Chapter 9 Molecular Geometry and Chemical Bonding Theory

Chapter 9 Molecular Geometry and Chemical Bonding Theory Copyright © Cengage Learning. All rights reserved. 10 | 1
Contents and Concepts Molecular Geometry and Directional Bonding 1. The Valence-Shell Electron-Pair Repulsion (VSEPR) Model 2. Dipole Moment and Molecular Geometry 3. Valence Bond Theory 4. Description of Multiple Bonding Molecular Orbital Theory 5. Principles of Molecular Orbital Theory 6. Electron Configurations of Diatomic Molecules of the Second-Period Elements 7. Molecular Orbitals and Delocalized Bonding
VSEPR model Molecular geometry is the general shape of a molecule, as determined by the relative positions of the atomic nuclei. The molecular geometry is the same as or a fragment of the electron domain (distinct electron regions) geometry

The valence-shell electron-pair repulsion (VSEPR) model predicts the shapes of molecules and ions by assuming that q the valence-shell electron pairs (domains) are arranged about each atom so that electron pairs are kept as far away from one another as possible, thereby minimizing electron pair (domains) repulsions q. The repulsions between electron domains increases in this order: lone pair < lone pair: bonded pair < bonded pair The diagram on the next slide illustrates this.
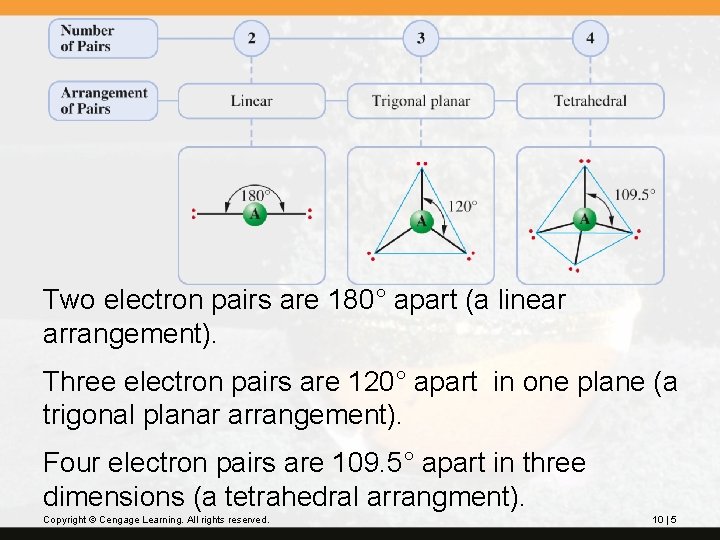
Two electron pairs are 180° apart (a linear arrangement). Three electron pairs are 120° apart in one plane (a trigonal planar arrangement). Four electron pairs are 109. 5° apart in three dimensions (a tetrahedral arrangment). Copyright © Cengage Learning. All rights reserved. 10 | 5
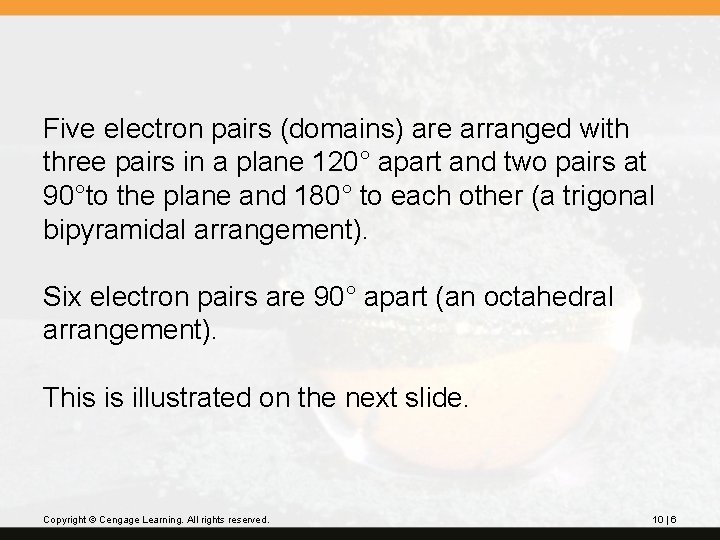
Five electron pairs (domains) are arranged with three pairs in a plane 120° apart and two pairs at 90°to the plane and 180° to each other (a trigonal bipyramidal arrangement). Six electron pairs are 90° apart (an octahedral arrangement). This is illustrated on the next slide. Copyright © Cengage Learning. All rights reserved. 10 | 6
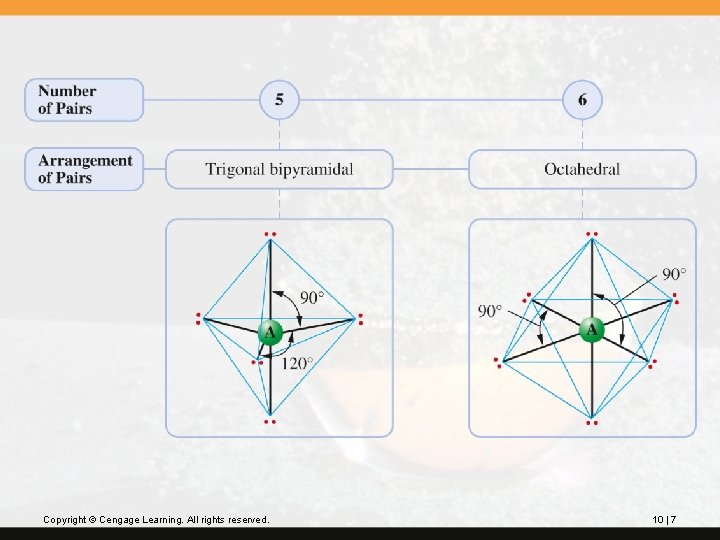
Copyright © Cengage Learning. All rights reserved. 10 | 7
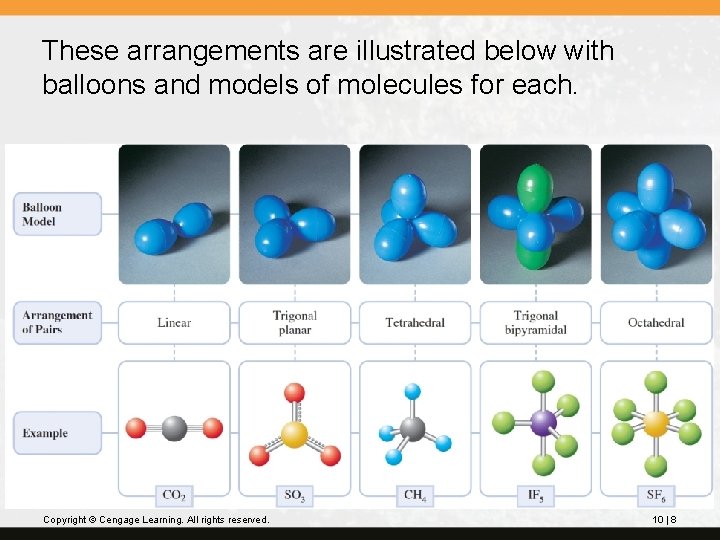
These arrangements are illustrated below with balloons and models of molecules for each. Copyright © Cengage Learning. All rights reserved. 10 | 8

To describe the molecular geometry, we describe the relative positions of the atoms, not the lone pairs. The direction in space of the bonding pairs gives the molecular geometry. Copyright © Cengage Learning. All rights reserved. 10 | 9
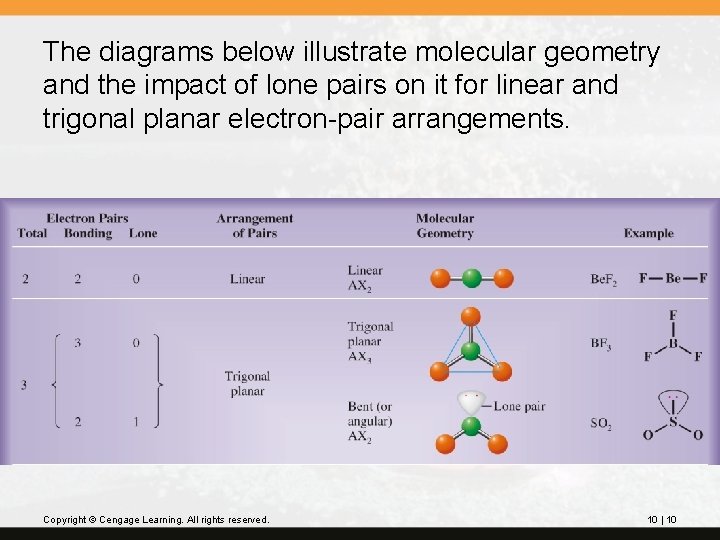
The diagrams below illustrate molecular geometry and the impact of lone pairs on it for linear and trigonal planar electron-pair arrangements. Copyright © Cengage Learning. All rights reserved. 10 | 10
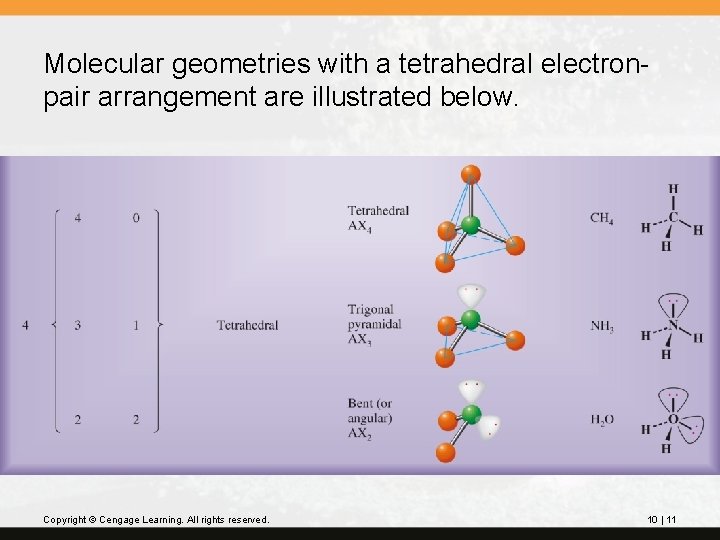
Molecular geometries with a tetrahedral electronpair arrangement are illustrated below. Copyright © Cengage Learning. All rights reserved. 10 | 11

