Chapter 9 Heat Honors Physics Temperature Adding or






















- Slides: 39

Chapter 9 “Heat” Honors Physics

Temperature • Adding or removing energy changes the temperature. • Temperature is the average KE of the atoms and molecules present. High avg. KE = high temp. • We use relative scales: ºF , ºC and absolute scales: K

Temperature • Internal energy (ΔU) is the energy associated with atomic motion and is proportional to the substance's temperature. • Temperature is changed by adding or removing energy.

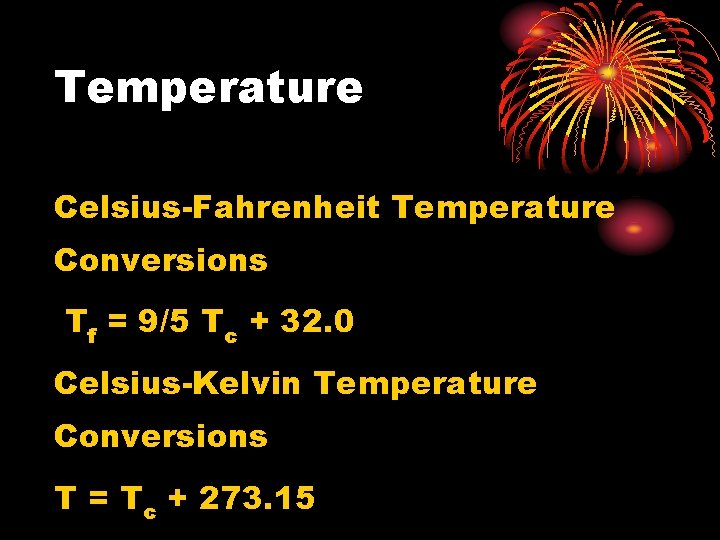
Temperature Celsius-Fahrenheit Temperature Conversions Tf = 9/5 Tc + 32. 0 Celsius-Kelvin Temperature Conversions T = Tc + 273. 15

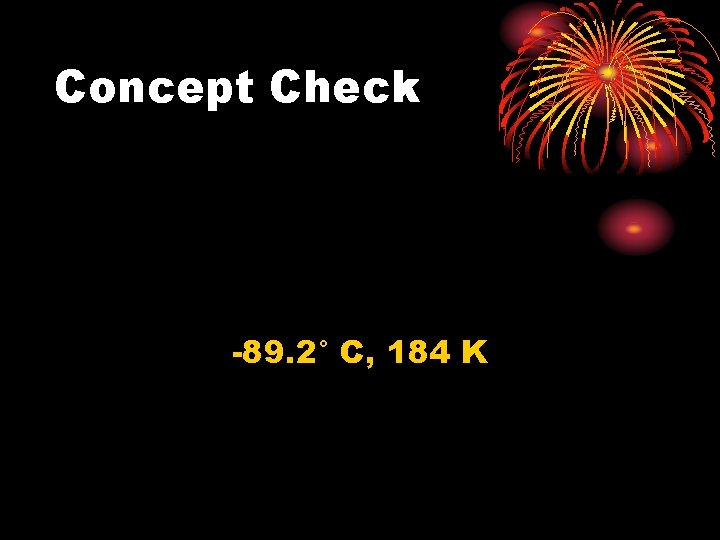
Concept Check The lowest outdoor temperature ever recorded on Earth is -128. 6°F, recorded at Vostok Station, Antarctica in 1983. What is this temperature on the Celsius and Kelvin scales?

Concept Check -89. 2° C, 184 K

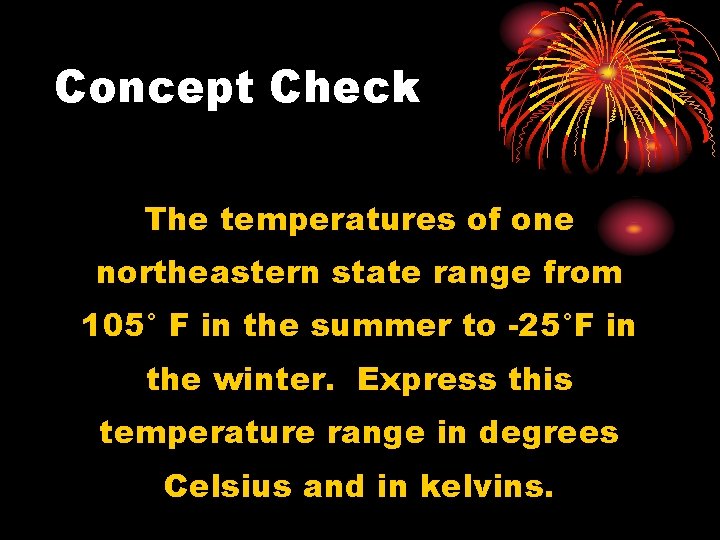
Concept Check The temperatures of one northeastern state range from 105° F in the summer to -25°F in the winter. Express this temperature range in degrees Celsius and in kelvins.

