Chapter 9 Energy and Hydrocarbons Chapter Outline Energy

Chapter 9 Energy and Hydrocarbons

Chapter Outline Energy from Fuels Alkanes: Backbone of Organic Chemistry Petroleum Refining Octane ratings
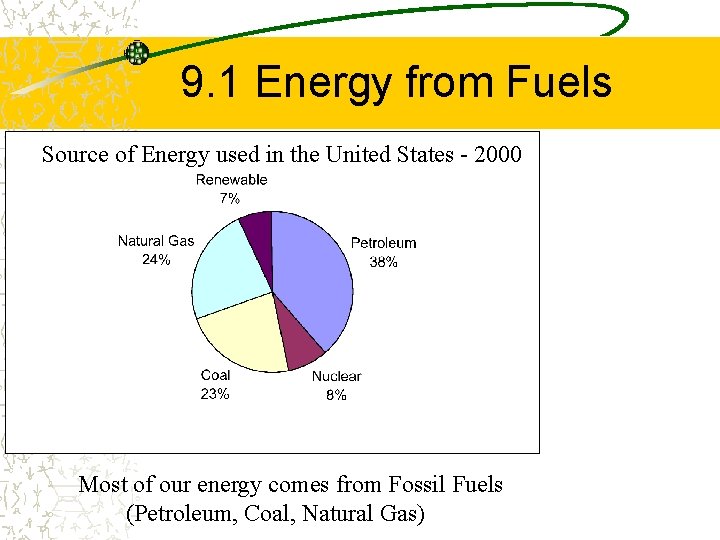
9. 1 Energy from Fuels Source of Energy used in the United States - 2000 Most of our energy comes from Fossil Fuels (Petroleum, Coal, Natural Gas)
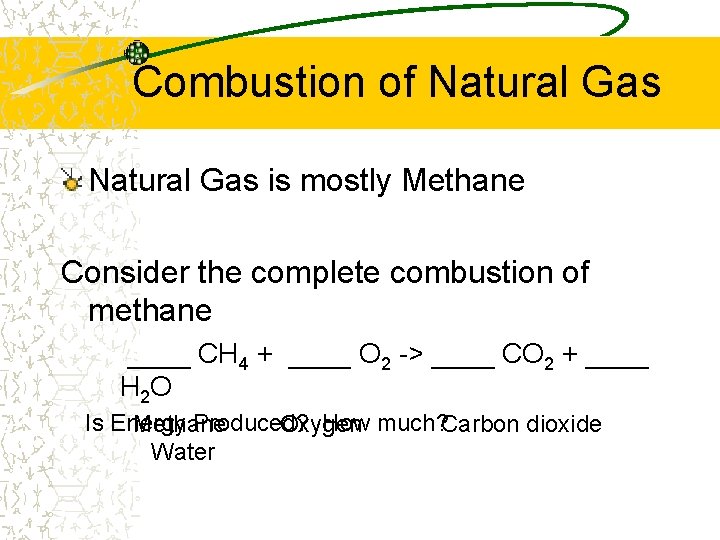
Combustion of Natural Gas is mostly Methane Consider the complete combustion of methane ____ CH 4 + ____ O 2 -> ____ CO 2 + ____ H 2 O Is Energy Produced? How much? Carbon dioxide Methane Oxygen Water