Molecular geometries for the trigonal bipyramidal electron-pair arrangement are shown on the next slide. Copyright © Cengage Learning. All rights reserved. 10 | 12
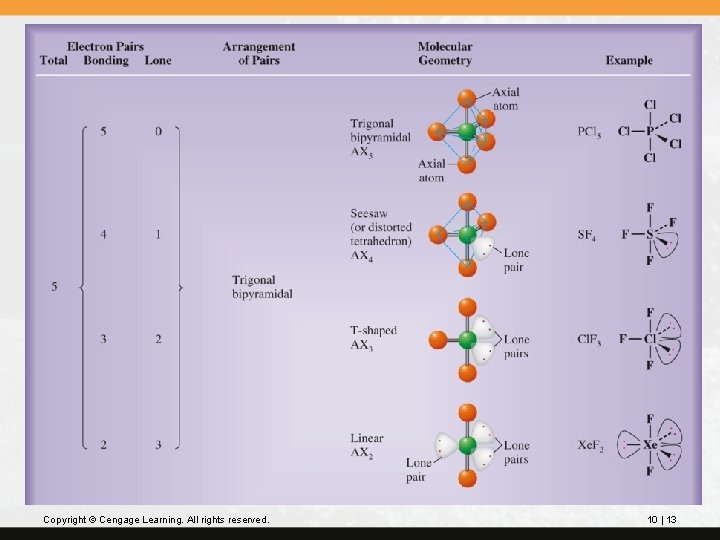
Copyright © Cengage Learning. All rights reserved. 10 | 13
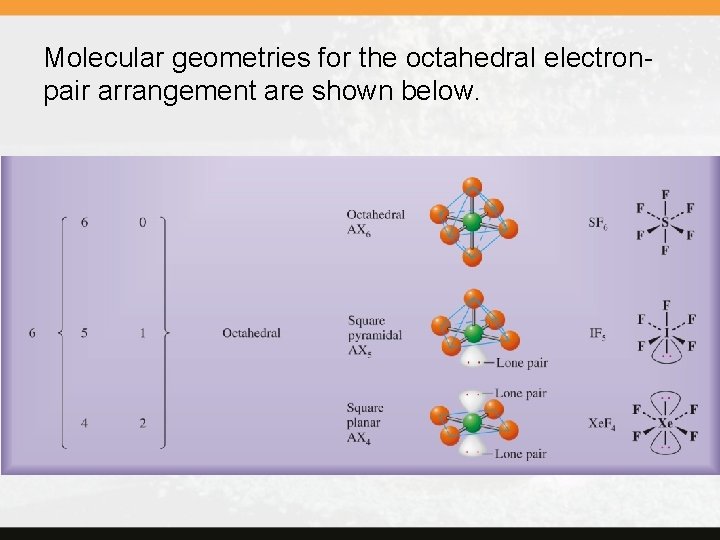
Molecular geometries for the octahedral electronpair arrangement are shown below.
The VSEPR model considers electron regions, thus a double or triple bond is treated as it were one lone pair. When resonance structures are required for the electron-dot diagram, you may choose any one to determine the electron-pair arrangement and the molecular geometry.
Predicting Molecular Geometry Using VSEPR 1. Write the electron-dot formula from the formula. 2. Based on the electron-dot formula, determine the number of distinct electron regions (domains) around the central atom (including bonding and nonbonding pairs). 3. Determine the arrangement of the electron pairs (domains) about the central atom (Figure 10. 3). This is the electron domain geometry. 4. Obtain the molecular geometry from the directions of the bonding pairs for this arrangement (Figure 10. 4).
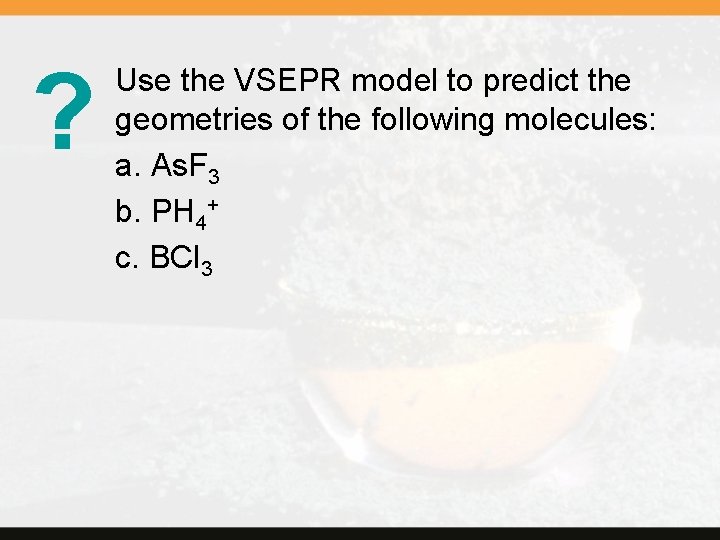
? Use the VSEPR model to predict the geometries of the following molecules: a. As. F 3 b. PH 4+ c. BCl 3
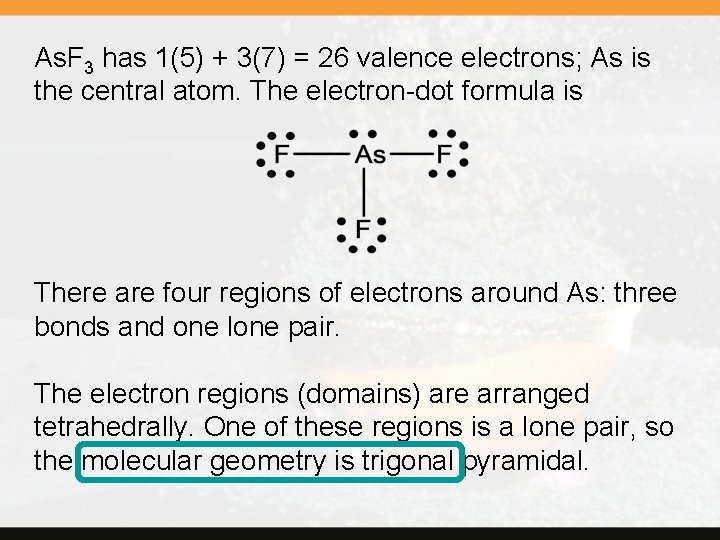
As. F 3 has 1(5) + 3(7) = 26 valence electrons; As is the central atom. The electron-dot formula is There are four regions of electrons around As: three bonds and one lone pair. The electron regions (domains) are arranged tetrahedrally. One of these regions is a lone pair, so the molecular geometry is trigonal pyramidal.
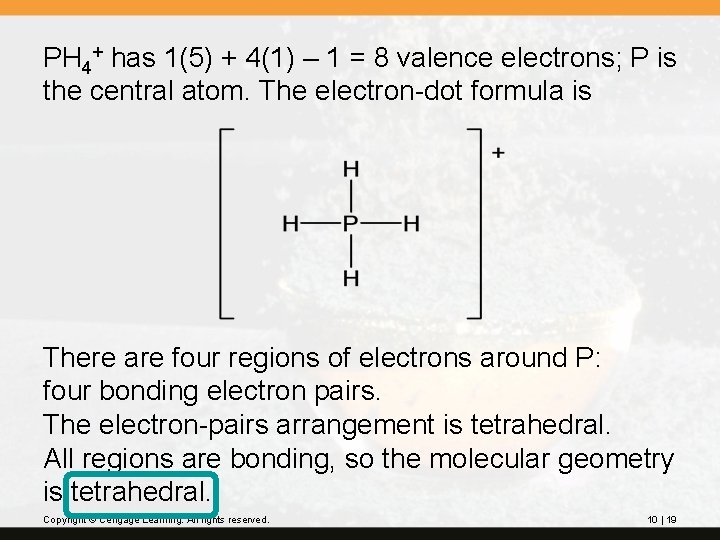
PH 4+ has 1(5) + 4(1) – 1 = 8 valence electrons; P is the central atom. The electron-dot formula is There are four regions of electrons around P: four bonding electron pairs. The electron-pairs arrangement is tetrahedral. All regions are bonding, so the molecular geometry is tetrahedral. Copyright © Cengage Learning. All rights reserved. 10 | 19
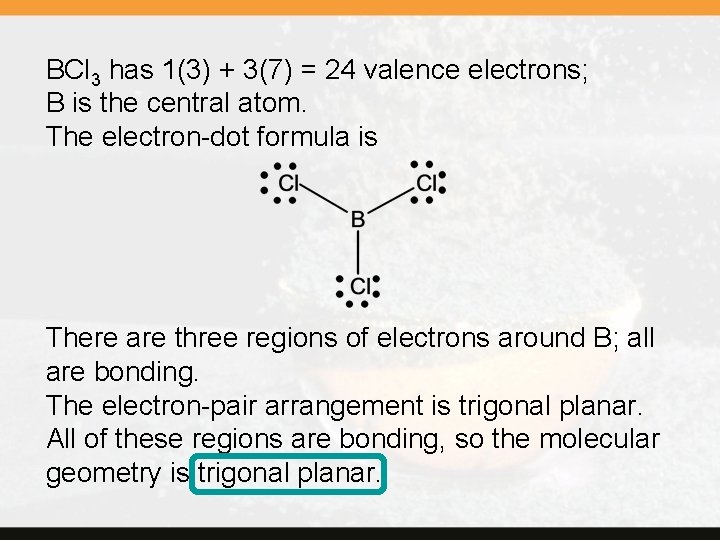
BCl 3 has 1(3) + 3(7) = 24 valence electrons; B is the central atom. The electron-dot formula is There are three regions of electrons around B; all are bonding. The electron-pair arrangement is trigonal planar. All of these regions are bonding, so the molecular geometry is trigonal planar.

Concept Check 10. 1 An atom in a molecule is surrounded by four pairs of electrons: one lone pair and three bonding pairs. Describe how the four electron pairs are arranged about the atom. How are any three of these pairs arranged in space? What is the geometry about this central atom, taking into account just the bonded atoms? The electron-pair arrangement is tetrahedral. Any three pairs are arranged as a trigonal pyramid. When one pair of the four is a lone pair, the geometry is trigonal pyramidal.
? Using the VSEPR model, predict the geometry of the following species: a. ICl 3 b. ICl 4 -
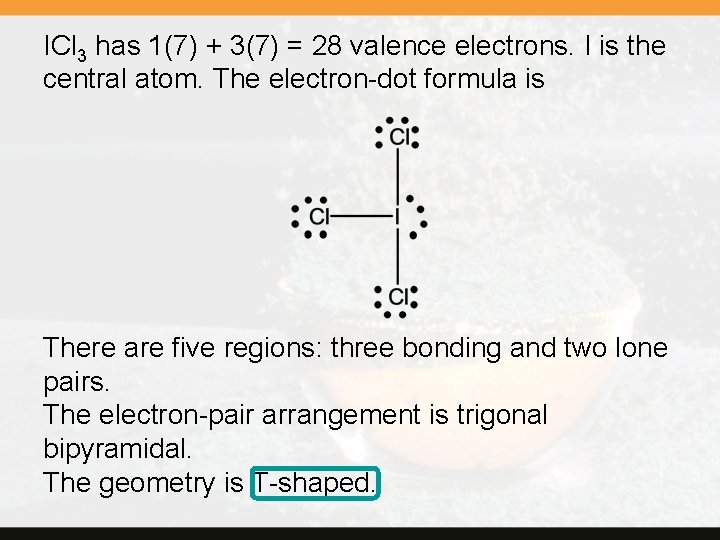
ICl 3 has 1(7) + 3(7) = 28 valence electrons. I is the central atom. The electron-dot formula is There are five regions: three bonding and two lone pairs. The electron-pair arrangement is trigonal bipyramidal. The geometry is T-shaped.
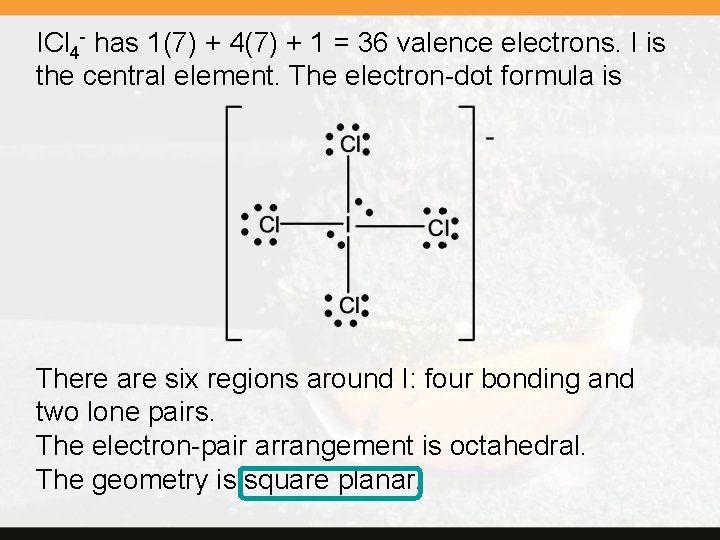
ICl 4 - has 1(7) + 4(7) + 1 = 36 valence electrons. I is the central element. The electron-dot formula is There are six regions around I: four bonding and two lone pairs. The electron-pair arrangement is octahedral. The geometry is square planar.
Predicting Bond Angles The angles 180°, 120°, 109. 5°, and so on are the bond angles when the central atom has no lone pair and all bonds are with the same other atom. When this is not the case, the bond angles deviate from these values in sometimes predictable ways. Because a lone pair tends to require more space than a bonding pair, it tends to reduce the bond angles.
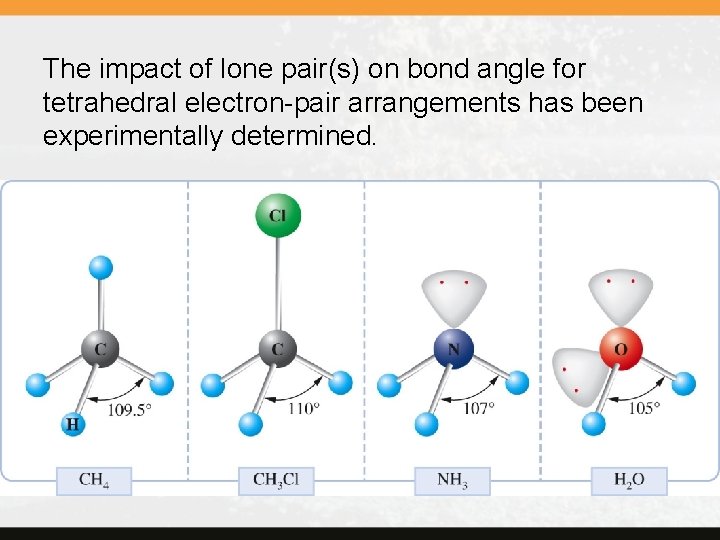
The impact of lone pair(s) on bond angle for tetrahedral electron-pair arrangements has been experimentally determined.
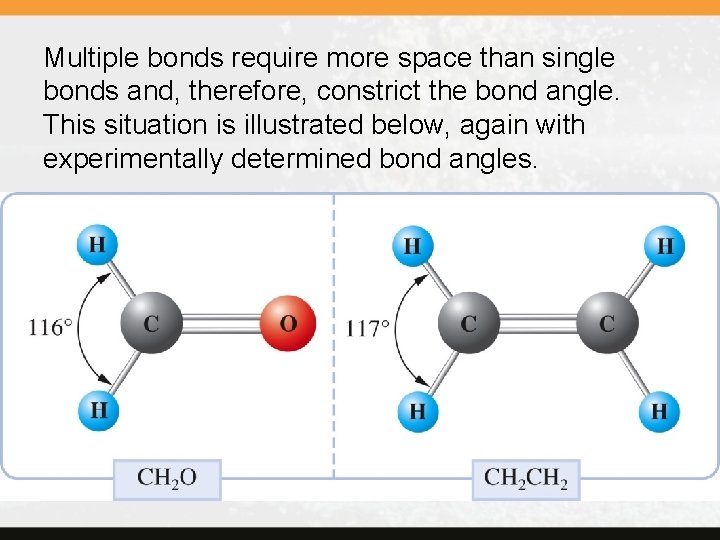
Multiple bonds require more space than single bonds and, therefore, constrict the bond angle. This situation is illustrated below, again with experimentally determined bond angles.

