CHAPTER 9 HEMOGLOBIN STRUCTURE SYNTHESIS FUNCTION AND MEASUREMENT
CHAPTER 9 HEMOGLOBIN STRUCTURE, SYNTHESIS, FUNCTION AND MEASUREMENT

Acknowledgements n n n n Addisa Ababa University Jimma University Hawassa University Haramaya University of Gondar American Society for Clinical Pathology Center for Disease Control and Prevention-Ethiopia

Objectives At the end of this chapter the students will be able to: § Define hemoglobin n Diagrammatically illustrate the structure of hemoglobin n Discribe the biosynthesis of heme and globin moieties of hemoglobin n Explain the functions of hemoglobin n State the principles of hemoglobin estimation in clinical practice n Explain the principle of the cyanmethemoglobin method of hemoglobin determination n Carry out calibration for the cyanmethemoglobin method of hemoglobin determination

Objective cont’d n n n Perform hemoglobin quantitation on a sample of blood using the cyanmethemoglobin method List the advantages of the cyanmethemoglobin method of hemoglobin determination Explain the principle of the Hemocue method of hemoglobin quantitation Descrive the advantages and disadvantages of the Hemocue method Perform hemoglobin quantitation on a sample of blood using the Sahli-Hellige method Explain why the Sahli-Hellige method is considered unreliable

Objective cont’d n Describe sources of specimen collection errors (preanalytic) that can cause inaccurate results n List the sources of error in various hemoglobin determination techniques n Discuss reference ranges and the significance of hemoglobin values on the basis of age and sex n Explain the correlation between red blood cell count, hemoglobin and hematocrit results n Discuss quality control and checks utilized to establish test validity and prevent erroneous results
9. 1 Structure of Hemoglobin n Hemoglobin is normally present in red cells Two primary structures ¨ Globin ¨ Heme which is composed of n Protoporphyrin n Iron The heme structure consists of a ring of C, H and N atoms called Protoporphyrin IX with an atom of Ferrous ( Fe 2+ ) iron attached ( ferroprotoporphyrin).
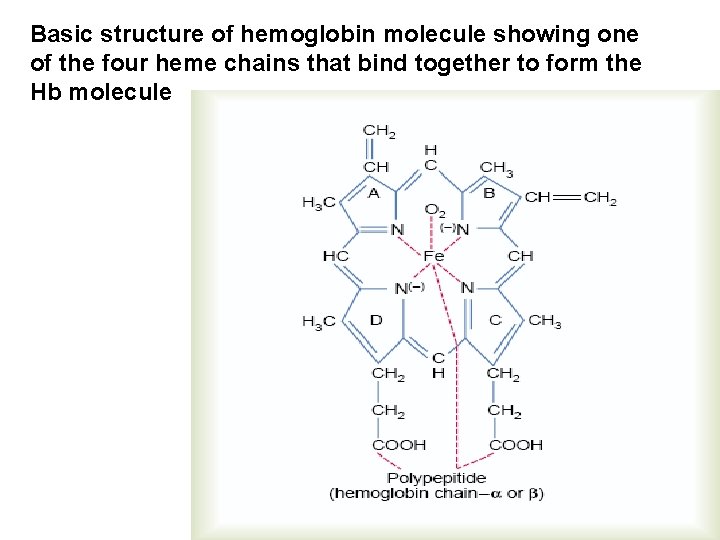
Basic structure of hemoglobin molecule showing one of the four heme chains that bind together to form the Hb molecule
Iron n n Iron is an essential component of hemoglobin ¨ Decreased tissue iron = cellular dysfunction ¨ Increased tissue iron = cellular destruction ¨ Regulated by absorption, not excretion Iron circulates in the plasma bound to transferrin
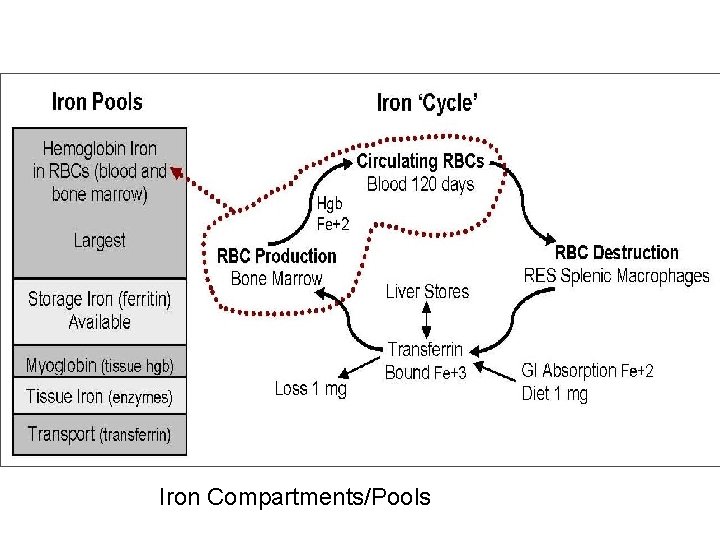
Iron Compartments/Pools
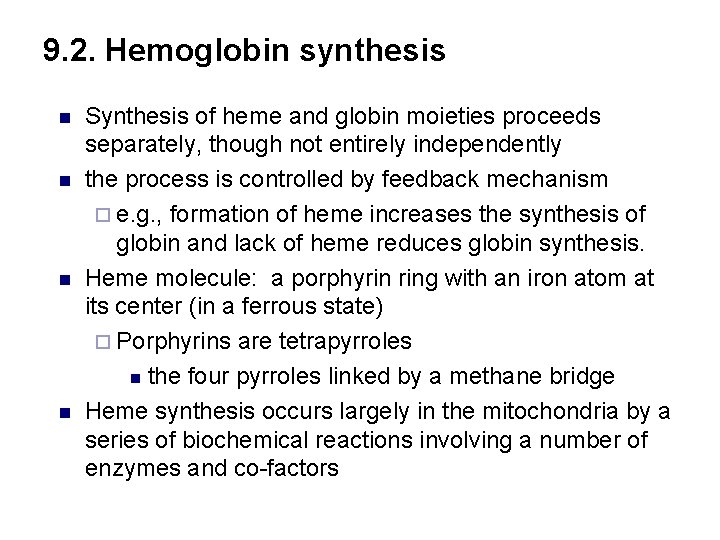
9. 2. Hemoglobin synthesis n n Synthesis of heme and globin moieties proceeds separately, though not entirely independently the process is controlled by feedback mechanism ¨ e. g. , formation of heme increases the synthesis of globin and lack of heme reduces globin synthesis. Heme molecule: a porphyrin ring with an iron atom at its center (in a ferrous state) ¨ Porphyrins are tetrapyrroles n the four pyrroles linked by a methane bridge Heme synthesis occurs largely in the mitochondria by a series of biochemical reactions involving a number of enzymes and co-factors
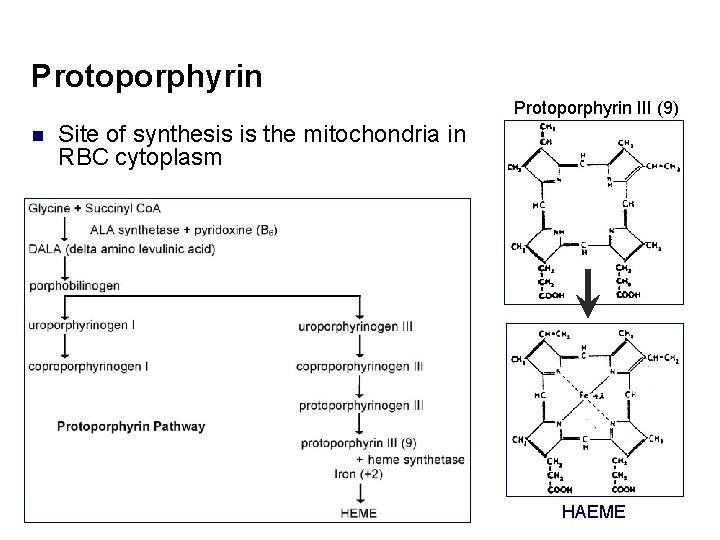
Protoporphyrin III (9) n Site of synthesis is the mitochondria in RBC cytoplasm HAEME

