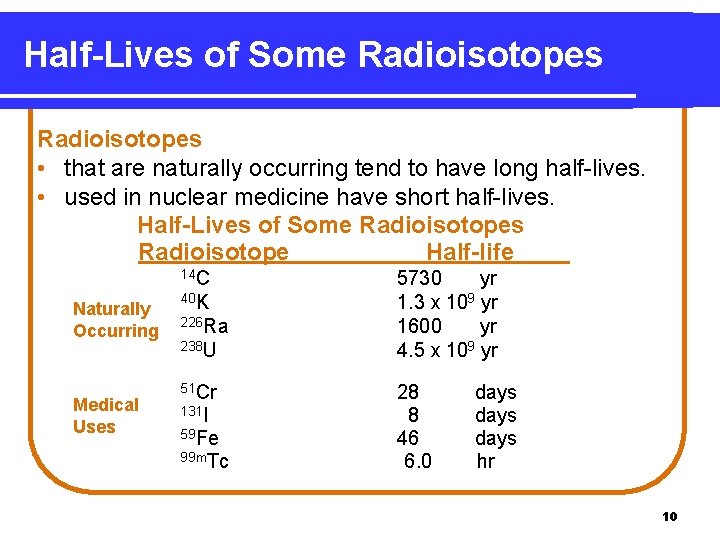
Half-Lives of Some Radioisotopes • that are naturally occurring tend to have long half-lives. • used in nuclear medicine have short half-lives. Half-Lives of Some Radioisotopes Radioisotope Half-life 14 C Naturally Occurring Medical Uses 40 K 226 Ra 238 U 51 Cr 131 I 59 Fe 99 m. Tc 5730 yr 1. 3 x 109 yr 1600 yr 4. 5 x 109 yr 28 8 46 6. 0 days hr 10

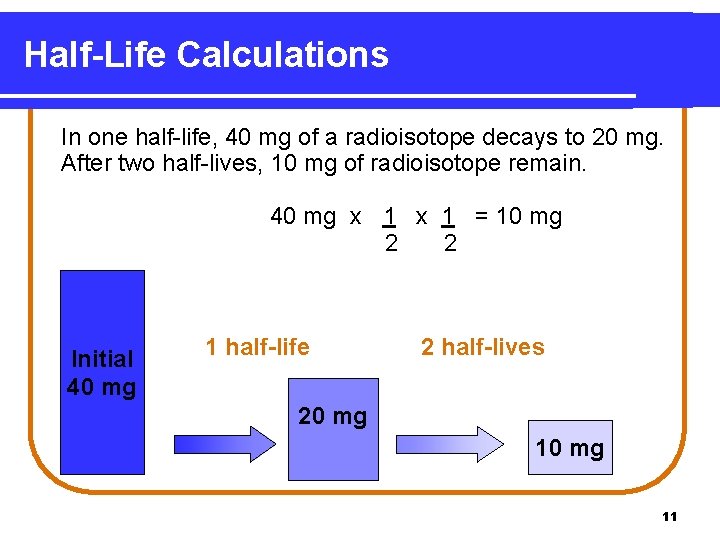
Half-Life Calculations In one half-life, 40 mg of a radioisotope decays to 20 mg. After two half-lives, 10 mg of radioisotope remain. 40 mg x 1 = 10 mg 2 2 Initial 40 mg 1 half-life 2 half-lives 20 mg 11

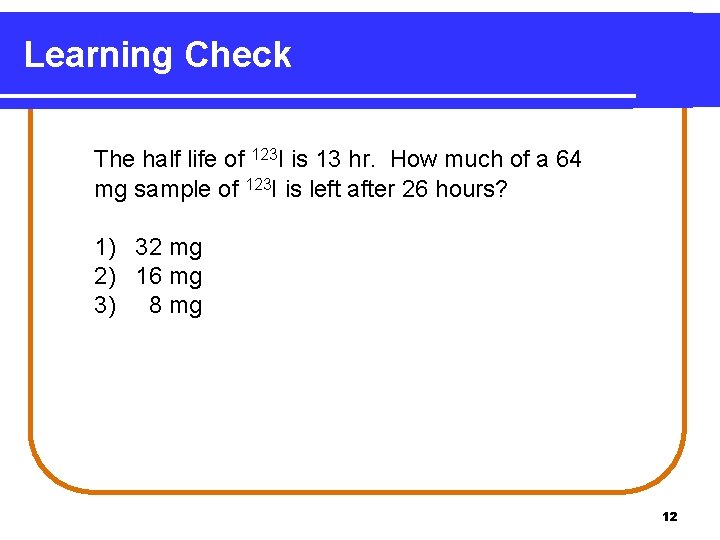
Learning Check The half life of 123 I is 13 hr. How much of a 64 mg sample of 123 I is left after 26 hours? 1) 32 mg 2) 16 mg 3) 8 mg 12