Dipole Moment A quantitative measure of the degree of partial charge separation (polarity) in a molecule.

Measurements are based on the fact that polar molecules are oriented by an electric field. This orientation affects the capacitance of the charged plates that create the electric field.
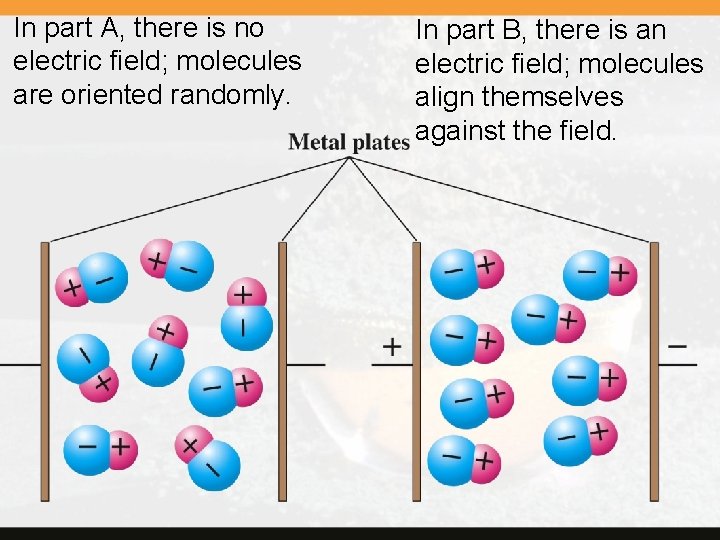
In part A, there is no electric field; molecules are oriented randomly. In part B, there is an electric field; molecules align themselves against the field.
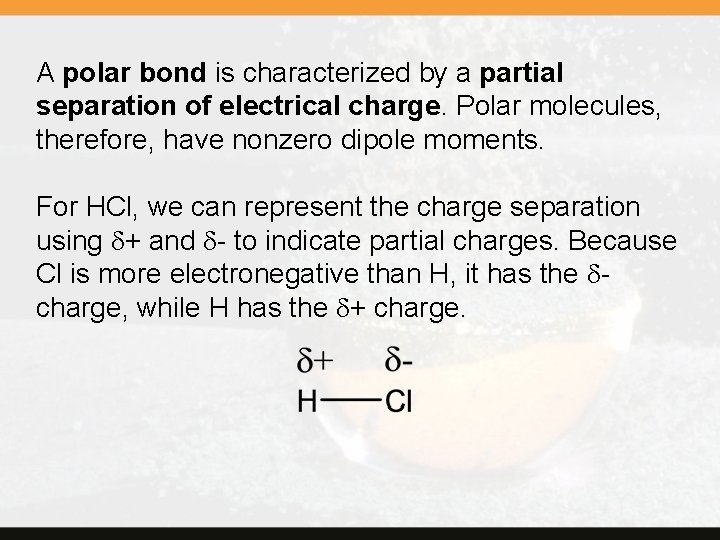
A polar bond is characterized by a partial separation of electrical charge. Polar molecules, therefore, have nonzero dipole moments. For HCl, we can represent the charge separation using d+ and d- to indicate partial charges. Because Cl is more electronegative than H, it has the dcharge, while H has the d+ charge.
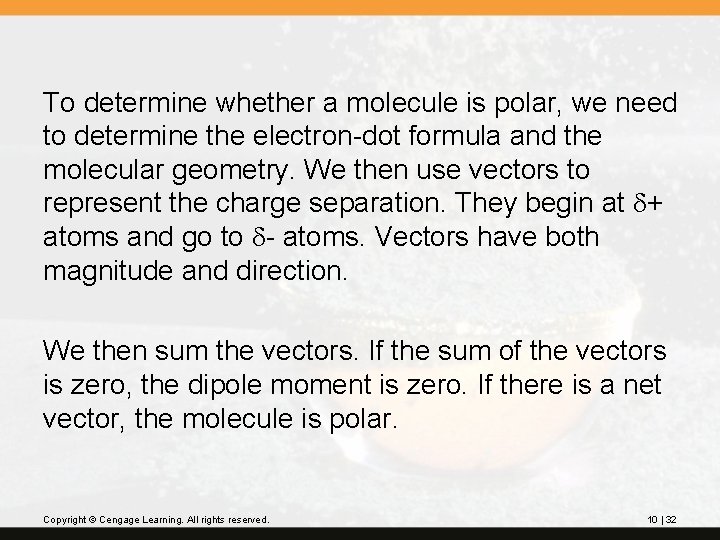
To determine whether a molecule is polar, we need to determine the electron-dot formula and the molecular geometry. We then use vectors to represent the charge separation. They begin at d+ atoms and go to d- atoms. Vectors have both magnitude and direction. We then sum the vectors. If the sum of the vectors is zero, the dipole moment is zero. If there is a net vector, the molecule is polar. Copyright © Cengage Learning. All rights reserved. 10 | 32
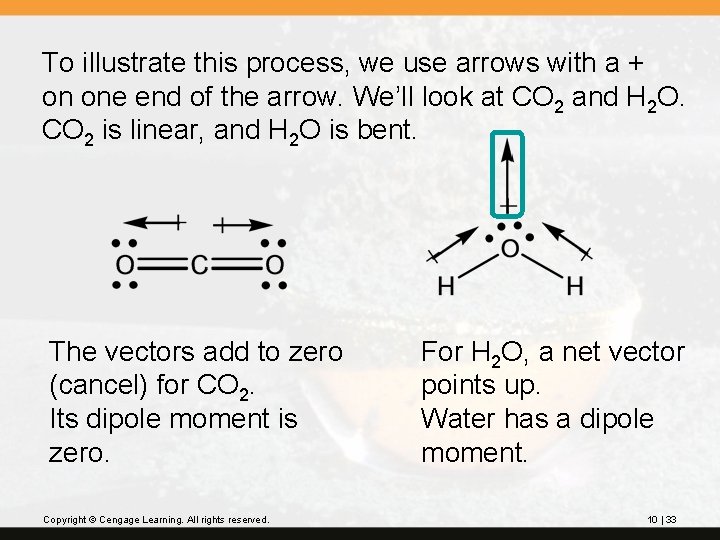
To illustrate this process, we use arrows with a + on one end of the arrow. We’ll look at CO 2 and H 2 O. CO 2 is linear, and H 2 O is bent. The vectors add to zero (cancel) for CO 2. Its dipole moment is zero. Copyright © Cengage Learning. All rights reserved. For H 2 O, a net vector points up. Water has a dipole moment. 10 | 33

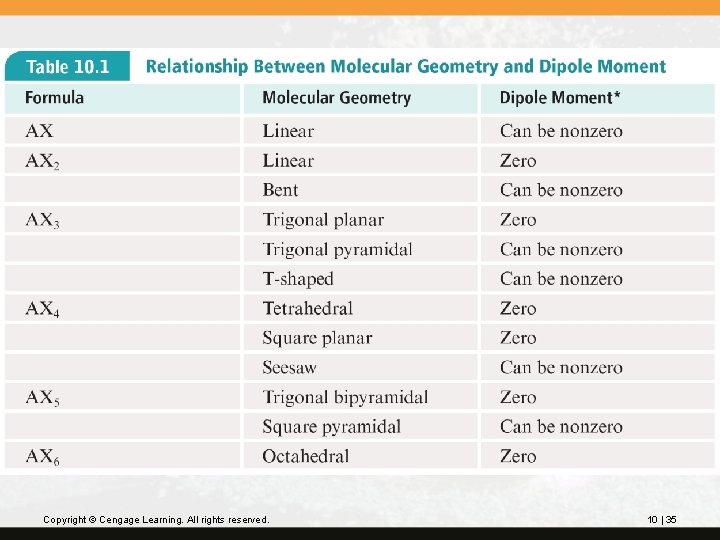
The relationship between molecular geometry and dipole moment is summarized in Table 10. 1. Copyright © Cengage Learning. All rights reserved. 10 | 34
Copyright © Cengage Learning. All rights reserved. 10 | 35
Polar molecules experience electrostatic attractive forces between molecules; in response, they orient themselves in a d+ to d- manner. This has an impact on molecular properties such as boiling point. The attractive forces due to the polarity lead the molecule to have a higher boiling point. Copyright © Cengage Learning. All rights reserved. 10 | 36
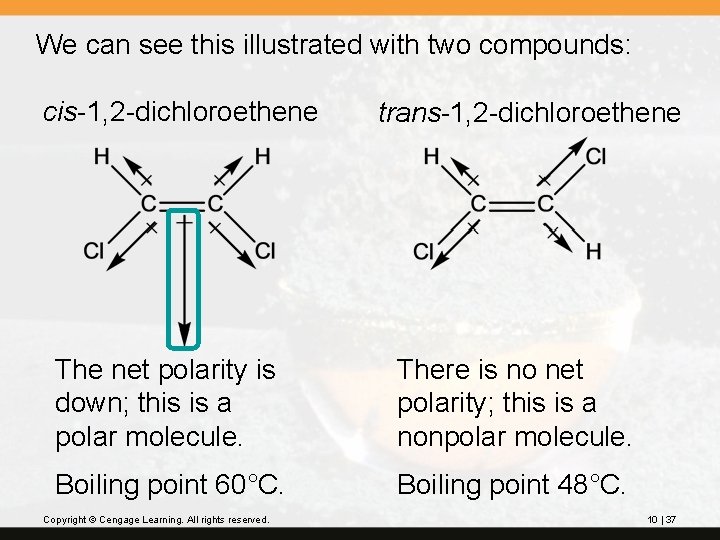
We can see this illustrated with two compounds: cis-1, 2 -dichloroethene trans-1, 2 -dichloroethene The net polarity is down; this is a polar molecule. There is no net polarity; this is a nonpolar molecule. Boiling point 60°C. Boiling point 48°C. Copyright © Cengage Learning. All rights reserved. 10 | 37

Concept Check 10. 2 Two molecules, each with the general formula AX 3, have different dipole moments. Molecule Y has a dipole moment of zero, whereas molecule Z has a nonzero dipole moment. From this information, what can you say about the geometries of Y and Z? Copyright © Cengage Learning. All rights reserved. 10 | 38
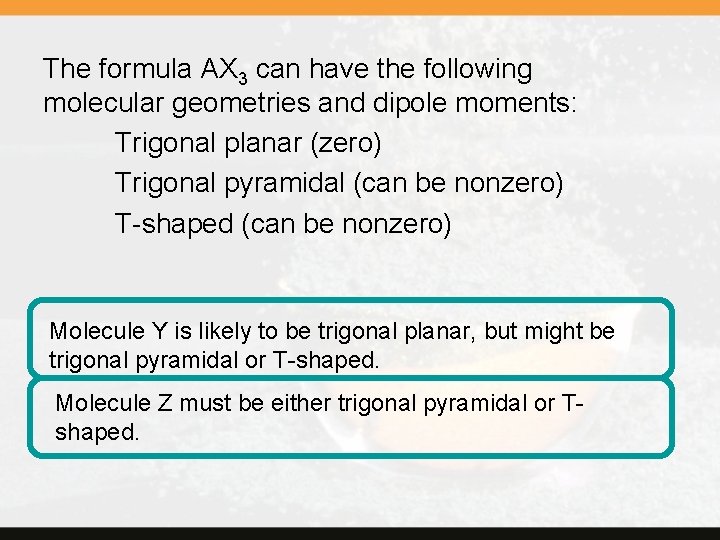
The formula AX 3 can have the following molecular geometries and dipole moments: Trigonal planar (zero) Trigonal pyramidal (can be nonzero) T-shaped (can be nonzero) Molecule Y is likely to be trigonal planar, but might be trigonal pyramidal or T-shaped. Molecule Z must be either trigonal pyramidal or Tshaped.