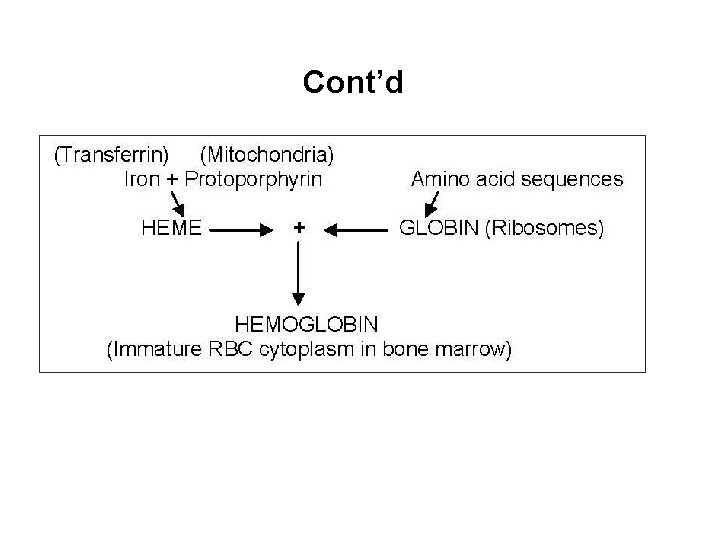
Globin n Globin chains are composed of amino acids arranged in a specific pattern Site of synthesis is the ribosomes 4 normal chain types are produced Alpha chain composed of 141 amino acid chains ¨ Beta chain 146 amino acid chains ¨ Gamma ¨ delta ¨
Cont’d
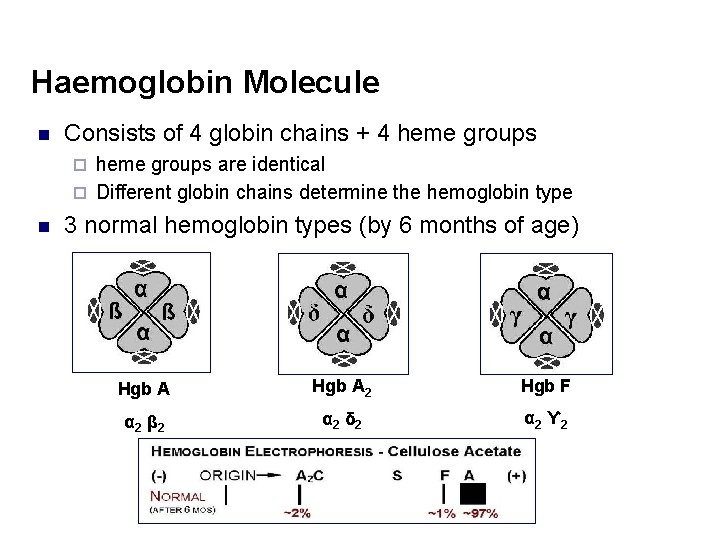
Haemoglobin Molecule n Consists of 4 globin chains + 4 heme groups are identical ¨ Different globin chains determine the hemoglobin type ¨ n 3 normal hemoglobin types (by 6 months of age) Hgb A 2 Hgb F α 2 β 2 α 2 δ 2 α 2 ϒ 2
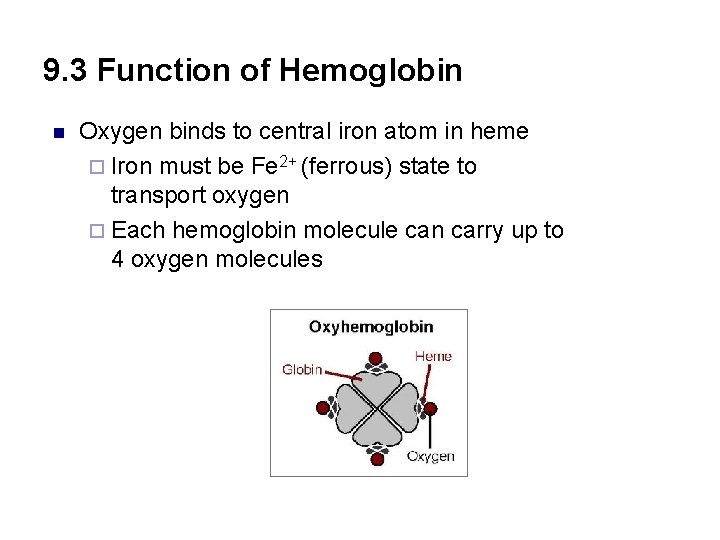
9. 3 Function of Hemoglobin n Oxygen binds to central iron atom in heme ¨ Iron must be Fe 2+ (ferrous) state to transport oxygen ¨ Each hemoglobin molecule can carry up to 4 oxygen molecules
Cont’d n Two normal Hgb forms ¨ Deoxyhemoglobin (Fe 2+ without oxygen), in tissues ¨ Oxyhemoglobin (Fe 2+ with oxygen), in lungs n Two abnormal Hgb forms ¨ Methemoglobin (Fe 3+ , oxidized) ¨ Carboxyhemoglobin (Fe 2+ with CO) n Both are reversible
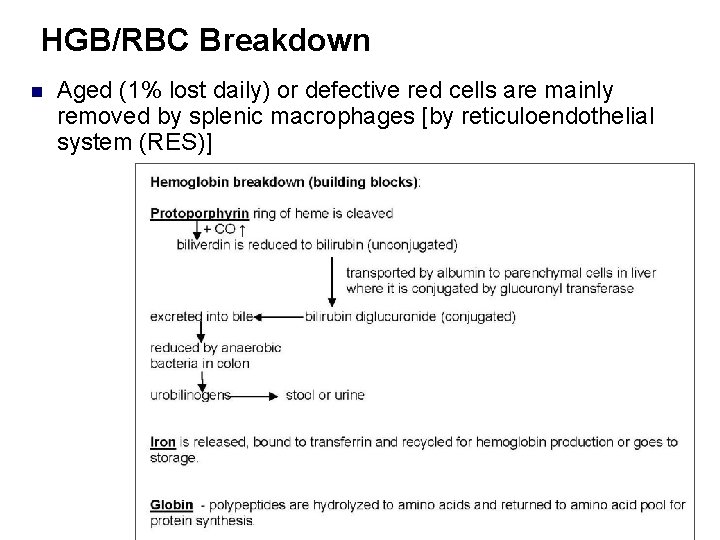
HGB/RBC Breakdown n Aged (1% lost daily) or defective red cells are mainly removed by splenic macrophages [by reticuloendothelial system (RES)]
9. 4. Methods of Hemoglobin Measurement • Is the measurement of concentration of Hgb in red cells (whole blood) • Hgb is reported in g/d. L There are different methods Spectrophotometric a) Cyanmethemoglobin b) Hemo-Cue c) Oxyhemoglobin d) Direct Read- Out (2. )Visual comparative methods a)Sahli - Hellige method b)BMS Hemoglobinometry ( 3) Cu SO 4 specific gravity (1)
I. Spectrophotometric 1. Cyanmethemoglobin method q ICSH recommended reference method ¨ EDTA anticoagulated whole blood or capillary samples ¨ All hemoglobin forms are measured EDTA whole blood