What determines if Energy is released or absorbed in a chemical reaction? Bond Energies!!
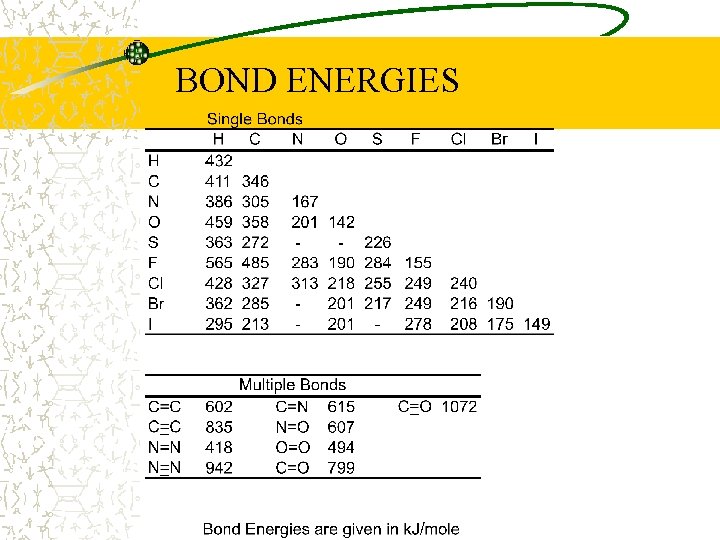
BOND ENERGIES
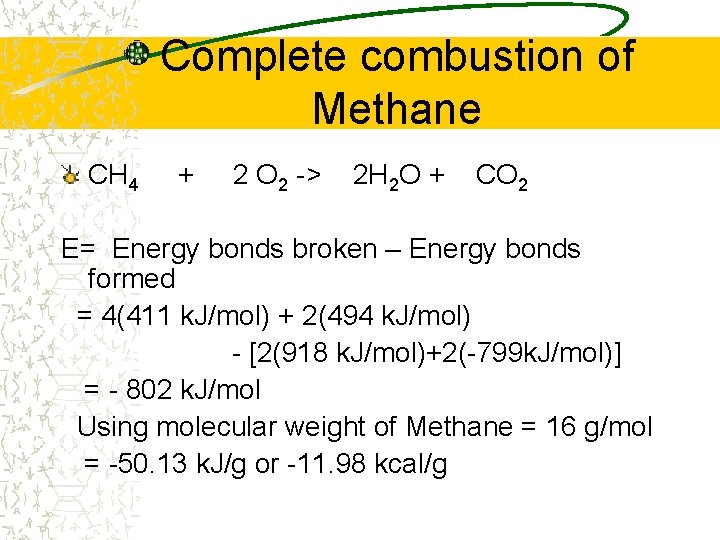
Complete combustion of Methane CH 4 + 2 O 2 -> 2 H 2 O + CO 2 E= Energy bonds broken – Energy bonds formed = 4(411 k. J/mol) + 2(494 k. J/mol) - [2(918 k. J/mol)+2(-799 k. J/mol)] = - 802 k. J/mol Using molecular weight of Methane = 16 g/mol = -50. 13 k. J/g or -11. 98 kcal/g
Your Turn Let’s try Propane and Gasohol Propane is C 3 H 8 Gasohol is ethanol CH 3 CH 2 OH
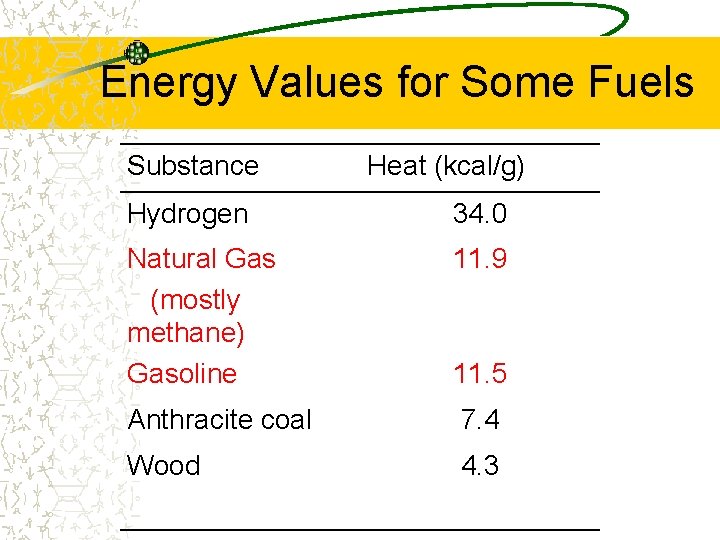
Energy Values for Some Fuels Substance Heat (kcal/g) Hydrogen 34. 0 Natural Gas (mostly methane) Gasoline 11. 9 Anthracite coal 7. 4 Wood 4. 3 11. 5

9. 2 Alkanes: Backbone of Organic Chemistry Naming Hydrocarbons!