? Which of the following molecules would be expected to have a zero dipole moment? a. Ge. F 4 b. SF 2 c. Xe. F 2 d. As. F 3
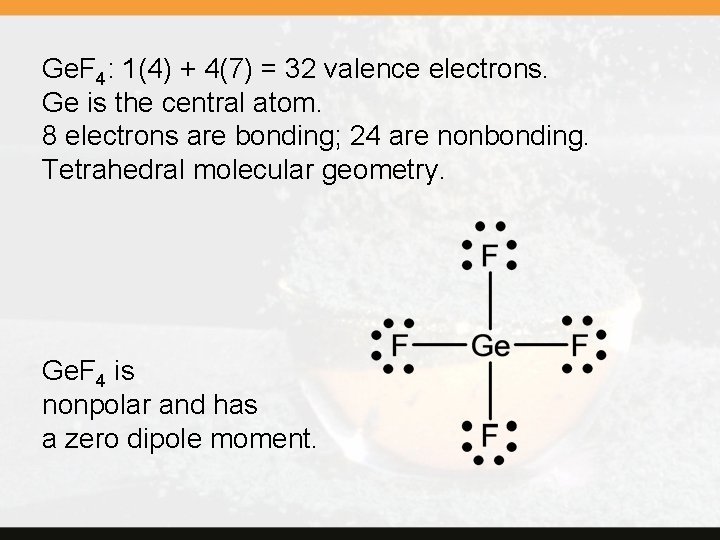
Ge. F 4: 1(4) + 4(7) = 32 valence electrons. Ge is the central atom. 8 electrons are bonding; 24 are nonbonding. Tetrahedral molecular geometry. Ge. F 4 is nonpolar and has a zero dipole moment.
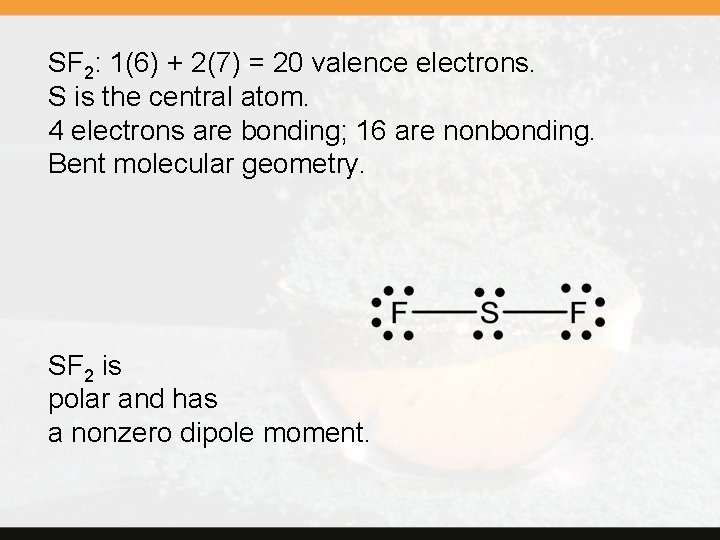
SF 2: 1(6) + 2(7) = 20 valence electrons. S is the central atom. 4 electrons are bonding; 16 are nonbonding. Bent molecular geometry. SF 2 is polar and has a nonzero dipole moment.
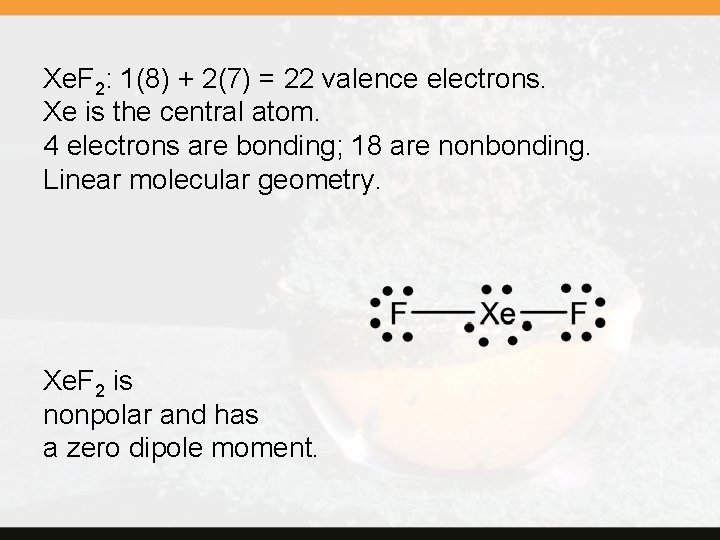
Xe. F 2: 1(8) + 2(7) = 22 valence electrons. Xe is the central atom. 4 electrons are bonding; 18 are nonbonding. Linear molecular geometry. Xe. F 2 is nonpolar and has a zero dipole moment.
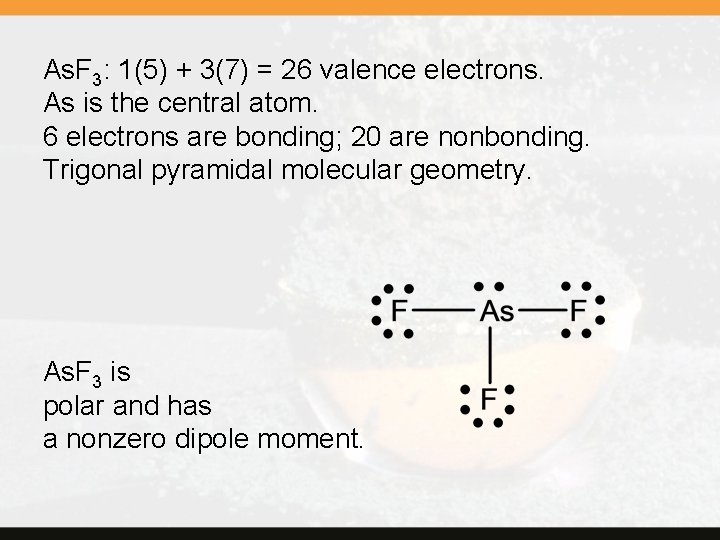
As. F 3: 1(5) + 3(7) = 26 valence electrons. As is the central atom. 6 electrons are bonding; 20 are nonbonding. Trigonal pyramidal molecular geometry. As. F 3 is polar and has a nonzero dipole moment.
Which of the following molecules would be expected to have a zero dipole moment? a. Ge. F 4 tetrahedral molecular geometry zero dipole moment b. SF 2 bent molecular geometry nonzero dipole moment c. Xe. F 2 linear molecular geometry zero dipole moment d. As. F 3 trigonal pyramidal molecular geometry nonzero dipole moment Copyright © Cengage Learning. All rights reserved. 10 | 45

Valence bond theory is an approximate theory put forth to explain the electron pair or covalent bond by quantum mechanics. Copyright © Cengage Learning. All rights reserved. 10 | 46

A bond forms when • An orbital on one atom comes to occupy a portion of the same region of space as an orbital on the other atom. The two orbitals are said to overlap. • The total number of electrons in both orbitals is no more than two. Copyright © Cengage Learning. All rights reserved. 10 | 47

The greater the orbital overlap, the stronger the bond. Orbitals (except s orbitals) bond in the direction in which they protrude or point, so as to obtain maximum overlap.
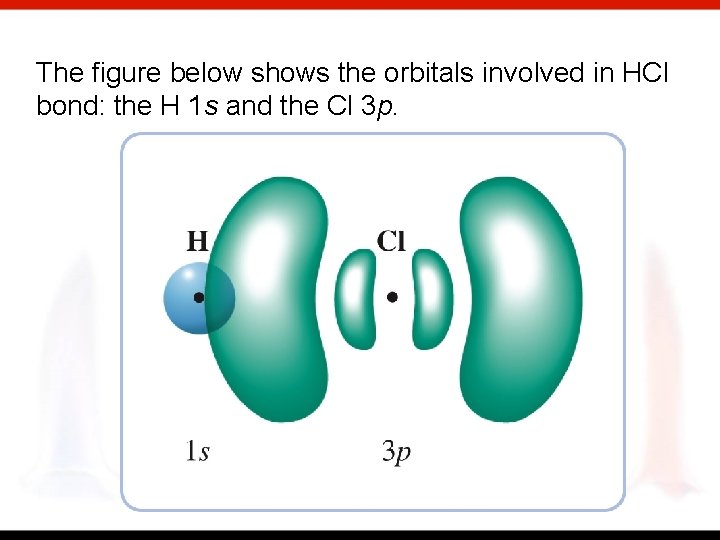
The figure below shows the orbitals involved in HCl bond: the H 1 s and the Cl 3 p.
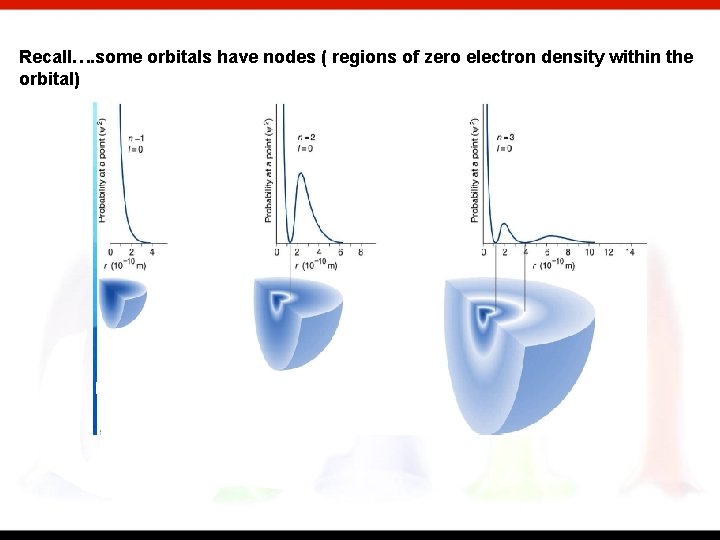
Recall…. some orbitals have nodes ( regions of zero electron density within the orbital)

Hybrid orbitals are orbitals used to describe the bonding that is obtained by taking combinations of the atomic orbitals of the isolated atoms. The number of hybrid orbitals formed always equals the number of atomic orbitals used.