Concept Check 41°C to -32°C, 314 K to 241 K

Concept Check A pan of water is heated from 23°C to 78°C. What is the change in its temperature on the Kelvin and Fahrenheit scales?

Concept Check 55 K, 99°F

Concept Check Liquid nitrogen is used to cool substances to very low temperatures. Express the boiling point of liquid nitrogen (77. 34 K at 1 atm of pressure) in degrees Celsius and in degrees Fahrenheit.

Concept Check -195. 81°C, -320. 5°F

Heat • Heat is the energy transferred between objects due to the difference in their temperatures. • Heat will naturally go from objects of high KE to objects of low KE. To go in the opposite direction requires work.

Thermal (Heat) Units • We’ll be mostly using joules (J) or calories (cal). However, you should be aware that there are others (p 307). • 1 calorie is equal to the amount of energy required to heat 1 gram of water from 4º to 5 ºC. • 1 cal = 4. 184 J

Total energy is conserved. • Conservation of energy states that the sum of the change in the potential energy and the change in kinetic energy and the change in internal energy must equal zero. PE + KE + U = 0

Concept Check Consider an arrangement similar to the one used to demonstrate energy conservation on p. 310. If a total mass of 11. 5 kg falls 6. 69 m and all of the mechanical energy is converted to internal energy, by how much will the internal energy of the water increase?

Concept Check 755 J

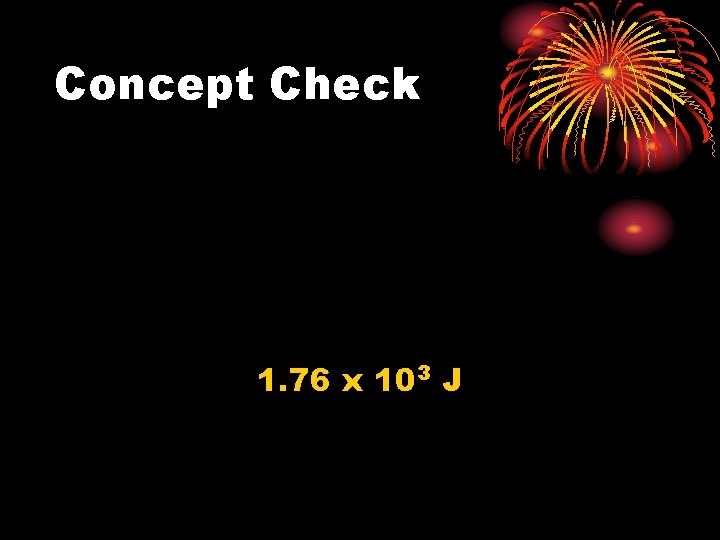
Concept Check A worker drives a 0. 500 kg spike into a rail tie with a 2. 50 kg sledgehammer. The hammer hits the spike with a speed of 65. 0 m/s. If one-third of the hammer's kinetic energy is converted to the internal energy of the hammer and spike, how much does the total internal energy increase?

Concept Check 1. 76 x 103 J

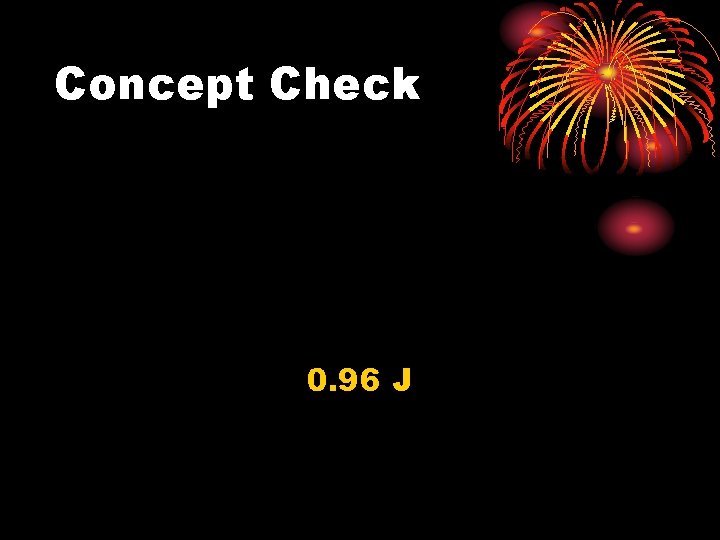
Concept Check A 3. 0 x 10 -3 kg copper penny drops a distance of 50. 0 m to the ground. If 65% of the initial potential energy goes into increasing the internal energy of the penny, determine the magnitude of that increase.