Cont. . Principle: q Blood is diluted in a solution of potassium ferricyanide and potassium cyanide (Drabkin’s solution). The potassium ferricyanide oxidizes hemoglobins to hemiglobin (Hi: methemoglobin) and the potassium cyanide provides CN - ions to form hemiglobin cyanide (Hi. CN) which has a maximum absorption at 540 nm. Finally absorbance of the solution is measured in a photometer or spectrophotometer at 540 nm and compared with that of a standard Hi. CN solution

Reagent n Drabkins solution ¨ Potassium Ferricyanide (Hexacyanoferrata)=K 3 Fe(CN)6 ¨ Potassium cyanide ¨ Potassium dihydrogen phosphate ¨ Highly poisonous; store securely in locked cupboard in light opaque container wrapped in silver foil
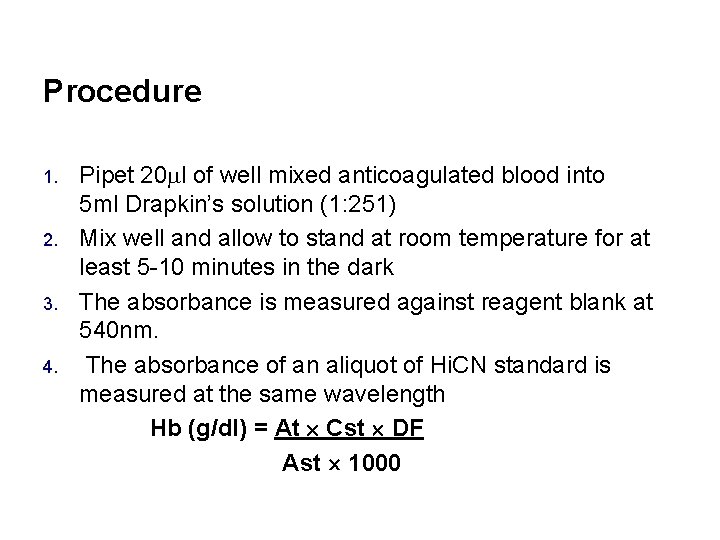
Procedure 1. 2. 3. 4. Pipet 20 l of well mixed anticoagulated blood into 5 ml Drapkin’s solution (1: 251) Mix well and allow to stand at room temperature for at least 5 -10 minutes in the dark The absorbance is measured against reagent blank at 540 nm. The absorbance of an aliquot of Hi. CN standard is measured at the same wavelength Hb (g/dl) = At Cst DF Ast 1000