Hybrid orbitals are named by using the atomic orbitals that combined: • one s orbital + one p orbital gives two sp orbitals • one s orbital + two p orbitals gives three sp 2 orbitals • one s orbital + three p orbitals gives four sp 3 orbitals • one s orbital + three p orbitals + one d orbital gives five sp 3 d orbitals • one s orbital + three p orbitals + two d orbitals gives six sp 3 d 2 orbitals Copyright © Cengage Learning. All rights reserved. 10 | 52
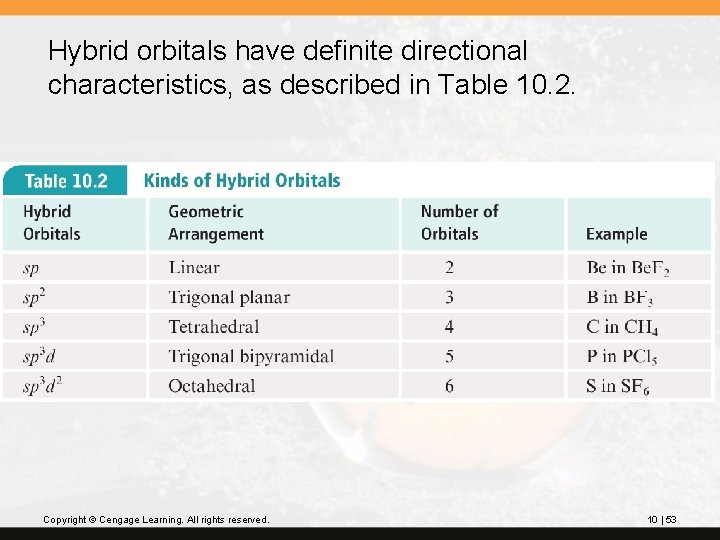
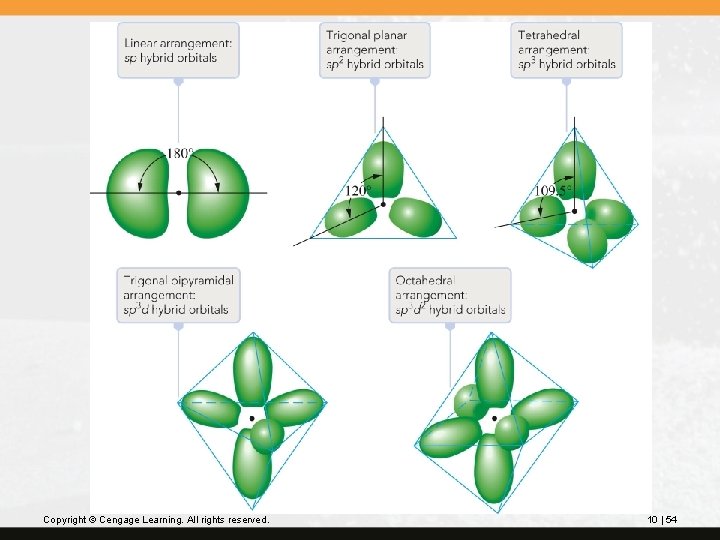
Hybrid orbitals have definite directional characteristics, as described in Table 10. 2. Copyright © Cengage Learning. All rights reserved. 10 | 53
Copyright © Cengage Learning. All rights reserved. 10 | 54

To obtain the bonding description about any atom in a molecule: 1. Write the Lewis electron-dot formula. 2. Use VSEPR to determine the electron arrangement about the atom. 3. From the arrangement, deduce the hybrid orbitals. 4. Assign the valence electrons to the hybrid orbitals one at a time, pairing only when necessary. 5. Form bonds by overlapping singly occupied hybrid orbitals with singly occupied orbitals of another atom. Copyright © Cengage Learning. All rights reserved. 10 | 55
Valence bond theory Let’s look at the methane molecule, CH 4. Simply using the atomic orbital diagram, it is difficult to explain its four identical C—H bonds. The valence bond theory allows us to explain this in two steps: promotion and hybridization.
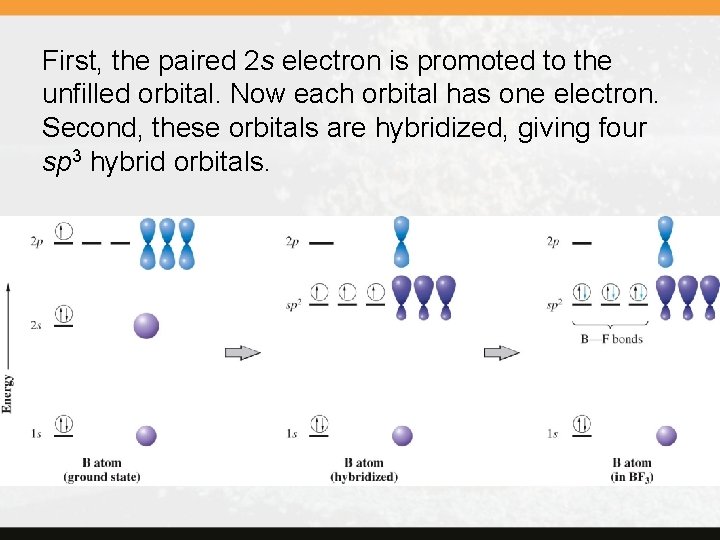
First, the paired 2 s electron is promoted to the unfilled orbital. Now each orbital has one electron. Second, these orbitals are hybridized, giving four sp 3 hybrid orbitals.
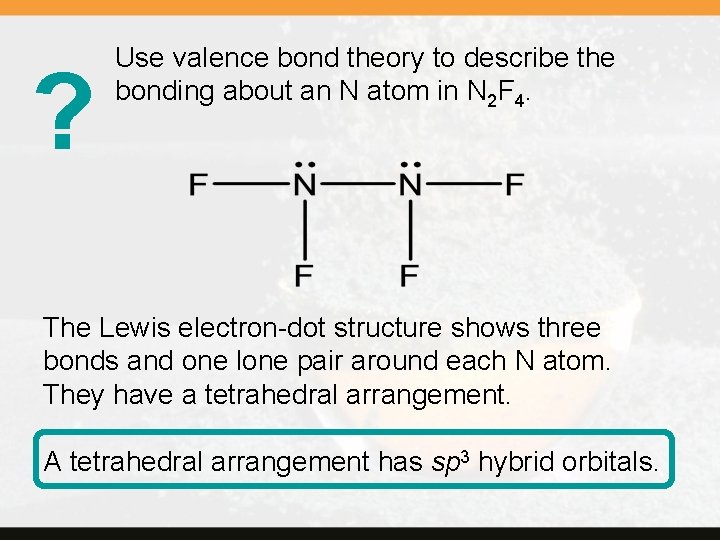
? Use valence bond theory to describe the bonding about an N atom in N 2 F 4. The Lewis electron-dot structure shows three bonds and one lone pair around each N atom. They have a tetrahedral arrangement. A tetrahedral arrangement has sp 3 hybrid orbitals.
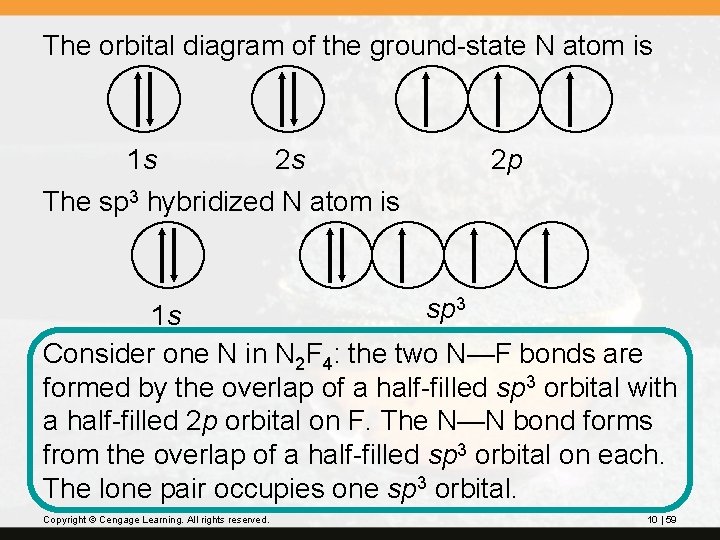
The orbital diagram of the ground-state N atom is 1 s 2 s 2 p The sp 3 hybridized N atom is sp 3 1 s Consider one N in N 2 F 4: the two N—F bonds are formed by the overlap of a half-filled sp 3 orbital with a half-filled 2 p orbital on F. The N—N bond forms from the overlap of a half-filled sp 3 orbital on each. The lone pair occupies one sp 3 orbital. Copyright © Cengage Learning. All rights reserved. 10 | 59
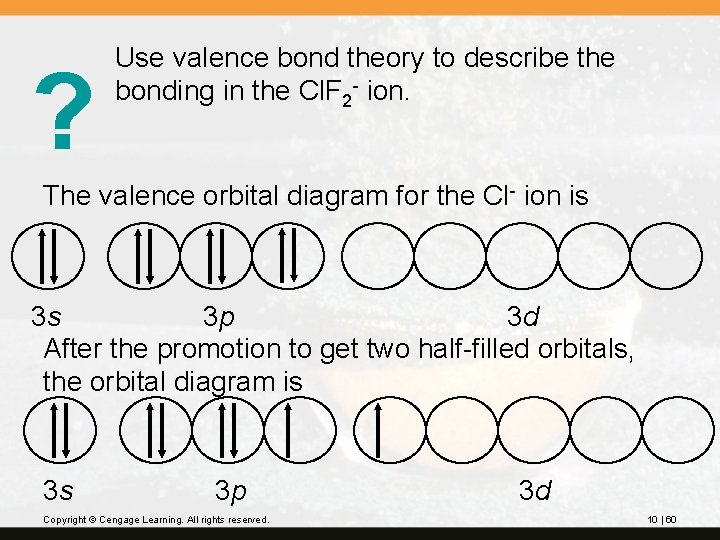
? Use valence bond theory to describe the bonding in the Cl. F 2 - ion. The valence orbital diagram for the Cl- ion is 3 s 3 p 3 d After the promotion to get two half-filled orbitals, the orbital diagram is 3 s 3 p Copyright © Cengage Learning. All rights reserved. 3 d 10 | 60
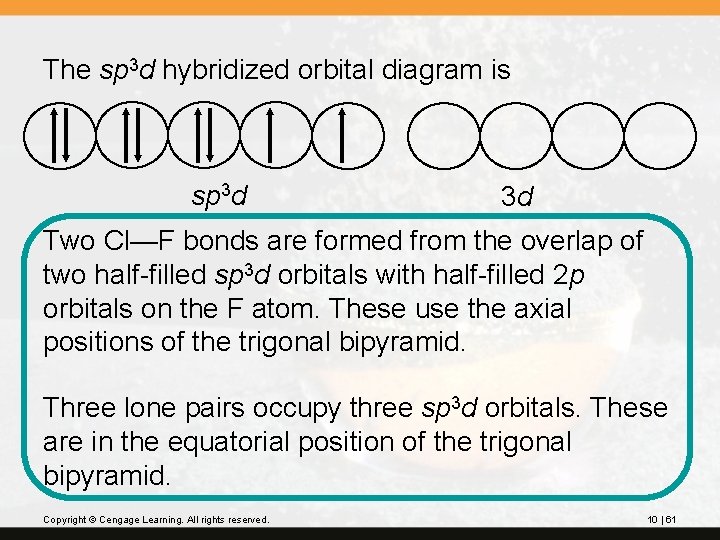
The sp 3 d hybridized orbital diagram is sp 3 d 3 d Two Cl—F bonds are formed from the overlap of two half-filled sp 3 d orbitals with half-filled 2 p orbitals on the F atom. These use the axial positions of the trigonal bipyramid. Three lone pairs occupy three sp 3 d orbitals. These are in the equatorial position of the trigonal bipyramid. Copyright © Cengage Learning. All rights reserved. 10 | 61

One hybrid orbital is required for each bond (whether a single or a multiple bond) and for each lone pair. Multiple bonding involves the overlap of one hybrid orbital and one (for a double bond) or two (for a triple bond) nonhybridized p orbitals. Copyright © Cengage Learning. All rights reserved. 10 | 62