Concept Check 0. 96 J

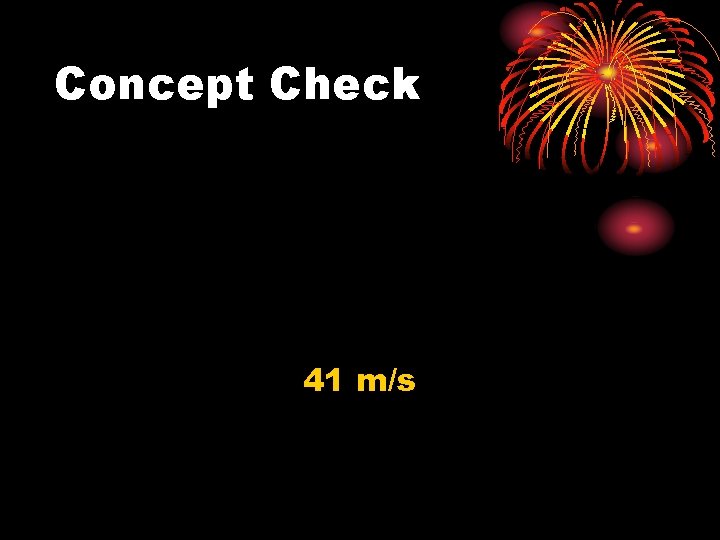
Concept Check The amount of internal energy needed to raise the temperature of 0. 25 kg of water by 0. 2ºC is 209. 3 J. How fast must a 0. 25 kg baseball travel in order for its kinetic energy to equal this internal energy?

Concept Check 41 m/s

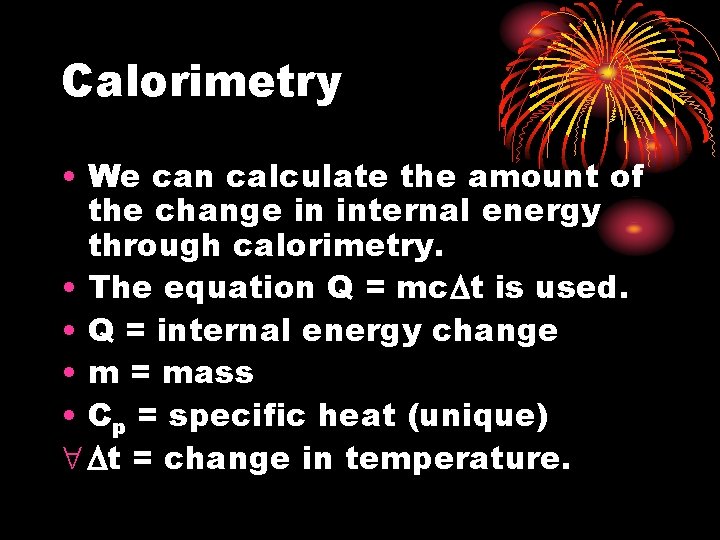
Calorimetry • We can calculate the amount of the change in internal energy through calorimetry. • The equation Q = mc t is used. • Q = internal energy change • m = mass • Cp = specific heat (unique) t = change in temperature.

Heat lost = Heat gained • In an insulated situation (no heat escapes into the surroundings): Q lost = -Q gained • m 1 cp 1 t 1 = -m 2 cp 2 t 2

Calorimetry • Generally, we use water as the substance that gains the heat since the specific heat of water is known.

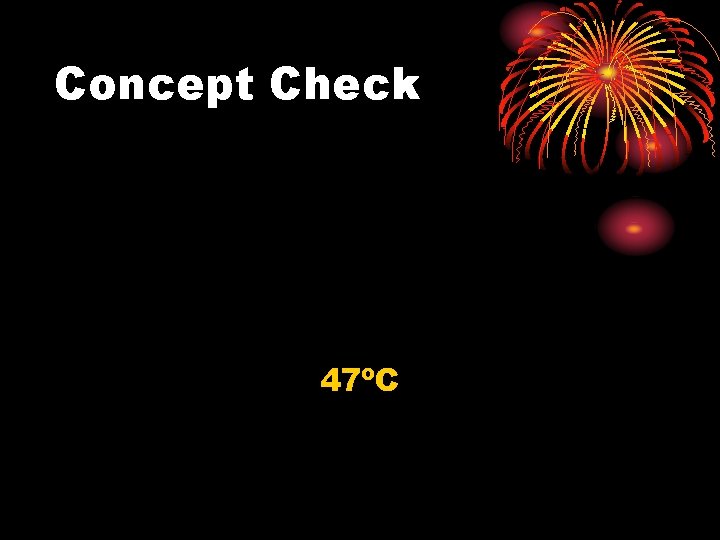
Concept Check What is the final temperature when a 3. 0 kg gold bar at 99ºC is dropped into 0. 22 kg of water at 25ºC? (Use the table on p. 314. )

Concept Check 47ºC

Concept Check A 0. 225 kg sample of tin initially at 97. 5ºC is dropped into 0. 115 kg of water. The initial temperature of the water is 10. 0ºC. If the specific heat capacity of tin is 230 J/kg*ºC, what is the final equilibrium temperature of the tin-water mixture?