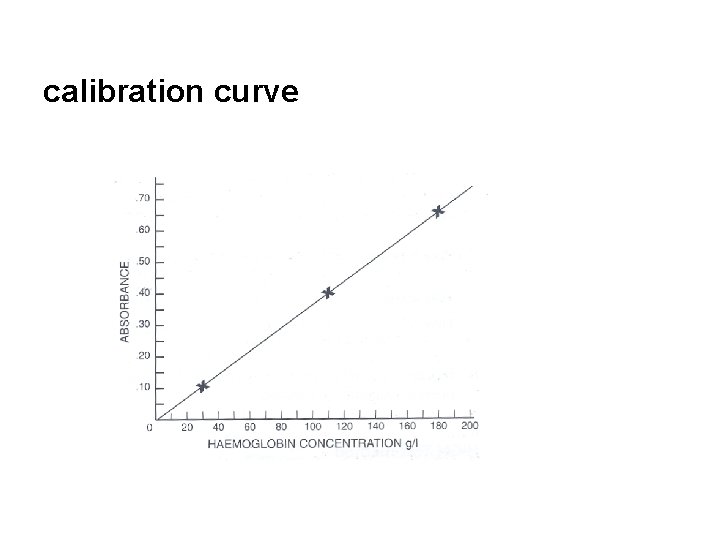
Calibration n n The Hemoglobin Standard is offered as a dry vial containing a standardized amount of methemoglobin prepared from human hemoglobin. Reconstituting the Hemoglobin Standard yields the Cyanmethemoglobin Standard solution. The solution will yield an absorbance equivalent to that of whole blood sample containing a hemoglobin level of 18 g/dl that has been diluted 1: 251 with Drabkin's solution. Dilutions of the Cyanmethemoglobin Standard Solution with Drabkin's solution are used to prepare a calibration curve as follows:
calibration curve

Reference range: n n Adult males: 13 -18 g/dl Adult females: 11 -16 g/dl Newborns: 14 -23 g/dl Note: reference values vary with age, sex, physiologic condition, altitude, etc. Thus local reference values should established.