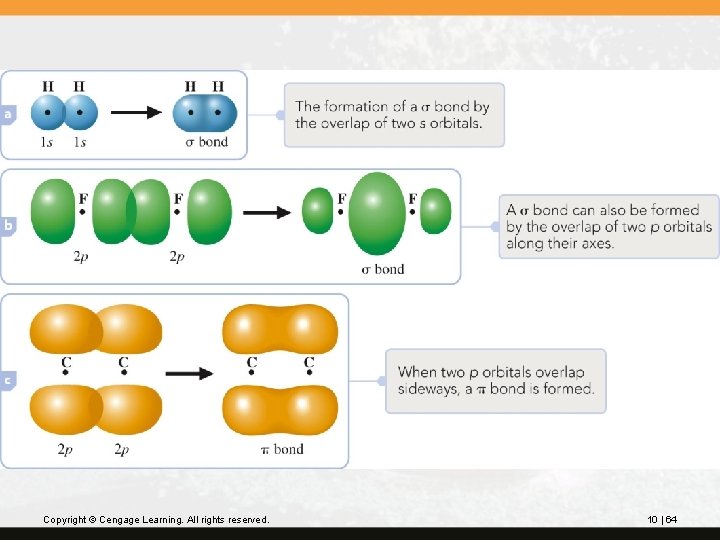
To describe a multiple bond, we need to distinguish between two kinds of bonds. A s bond (sigma) has a cylindrical shape about the bond axis. It is formed either when two s orbitals overlap or with directional orbitals (p or hybrid), when they overlap along their axis. A p bond (pi) has an electron distribution above and below the bond axis. It is formed by the sideways overlap of two parallel p orbitals. This overlap occurs when two parallel half-filled p orbitals are available after s bonds have formed. Copyright © Cengage Learning. All rights reserved. 10 | 63
Copyright © Cengage Learning. All rights reserved. 10 | 64
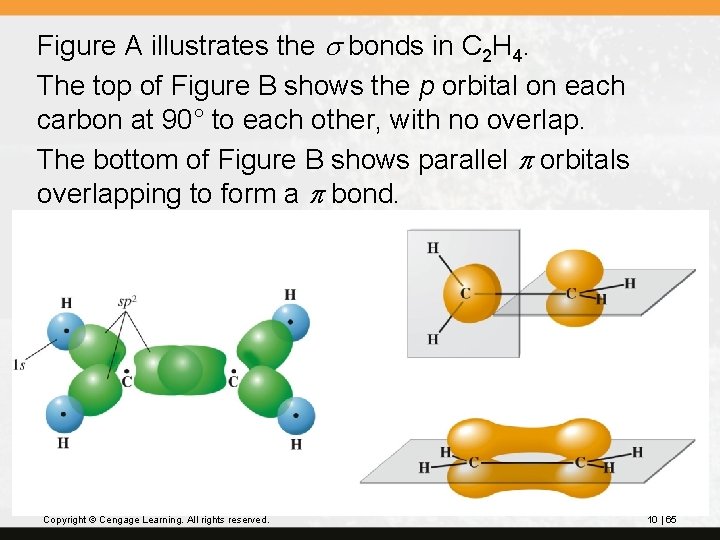
Figure A illustrates the s bonds in C 2 H 4. The top of Figure B shows the p orbital on each carbon at 90° to each other, with no overlap. The bottom of Figure B shows parallel p orbitals overlapping to form a p bond. Copyright © Cengage Learning. All rights reserved. 10 | 65
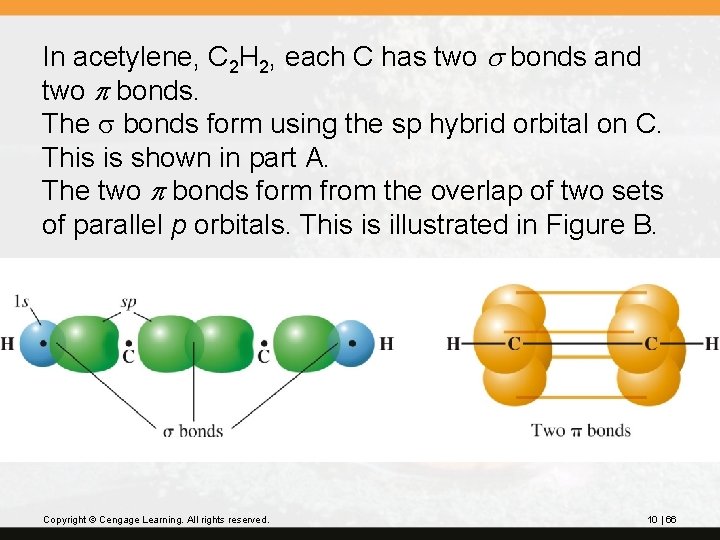
In acetylene, C 2 H 2, each C has two s bonds and two p bonds. The s bonds form using the sp hybrid orbital on C. This is shown in part A. The two p bonds form from the overlap of two sets of parallel p orbitals. This is illustrated in Figure B. Copyright © Cengage Learning. All rights reserved. 10 | 66
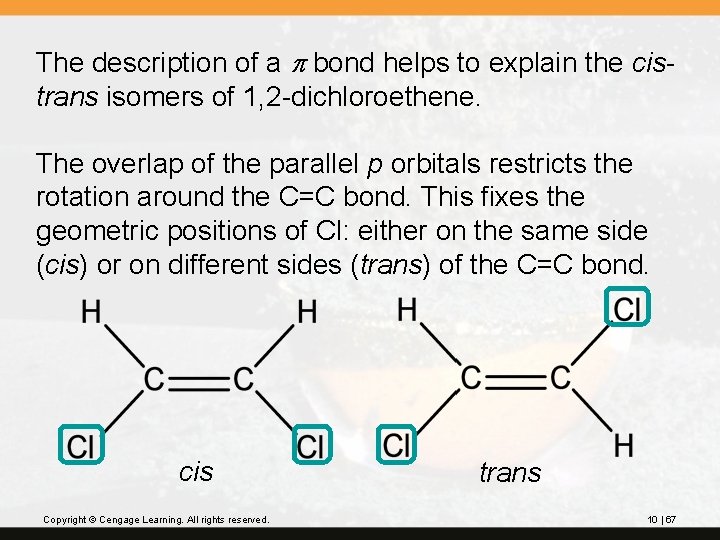
The description of a p bond helps to explain the cistrans isomers of 1, 2 -dichloroethene. The overlap of the parallel p orbitals restricts the rotation around the C=C bond. This fixes the geometric positions of Cl: either on the same side (cis) or on different sides (trans) of the C=C bond. cis Copyright © Cengage Learning. All rights reserved. trans 10 | 67
Concept Check 10. 3 An atom in a molecule has one single bond and one triple bond to other atoms. What hybrid orbitals do you expect for this atom? Describe how you arrive at your answer. One single bond and one triple bond requires two hybrid orbitals and two sets of two parallel p orbitals. That requires sp hybridization. Copyright © Cengage Learning. All rights reserved. 10 | 68
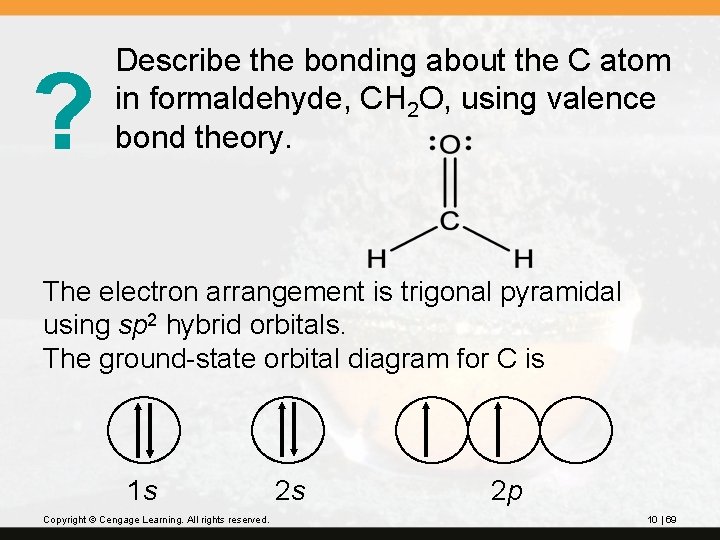
? Describe the bonding about the C atom in formaldehyde, CH 2 O, using valence bond theory. The electron arrangement is trigonal pyramidal using sp 2 hybrid orbitals. The ground-state orbital diagram for C is 1 s Copyright © Cengage Learning. All rights reserved. 2 s 2 p 10 | 69
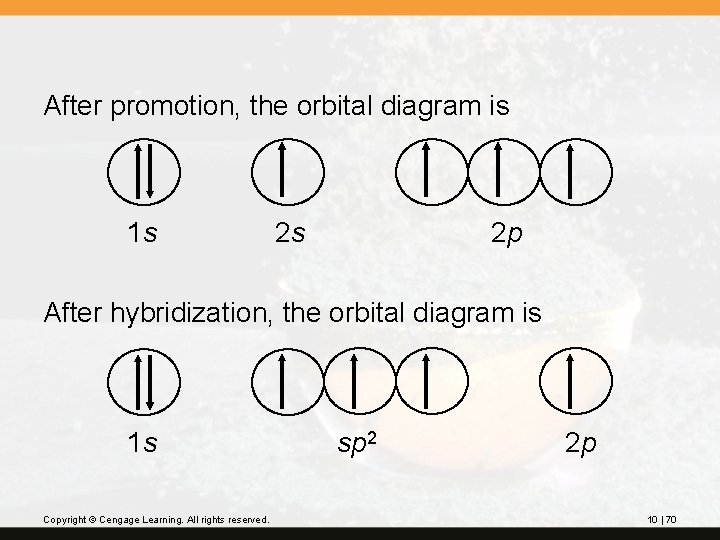
After promotion, the orbital diagram is 1 s 2 s 2 p After hybridization, the orbital diagram is 1 s Copyright © Cengage Learning. All rights reserved. sp 2 2 p 10 | 70

The C—H s bonds are formed from the overlap of two C sp 2 hybrid orbitals with the 1 s orbital on the H atoms. The C—O s bond is formed from the overlap of one sp 2 hybrid orbital and one O half-filled p orbital. The C—O p bond is formed from the sideways overlap of the C 2 p orbital and an O 2 p orbital.
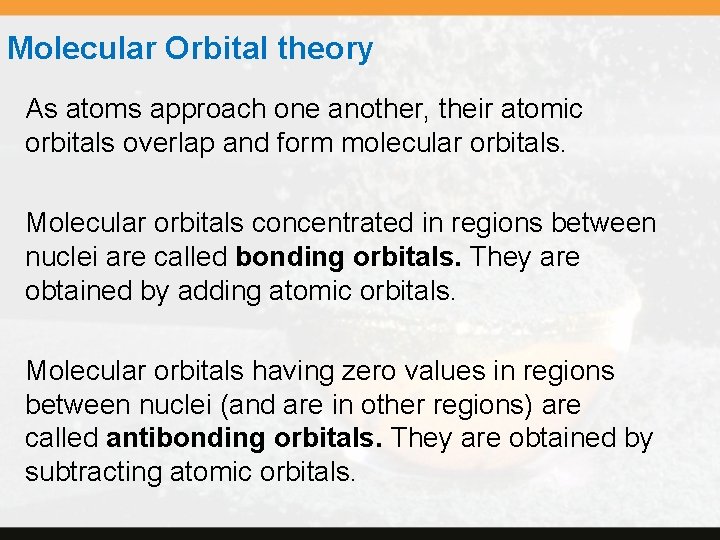
Molecular Orbital theory As atoms approach one another, their atomic orbitals overlap and form molecular orbitals. Molecular orbitals concentrated in regions between nuclei are called bonding orbitals. They are obtained by adding atomic orbitals. Molecular orbitals having zero values in regions between nuclei (and are in other regions) are called antibonding orbitals. They are obtained by subtracting atomic orbitals.

The figure on the next slide illustrates the combination of two 1 s orbitals. The top shows the formation of the bonding molecular orbital; the bottom shows the formation of the antibonding molecular orbital. Copyright © Cengage Learning. All rights reserved. 10 | 73
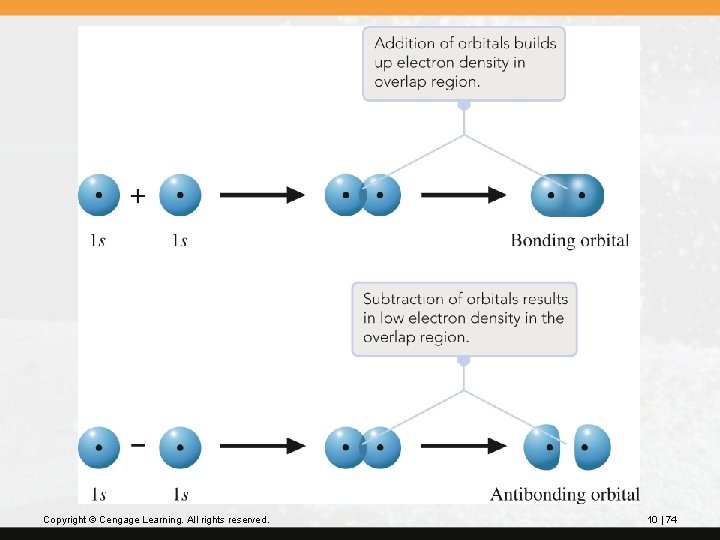
Copyright © Cengage Learning. All rights reserved. 10 | 74