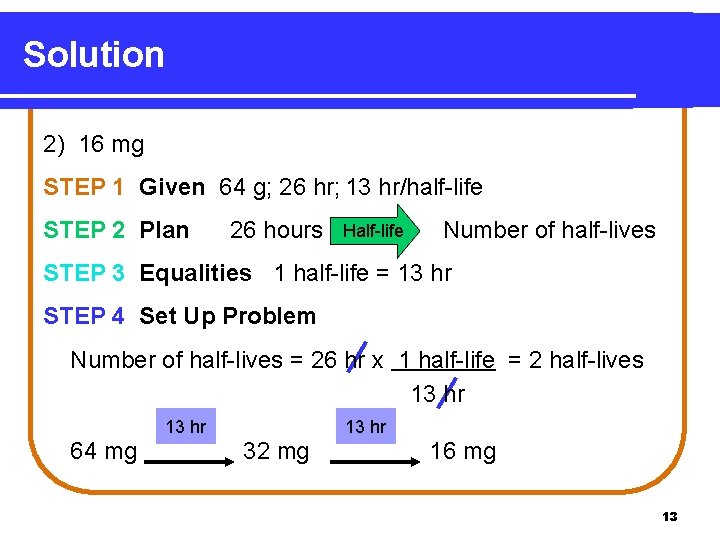
Solution 2) 16 mg STEP 1 Given 64 g; 26 hr; 13 hr/half-life STEP 2 Plan 26 hours Half-life Number of half-lives STEP 3 Equalities 1 half-life = 13 hr STEP 4 Set Up Problem Number of half-lives = 26 hr x 1 half-life = 2 half-lives 13 hr 64 mg 13 hr 32 mg 16 mg 13

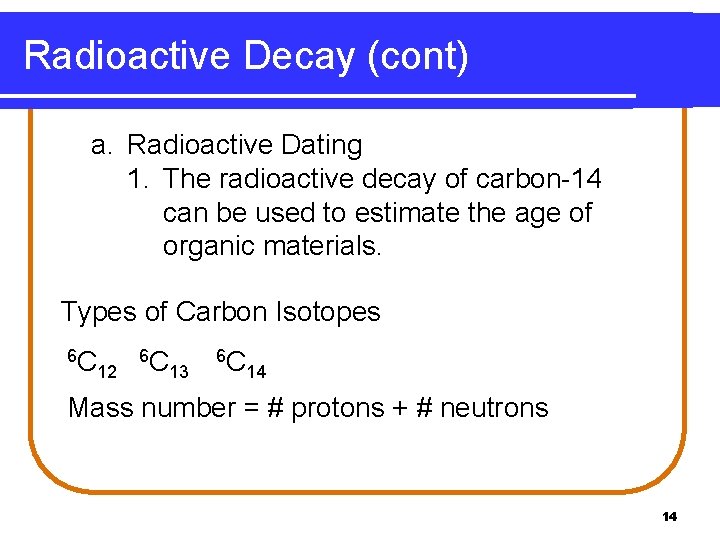
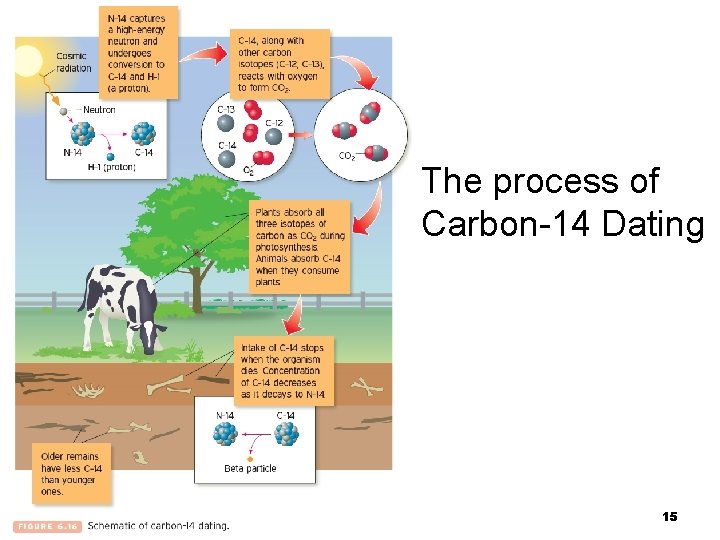
Radioactive Decay (cont) a. Radioactive Dating 1. The radioactive decay of carbon-14 can be used to estimate the age of organic materials. Types of Carbon Isotopes 6 C 12 6 C 13 6 C 14 Mass number = # protons + # neutrons 14