Advantages: n n Stable Hemiglobincyanide standard available to calibrate instrument Convenient method Readily available and stable standard solution (readings need not be made immediately after dilution) All forms of hemoglobin except sulfhemoglobin (SHb) are readily converted to Hi. CN.
Sources of error when measuring Hemoglobin photometrically n n n n Not measuring the correct volume of blood due to poor technique or using a wet or chipped pipet. When using anticoaglulated venous blood, not mixing the sample sufficiently. Not ensuring that the optical surfaces of a cuvet are clean and dry Air bubbles in the solution to be measured Wrong wavelength Improper instrument calibration Reagent exposed to light
Sources of error cont’d n n Lipemia Extremely high WBC count causes cloudiness Presence of abnormal Hemoglobins Presence of abnormal proteins Note: n Lipemia causes an increase in the Hb result due to cloudiness in the solution read by the spectrophotometer. ¨ In lipemia, centrifugation can clear the specimen and the supernatant reading will be accurate. n Abnormal Hemoglobins or proteins are not lysed by the reagent, so again the solution is cloudier which makes the instrument read the Hemoglobin result higher than it is.
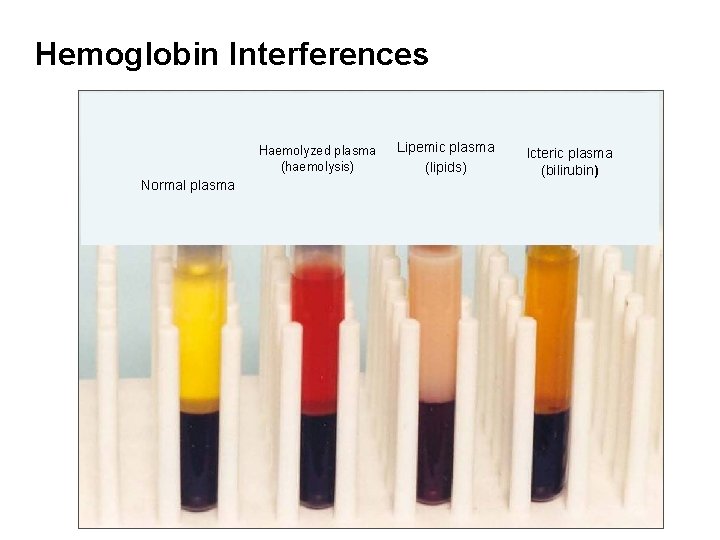
Hemoglobin Interferences Haemolyzed plasma (haemolysis) Normal plasma Lipemic plasma (lipids) Icteric plasma (bilirubin)
2. Hemoglobin - Hemo. Cue® n Hemo. Cue® photometer ¨ Uses dry reagent system (cuvetes) ¨ Determines concentration of azide methemoglobin photometrically ¨ Electronic check and whole blood control samples must be run to monitor instrument function and reagent
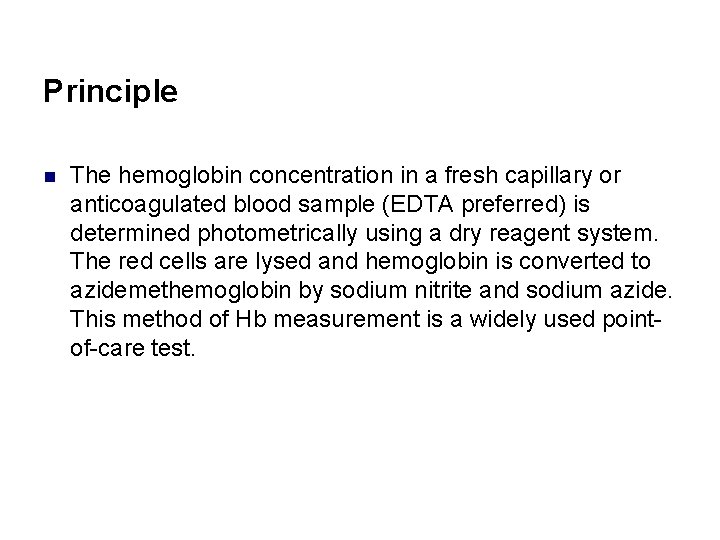
Principle n The hemoglobin concentration in a fresh capillary or anticoagulated blood sample (EDTA preferred) is determined photometrically using a dry reagent system. The red cells are lysed and hemoglobin is converted to azidemethemoglobin by sodium nitrite and sodium azide. This method of Hb measurement is a widely used pointof-care test.
Hemo. Cue® cont’d Procedure 1. Turn on Hemo. Cue® instrument 2. Run electronic calibration check (red control cuvette) 3. Fill specimen cuvette with EDTA or capillary blood in a continuous process without bubbles. 4. Place cuvette in instrument, insert to ‘measure’ position ¨ Hemoglobin result will be displayed in g/d. L n
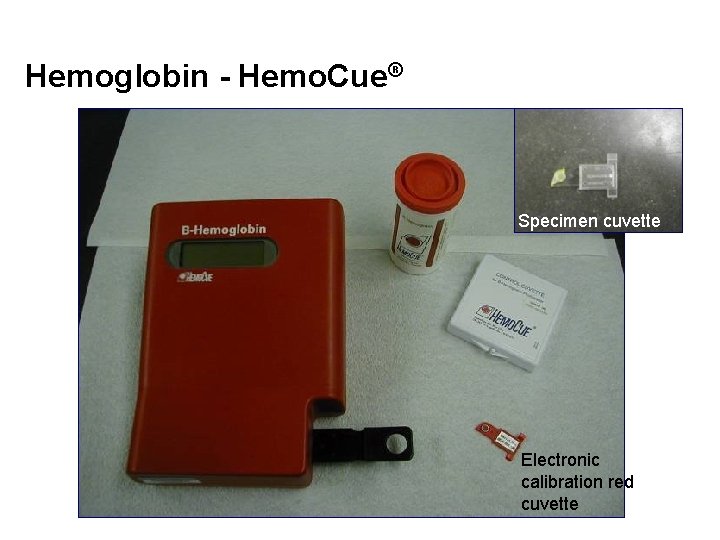
Hemoglobin - Hemo. Cue® Specimen cuvette Electronic calibration red cuvette

Hemocue Cont’d n n Advantage Hemo. Cue® system ¨ No dilution necessary ¨ The instrument reads the result when it is ready (no need to let stand for lysing of RBCs) and result is reported directly eliminating errors in reading from a calibration chart ¨ High accuracy ¨ No expensive instrument needed Disadvantage: ¨ Test cuvettes are expensive ¨ Finger prick technique must be good
Sources of error Hemo. Cue® method ¨ Failure to properly collect the blood sample if done as a capillary collection n Blood not collected from a free flowing finger prick ¨ Failure to fill cuvette properly ¨ If not, read within 10 minutes of collection