Once a molecular orbital is formed, it can be occupied in the same way as are atomic orbitals. As an example, we’ll study hydrogen, H 2. Each hydrogen atom has one electron in a 1 s orbital, for a total of two electrons. When the two 1 s orbitals combine, they form two s molecular orbitals. Copyright © Cengage Learning. All rights reserved. 10 | 75
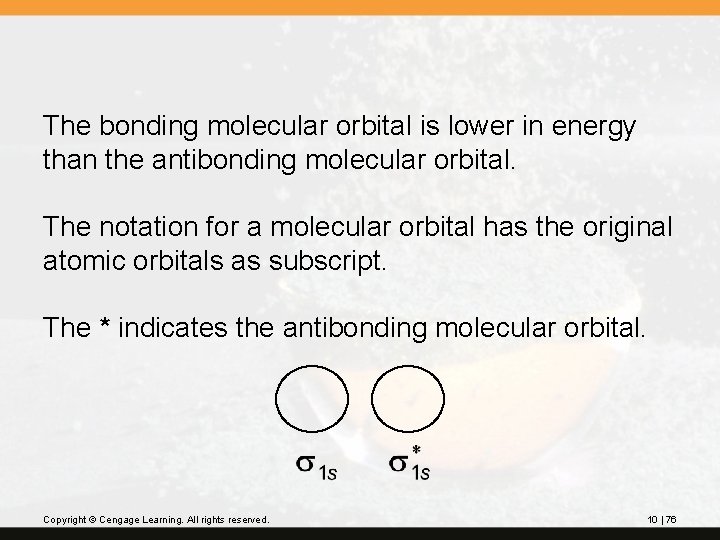
The bonding molecular orbital is lower in energy than the antibonding molecular orbital. The notation for a molecular orbital has the original atomic orbitals as subscript. The * indicates the antibonding molecular orbital. Copyright © Cengage Learning. All rights reserved. 10 | 76
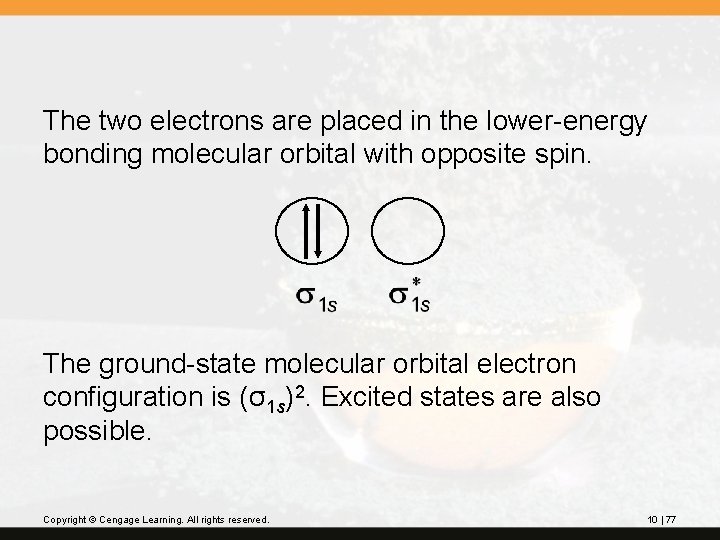
The two electrons are placed in the lower-energy bonding molecular orbital with opposite spin. The ground-state molecular orbital electron configuration is (σ1 s)2. Excited states are also possible. Copyright © Cengage Learning. All rights reserved. 10 | 77
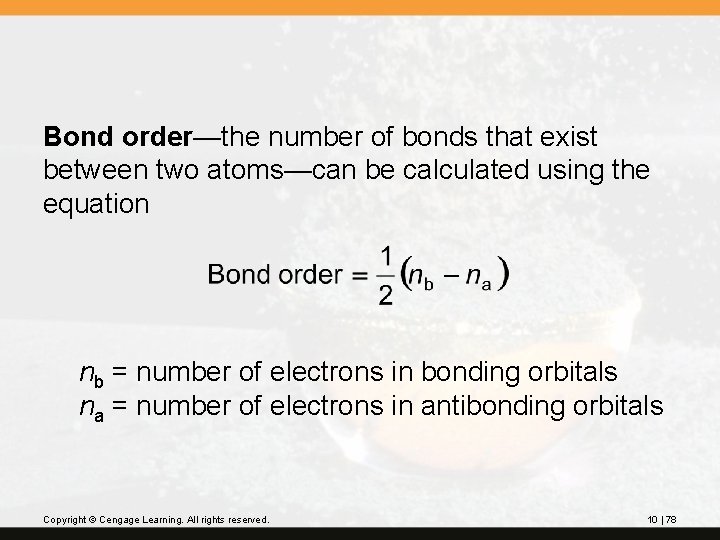
Bond order—the number of bonds that exist between two atoms—can be calculated using the equation nb = number of electrons in bonding orbitals na = number of electrons in antibonding orbitals Copyright © Cengage Learning. All rights reserved. 10 | 78
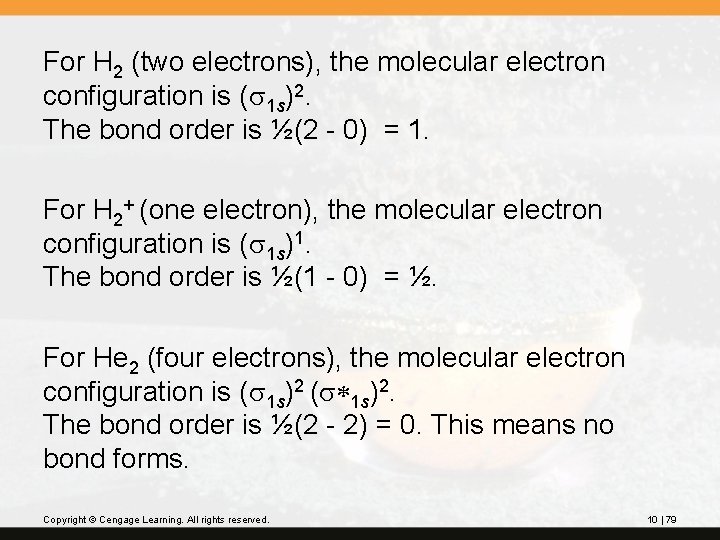
For H 2 (two electrons), the molecular electron configuration is (s 1 s)2. The bond order is ½(2 - 0) = 1. For H 2+ (one electron), the molecular electron configuration is (s 1 s)1. The bond order is ½(1 - 0) = ½. For He 2 (four electrons), the molecular electron configuration is (s 1 s)2 (s*1 s)2. The bond order is ½(2 - 2) = 0. This means no bond forms. Copyright © Cengage Learning. All rights reserved. 10 | 79

The strength of the interaction between two atomic orbitals to form molecular orbitals is determined by two factors: • The energy difference between the interacting orbitals • The magnitude of the overlap For the interaction to be strong, the energies of the two orbitals must be approximately equal and the overlap must be large. Copyright © Cengage Learning. All rights reserved. 10 | 80
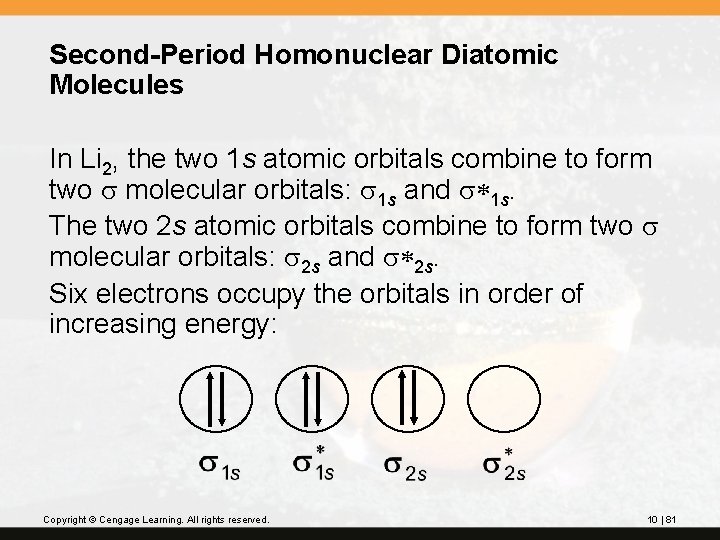
Second-Period Homonuclear Diatomic Molecules In Li 2, the two 1 s atomic orbitals combine to form two s molecular orbitals: s 1 s and s*1 s. The two 2 s atomic orbitals combine to form two s molecular orbitals: s 2 s and s*2 s. Six electrons occupy the orbitals in order of increasing energy: Copyright © Cengage Learning. All rights reserved. 10 | 81
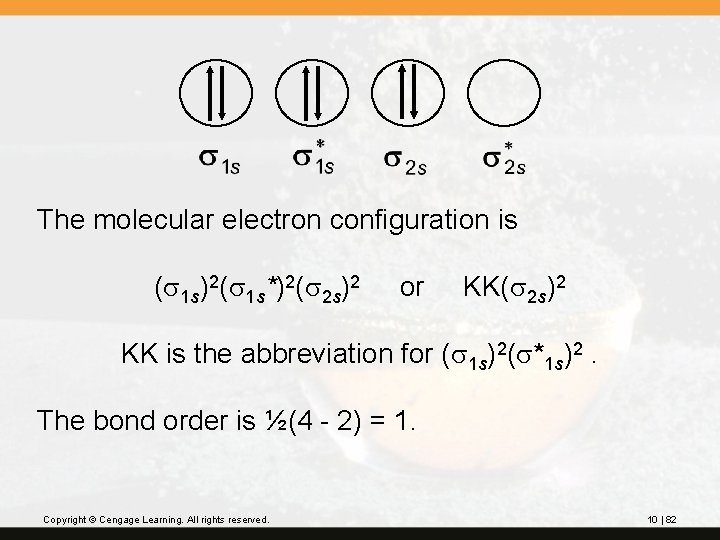
The molecular electron configuration is (s 1 s)2(s 1 s*)2(s 2 s)2 or KK(s 2 s)2 KK is the abbreviation for (s 1 s)2(s*1 s)2. The bond order is ½(4 - 2) = 1. Copyright © Cengage Learning. All rights reserved. 10 | 82
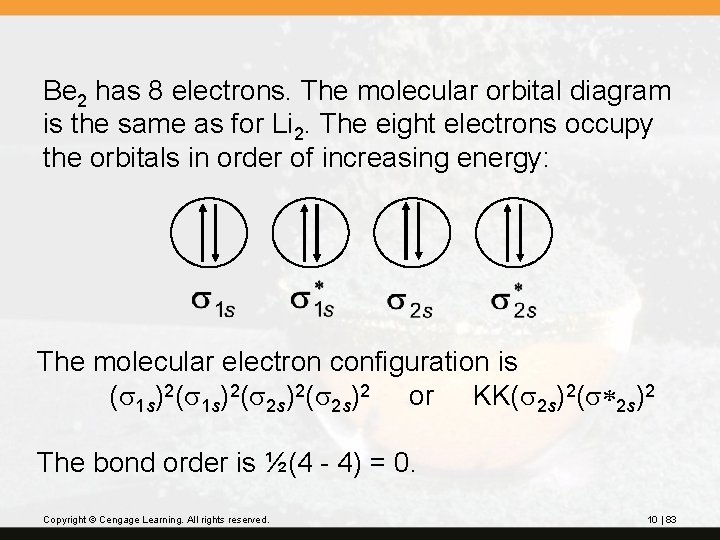
Be 2 has 8 electrons. The molecular orbital diagram is the same as for Li 2. The eight electrons occupy the orbitals in order of increasing energy: The molecular electron configuration is (s 1 s)2(s 2 s)2 or KK(s 2 s)2(s*2 s)2 The bond order is ½(4 - 4) = 0. Copyright © Cengage Learning. All rights reserved. 10 | 83

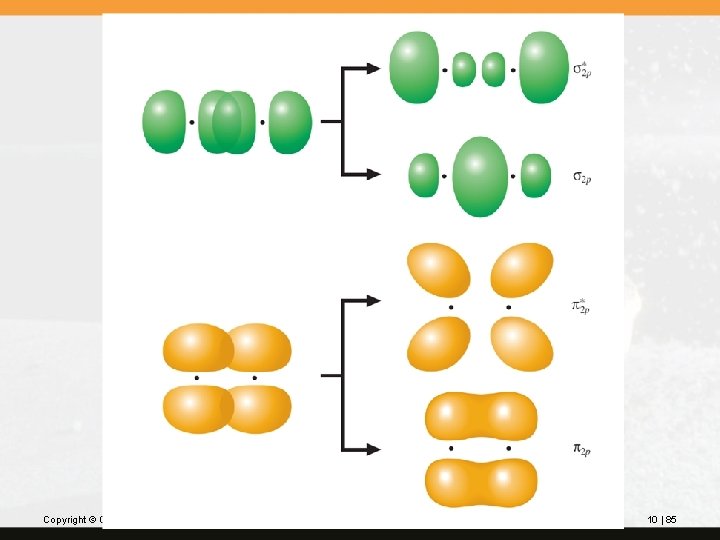
Molecular orbitals formed by the overlap of atomic p orbitals are of two types. When two p orbitals overlap end-to-end, two s molecular orbitals form: s 2 p and s*2 p. When two p orbitals overlap sideways, two p molecular orbitals form: p 2 p and p*2 p. Because two p orbitals on each atom overlap with two p orbitals on another atom, a total of four molecular orbitals form: two bonding and two antibonding. Copyright © Cengage Learning. All rights reserved. 10 | 84
Copyright © Cengage Learning. All rights reserved. 10 | 85
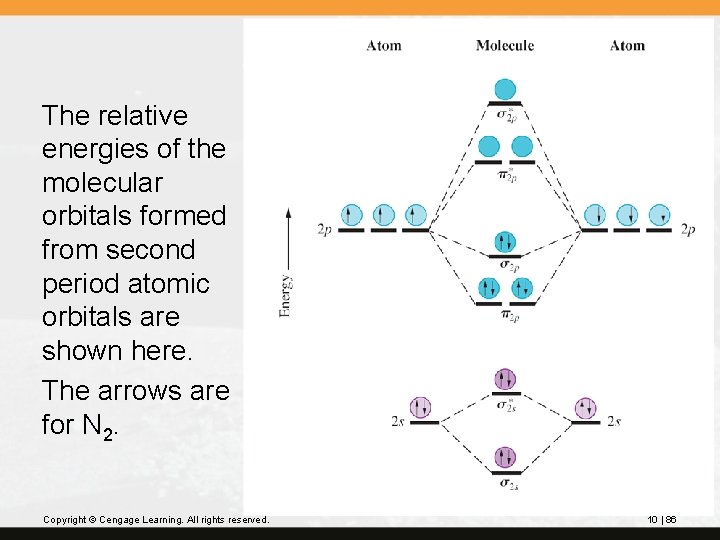
The relative energies of the molecular orbitals formed from second period atomic orbitals are shown here. The arrows are for N 2. Copyright © Cengage Learning. All rights reserved. 10 | 86