The process of Carbon-14 Dating 15

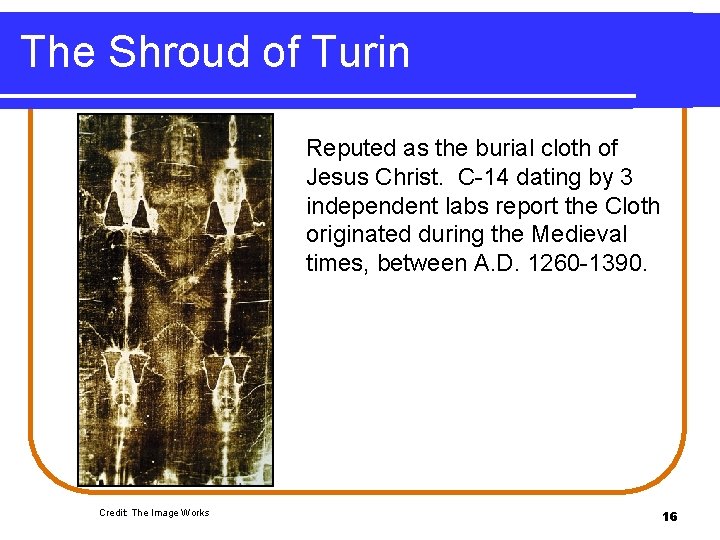
The Shroud of Turin Reputed as the burial cloth of Jesus Christ. C-14 dating by 3 independent labs report the Cloth originated during the Medieval times, between A. D. 1260 -1390. Credit: The Image Works 16

Mummified remains found frozen in the Italian Alps At least 5000 years old By carbon-14 dating Credit: Landov 17

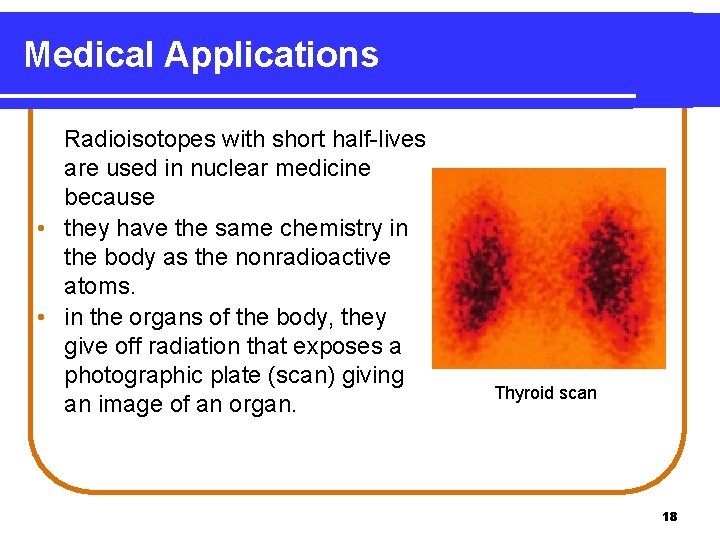
Medical Applications Radioisotopes with short half-lives are used in nuclear medicine because • they have the same chemistry in the body as the nonradioactive atoms. • in the organs of the body, they give off radiation that exposes a photographic plate (scan) giving an image of an organ. Thyroid scan 18

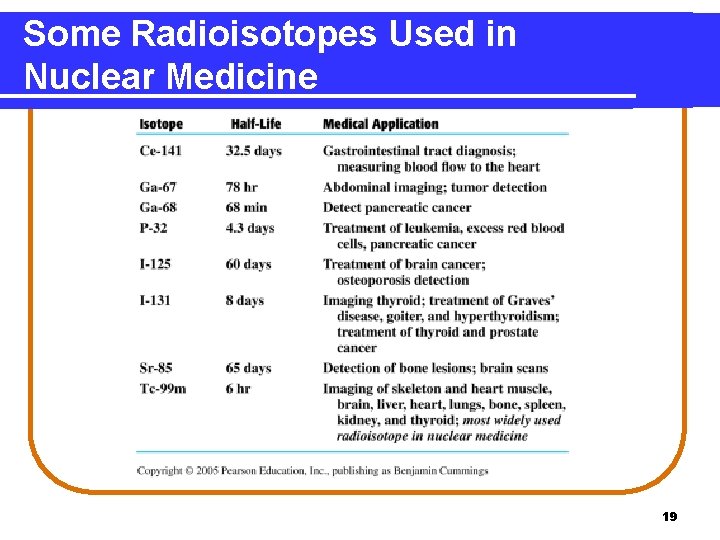
Some Radioisotopes Used in Nuclear Medicine 19

Learning Check Which of the following radioisotopes are most likely to be used in nuclear medicine? 1) 2) 3) 40 K half-life 1. 3 x 109 years 42 K half-life 12 hours 131 I half-life 8 days 20

Solution Which of the following radioisotopes are most likely to be used in nuclear medicine? Radioisotopes with short half-lives are used in nuclear medicine. 2) 3) 42 K half-life 12 hours 131 I half-life 8 days 21