Concept Check 18ºC

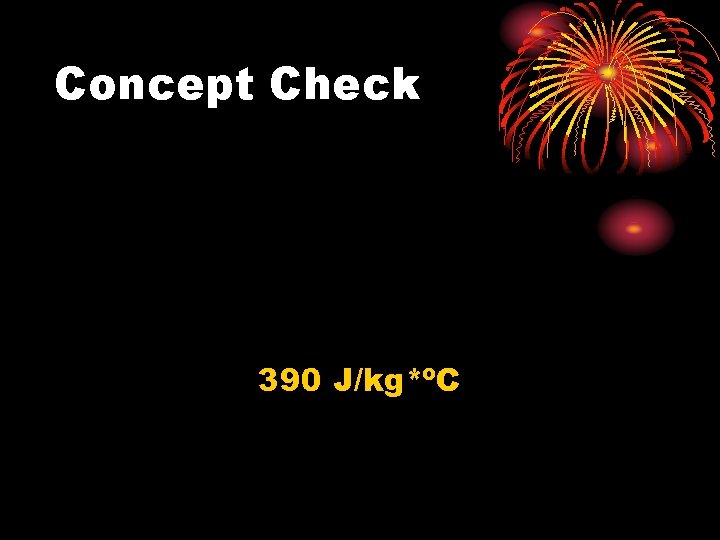
Concept Check Brass is an alloy made from copper and zinc. A 0. 59 kg brass sample at 98. 0ºC is dropped into 2. 80 kg of water at 5. 0ºC. If the equilibrium temperature is 6. 8ºC, what is the specific heat capacity of brass?

Concept Check 390 J/kg*ºC

Concept Check A hot, just minted copper coin is placed in 101 g of water to cool. The water temperature changes by 8. 39ºC, and the temperature of the coin changes by 68. 0ºC. What is the mass of the coin?

Concept Check 135 g

Phase Changes • Phase changes occur when an object goes from one phase of matter to another. • When an object is going through a phase change, added energy is used to make the change without raising the temperature. This is latent heat.

Equations for Phase Changes. • During heating (temperature change) use Q = mc t • During phase changes use heat of fusion or heat of vaporization. • Q = m. Lf or Q = m. Lv • L = latent heat (unique)

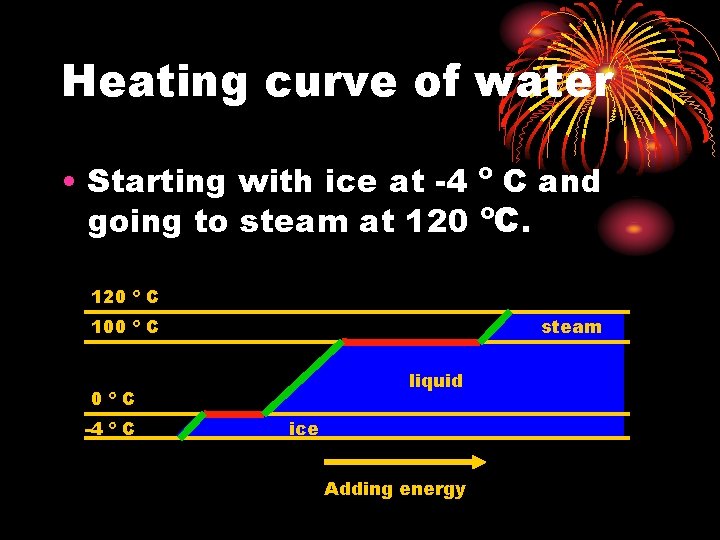
Heating curve of water • Starting with ice at -4 º C and going to steam at 120 ºC. 120 º C 100 º C steam liquid 0ºC -4 º C ice Adding energy

Types of Heat Transfer • Thermal Conduction-requires objects to touch in order to transfer heat. • Convection-requires the motion of a fluid between objects in order to transfer heat. • Radiation-energy moves in the form of electromagnetic radiation in order to transfer heat.

Conduction vs insulation • The ability to conduct heat is a gradient from good conductors such as metals to poor conductors such as ceramics or plastics. Poor conductors are good insulators. An insulator doesn’t actually inhibit conduction, it is just a poor conductor.