Because there are two bonding p orbitals, together they can hold four electrons. They are filled one electron per orbital before a second electron is added to the same orbital with opposite spin. Copyright © Cengage Learning. All rights reserved. 10 | 87

? Give the orbital diagram and electron configuration of the F 2 molecule. Is the molecular substance diamagnetic or paramagnetic? What is the order of the bond in F 2? Copyright © Cengage Learning. All rights reserved. 10 | 88
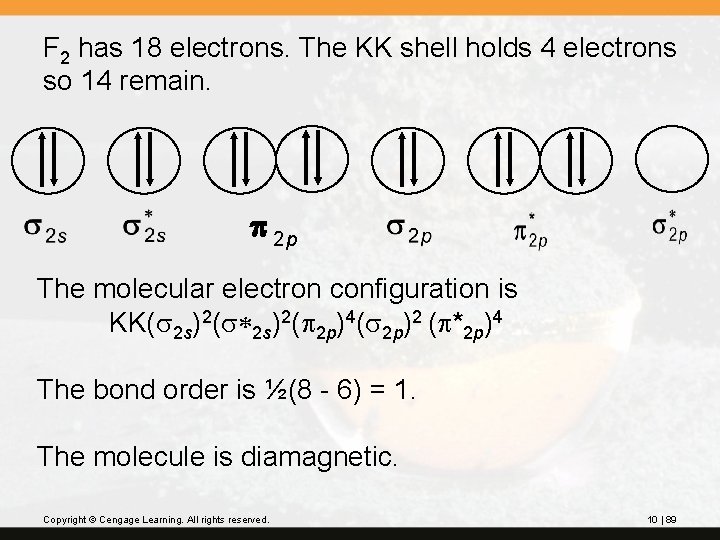
F 2 has 18 electrons. The KK shell holds 4 electrons so 14 remain. p 2 p The molecular electron configuration is KK(s 2 s)2(s*2 s)2(p 2 p)4(s 2 p)2 (p*2 p)4 The bond order is ½(8 - 6) = 1. The molecule is diamagnetic. Copyright © Cengage Learning. All rights reserved. 10 | 89

? A number of compounds of the nitrosonium ion, NO+, are known, including nitrosonium hydrogen sulfate, (NO+)(HSO 4 -). Use the molecular orbitals similar to those of homonuclear diatomic molecules and obtain the orbital diagram, electron configuration, bond order, and magnetic characteristics of the NO+ ion. Note: The stability of the ion results from the loss of an antibonding electron from NO. Copyright © Cengage Learning. All rights reserved. 10 | 90
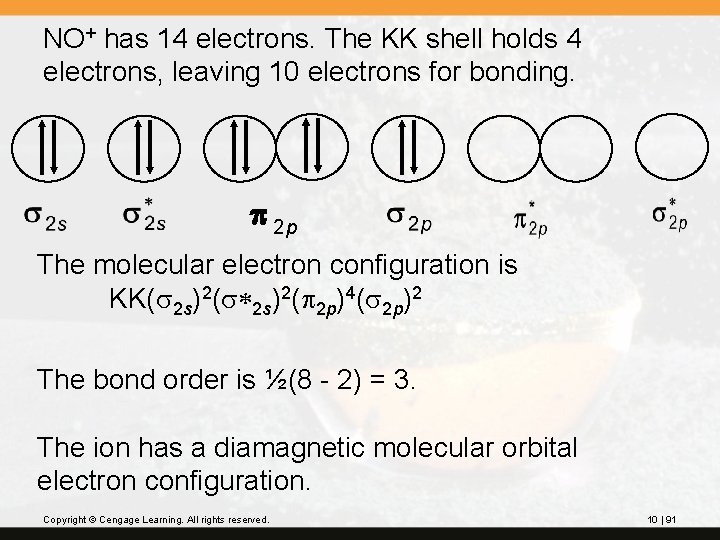
NO+ has 14 electrons. The KK shell holds 4 electrons, leaving 10 electrons for bonding. p 2 p The molecular electron configuration is KK(s 2 s)2(s*2 s)2(p 2 p)4(s 2 p)2 The bond order is ½(8 - 2) = 3. The ion has a diamagnetic molecular orbital electron configuration. Copyright © Cengage Learning. All rights reserved. 10 | 91

Table 10. 3 (next slide) compares theoretical bond order to the experimental bond length, bond dissociation energy, and magnetic character for the second-period homonuclear diatomic molecules. Copyright © Cengage Learning. All rights reserved. 10 | 92
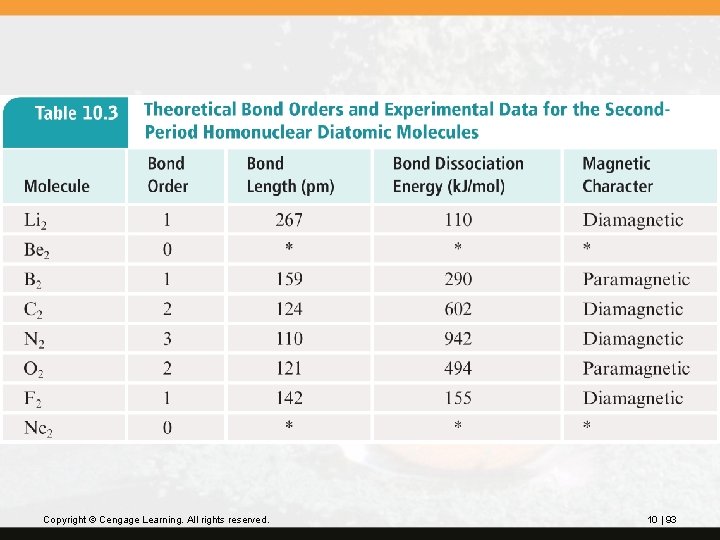
Copyright © Cengage Learning. All rights reserved. 10 | 93

Molecular orbital theory can describe delocalized bonding in terms of a single electron configuration. Besides having the conventional s bonds between two atoms, these molecules have molecular orbitals formed by more than two atomic orbitals. Ozone, O 3, is one example. Copyright © Cengage Learning. All rights reserved. 10 | 94
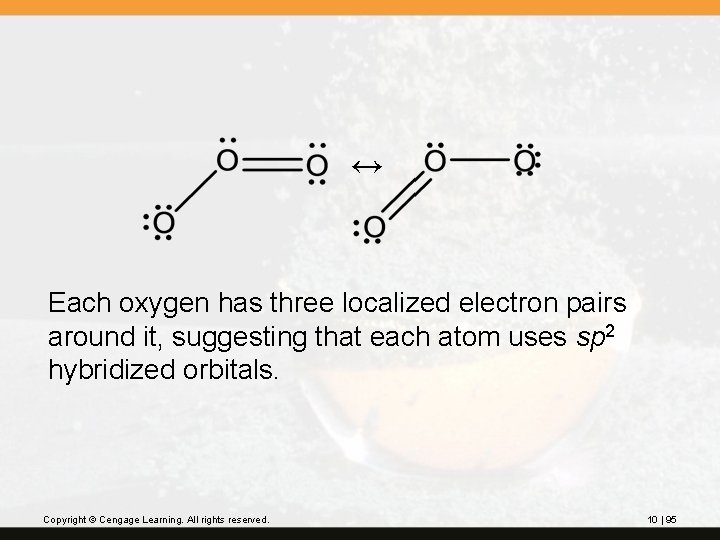
↔ Each oxygen has three localized electron pairs around it, suggesting that each atom uses sp 2 hybridized orbitals. Copyright © Cengage Learning. All rights reserved. 10 | 95

The overlap of one hybrid orbital on the central O atom with a hybrid orbital on each terminal O forms the two O—O s bonds. This leaves one hybrid orbital on the central O atom and two hybrid orbitals on the terminal O atoms. Copyright © Cengage Learning. All rights reserved. 10 | 96
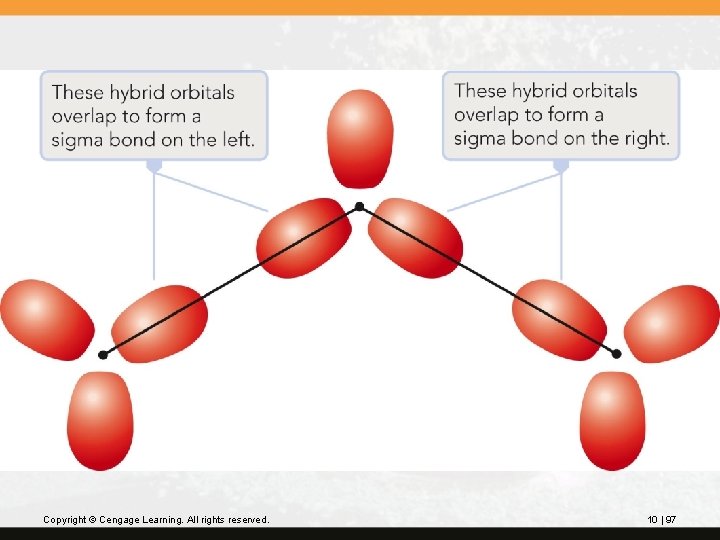
Copyright © Cengage Learning. All rights reserved. 10 | 97
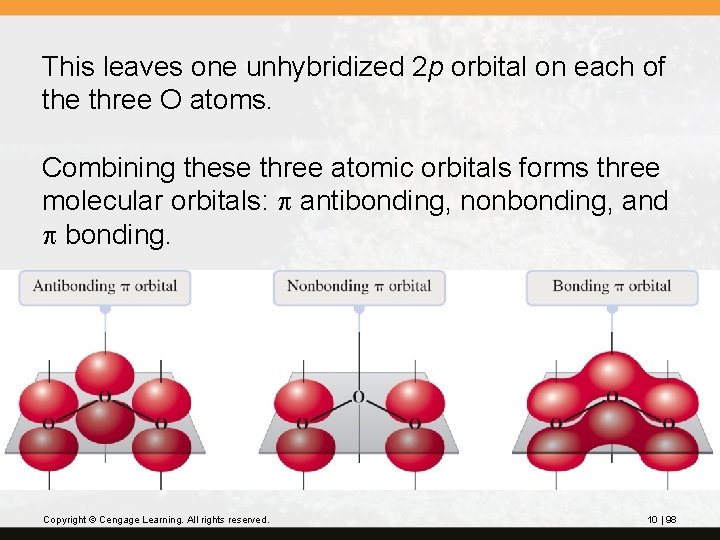
This leaves one unhybridized 2 p orbital on each of the three O atoms. Combining these three atomic orbitals forms three molecular orbitals: p antibonding, nonbonding, and p bonding. Copyright © Cengage Learning. All rights reserved. 10 | 98
All three of the molecular orbitals span the entire molecule. Both the bonding orbitals and the nonbonding orbitals (same energy as isolated atoms) are doubly occupied. This agrees with the resonance structure in which one delocalized pair is bonding and one is a lone pair (nonbonding) on the terminal atom. Copyright © Cengage Learning. All rights reserved. 10 | 99
- Slides: 99