Quality control n n Spectrophotometer/photometer ¨ Whole blood control must be performed ¨ Performed in duplicate; should match within plus/minus 0. 5 g/dl ¨ Calibrator for making a standard curve Hemo. Cue® ¨ Calibrator cartridge ¨ Whole blood control
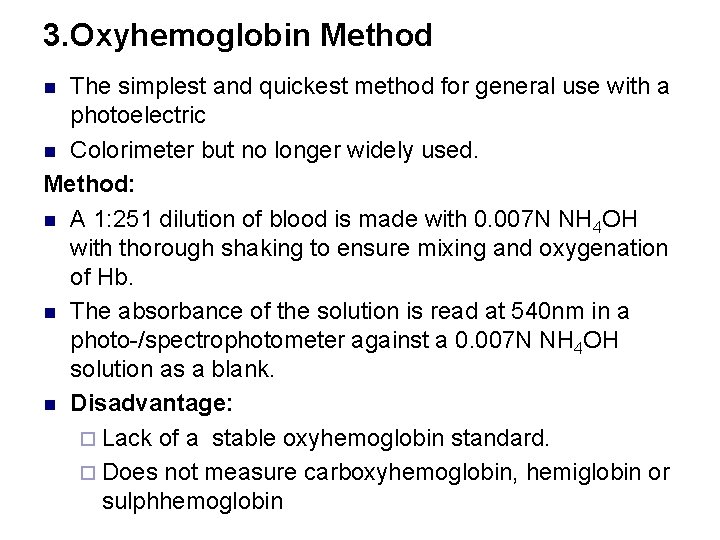
3. Oxyhemoglobin Method The simplest and quickest method for general use with a photoelectric n Colorimeter but no longer widely used. Method: n A 1: 251 dilution of blood is made with 0. 007 N NH 4 OH with thorough shaking to ensure mixing and oxygenation of Hb. n The absorbance of the solution is read at 540 nm in a photo-/spectrophotometer against a 0. 007 N NH 4 OH solution as a blank. n Disadvantage: ¨ Lack of a stable oxyhemoglobin standard. ¨ Does not measure carboxyhemoglobin, hemiglobin or sulphhemoglobin n
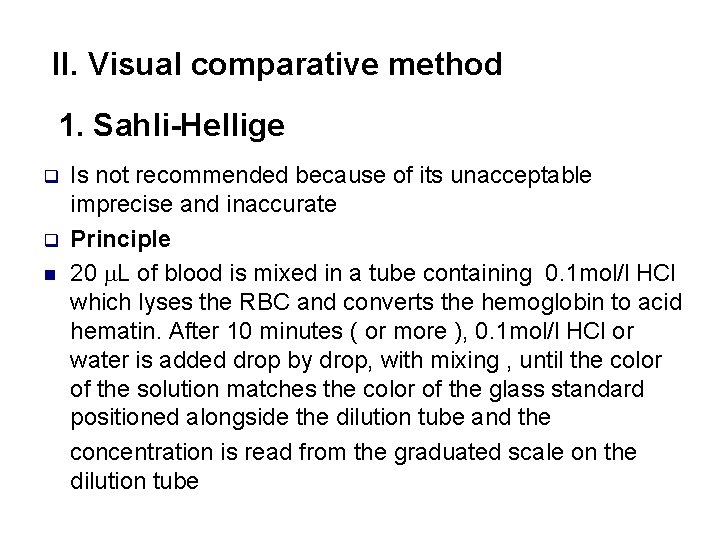
II. Visual comparative method 1. Sahli-Hellige q q n Is not recommended because of its unacceptable imprecise and inaccurate Principle 20 L of blood is mixed in a tube containing 0. 1 mol/l HCl which lyses the RBC and converts the hemoglobin to acid hematin. After 10 minutes ( or more ), 0. 1 mol/l HCl or water is added drop by drop, with mixing , until the color of the solution matches the color of the glass standard positioned alongside the dilution tube and the concentration is read from the graduated scale on the dilution tube

Procedure: 1. Fill the graduated tube to the ''20'' mark of the red graduation or to the 3 g/l mark of the yellow graduation with 0. 1 N HCl. 2. Draw venous or capillary blood to the 20μl mark of the Sahli pipet. Do not allow air bubbles to enter into the Sahli pipet. ¨ With EDTA anticoagulated venous blood ensure that it is well mixed by inverting the tube repeatedly for about 1 minute immediately before pipetting it. ¨ If using capillary blood, wipe away the first drop of blood from the finger.

Cont. . 3. Wipe the outside of the pipet with absorbent paper. ¨ Check that the blood is still on the “ 20” mark. 4. Blow the blood from the pipet into the graduated tube containing the 0. 1 N hydrochloric acid solution. 5. Rinse the pipet by drawing and blowing out the acid solution 3 times.

Cont. . 6. Place the graduated tube in the hemoglobinometer stand facing a window. 7. Allow 10 minutes for RBC lysis and formation of acid hematin 8. Compare the color of the tube containing diluted blood with the color of the standard § If the color of the diluted sample is darker than that of the reference, continue to dilute by adding 0. 1 N HCl or distilled water drop by drop. 9. Stir with the glass rod after adding each drop.

Cont. . 10. Remove the rod and compare the color of the tube with the standard columns. 11. Stop when the colors match. 12. Note the mark reached. n Depending on the type of hemoglobinometer, this gives the hemoglobin concentration either in g/dl or as a percentage of ''normal''. n To convert percentages to g/dl, multiply the reading by 0. 146.

Disadvantage n n n . Fading of the color glass standard and difficulty in matching it to the acid hematin solution. Conversion to acid –hematin is slow ¨ Because of this, all red cells may not lyse and Hb may not converted to Acid Hematin in specified lesser time resulting a falsely decreased Hgb value Acid Hematin is unstable
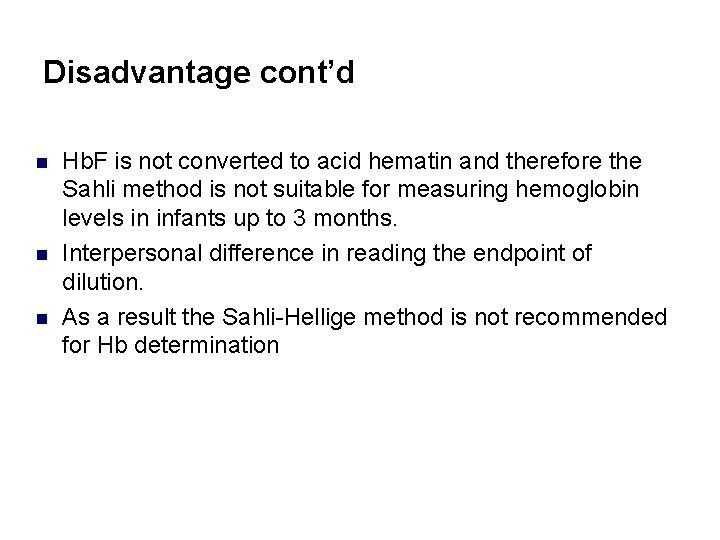
Disadvantage cont’d n n n Hb. F is not converted to acid hematin and therefore the Sahli method is not suitable for measuring hemoglobin levels in infants up to 3 months. Interpersonal difference in reading the endpoint of dilution. As a result the Sahli-Hellige method is not recommended for Hb determination

Materials: n n n Sahli hemoglobinometer Sahli pipet Stirring glass rod Dropping pipet Absorbent cotton 0. 1 N HCl
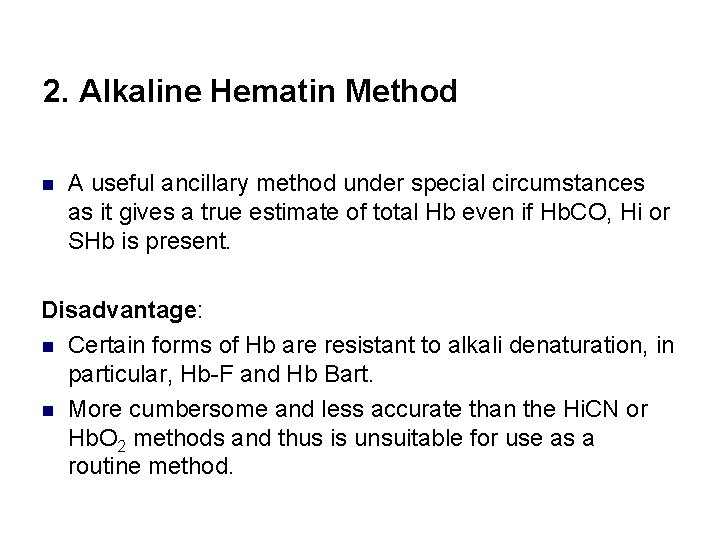
2. Alkaline Hematin Method n A useful ancillary method under special circumstances as it gives a true estimate of total Hb even if Hb. CO, Hi or SHb is present. Disadvantage: n Certain forms of Hb are resistant to alkali denaturation, in particular, Hb-F and Hb Bart. n More cumbersome and less accurate than the Hi. CN or Hb. O 2 methods and thus is unsuitable for use as a routine method.
3. BMS Hemoglobinometer n n Requires no dilution of blood Use in clinics with a few Hb tests are performed Principle n A drop of blood is mixed with a saponin impregnated stick. This lyses the red cells giving a clear solution of Hb. The absorption of light by the hemolysed patient blood sample ( existing in one half of the field of view of the meter ) is matched with a scale on the meter( that of the standard in the other half). The Hb value in g/dl is obtained from a scale on the meter

WHO Hb colour scale n The method is based on comparing the color of a drop of blood absorbed on a particular type of chromatographic paper against a printed scale of colors corresponding to different levels of Hb

III. Copper Sulphate Densitometery n n n This is a qualitative method based on the capacity of a standard solution of copper sulphate to cause the suspension or sinking of a drop of blood. The measurement of specific gravity of a sample of blood corresponds to its hemoglobin concentration. The method is routinely utilized in some blood banking laboratories while screening blood donors for the presence of anemia.

Sources of Error n Pre-analytical errors are a common cause of inaccurate results ¨ wrong patient identification ¨ improper venous blood sample collection ¨ Wrong anticoagulant type and concentration ¨ Failure to mix blood with anticoagulant ¨ Mislabeling ¨ improper capillary blood collection ¨ Clotted sample
Cont’d ¨ Hemoconcentration ¨ Hemodilution ¨ Hemolysis does not cause hemoglobin error ¨ Technical errors (failure to adhere to SOP) ¨ Post analytic errors Do controls detect specimen collection errors?
Quality Control n n n Control samples monitor the correct functioning of equipment, stability of reagents and testing technique Controls do not detect specimen collection errors Control samples with known assayed values are used to check result reliability ¨ Controls detect invalid results caused by errors in testing technique, reagents or instrument malfunction
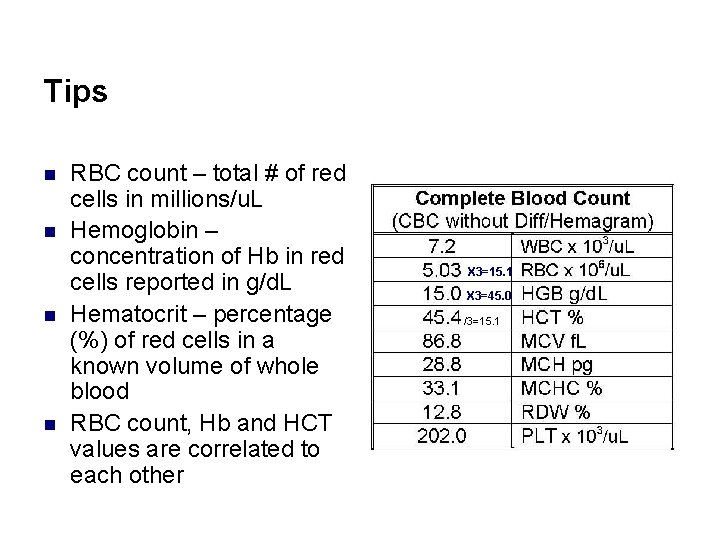
Tips n n RBC count – total # of red cells in millions/u. L Hemoglobin – concentration of Hb in red cells reported in g/d. L Hematocrit – percentage (%) of red cells in a known volume of whole blood RBC count, Hb and HCT values are correlated to each other X 3=15. 1 X 3=45. 0 /3=15. 1
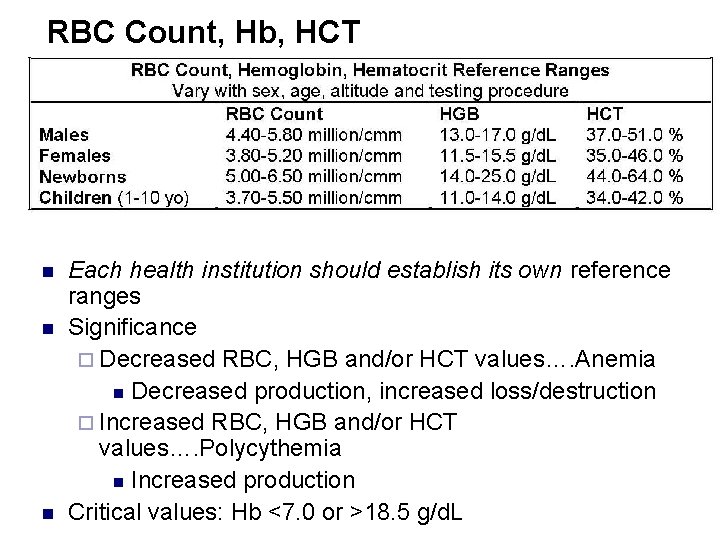
RBC Count, Hb, HCT n n n Each health institution should establish its own reference ranges Significance ¨ Decreased RBC, HGB and/or HCT values…. Anemia n Decreased production, increased loss/destruction ¨ Increased RBC, HGB and/or HCT values…. Polycythemia n Increased production Critical values: Hb <7. 0 or >18. 5 g/d. L
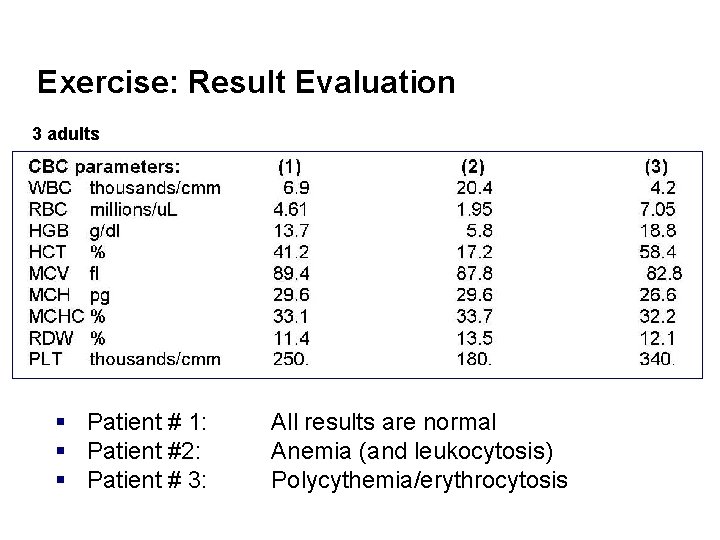
Exercise: Result Evaluation 3 adults § Patient # 1: § Patient #2: § Patient # 3: All results are normal Anemia (and leukocytosis) Polycythemia/erythrocytosis
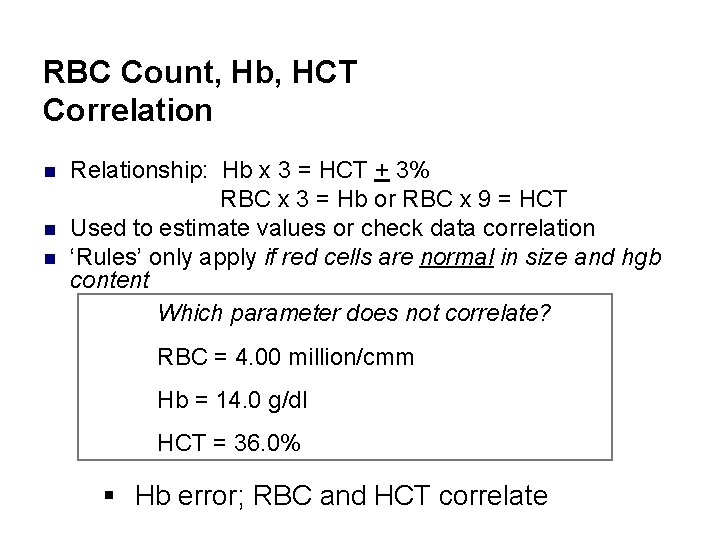
RBC Count, Hb, HCT Correlation n Relationship: Hb x 3 = HCT + 3% RBC x 3 = Hb or RBC x 9 = HCT Used to estimate values or check data correlation ‘Rules’ only apply if red cells are normal in size and hgb content Which parameter does not correlate? RBC = 4. 00 million/cmm Hb = 14. 0 g/dl HCT = 36. 0% § Hb error; RBC and HCT correlate
Summary n n n Biochemistry of the hemoglobin molecule Cyanmethaemoglobin method (or modifications) of hemoglobin determination, including specimen requirements Sources of specimen collection error (pre-analytic) that can cause inaccurate results Potential sources of error when measuring hemoglobin photometrically (i. e. , substances that interfere with photometric measurements) Quality control and checks utilized to establish test validity and prevent erroneous results

Review Questions 1. 2. 3. 4. 5. Describe synthesis of the heme and globin moieties of hemoglobin Summarize the functions of hemoglobin in the body. What are the two most commonly applied color comparison methods for measurement of hemoglobin in a sample of blood? Write the test principle of each of these methods. Compare and contrast (in terms of accuracy, advantage, drawbacks, etc. ) the two routine methods of hemoglobin quantitation. What is the clinical implication of hemoglobin measurement in a sample of blood?

Review Questions cont’d 6. 7. List at least five pre-analytic errors and their remedies in Hb determination List at least five possible sources of error and their remedies in Hb determination using photomertic methods
- Slides: 59