Alkanes The simplest alkane is methane – the primary component in Natural Gas. Some Definitions: Alkanes - contain carbon-carbon single bonds Alkenes – contain carbon-carbon double bonds Alkynes – contain carbon-carbon triple
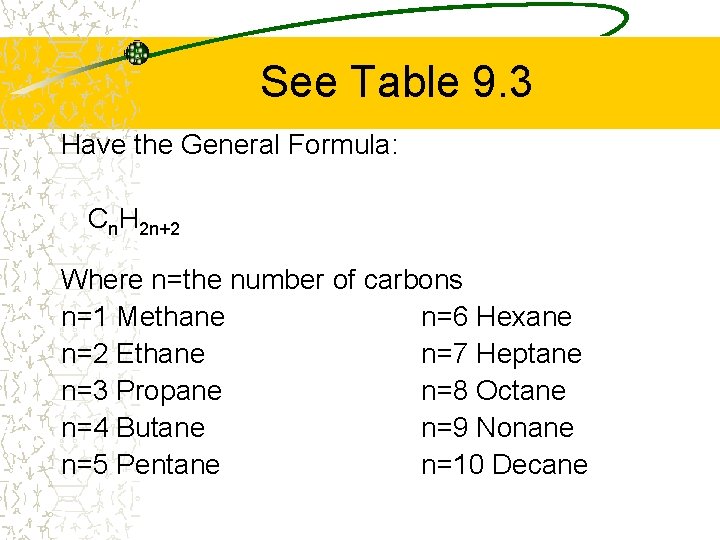
See Table 9. 3 Have the General Formula: Cn. H 2 n+2 Where n=the number of carbons n=1 Methane n=6 Hexane n=2 Ethane n=7 Heptane n=3 Propane n=8 Octane n=4 Butane n=9 Nonane n=5 Pentane n=10 Decane
Straight and Branched-Chain Isomers of Alkanes Methyl – Ethyl Propyl Isopropyl – Butyl – tert-Butyl -

Naming Branched Chain Alkanes IUPAC (International Union of Pure and Applied Chemistry) Find the longest chain of Carbons(this determines the parent name) Number them from the end with the most branching. When two or more substituents are identical, indicate this by the use of prefixes di, tri, tetra, etc. If there are more different groups , the groups
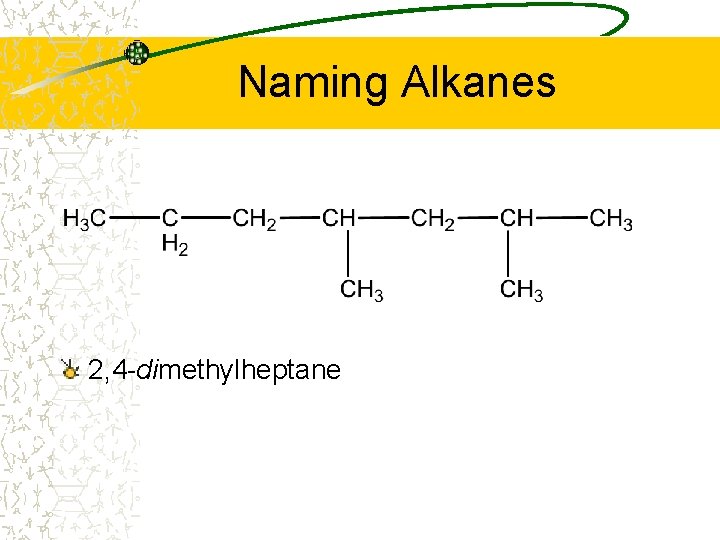
Naming Alkanes 2, 4 -dimethylheptane
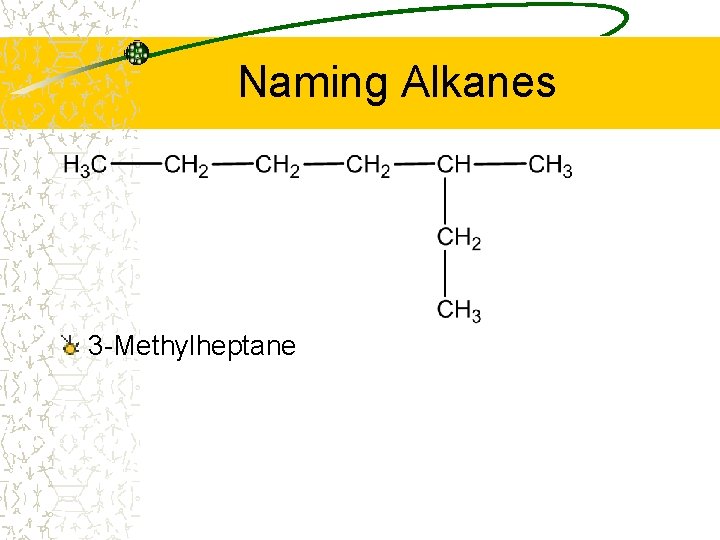
Naming Alkanes 3 -Methylheptane
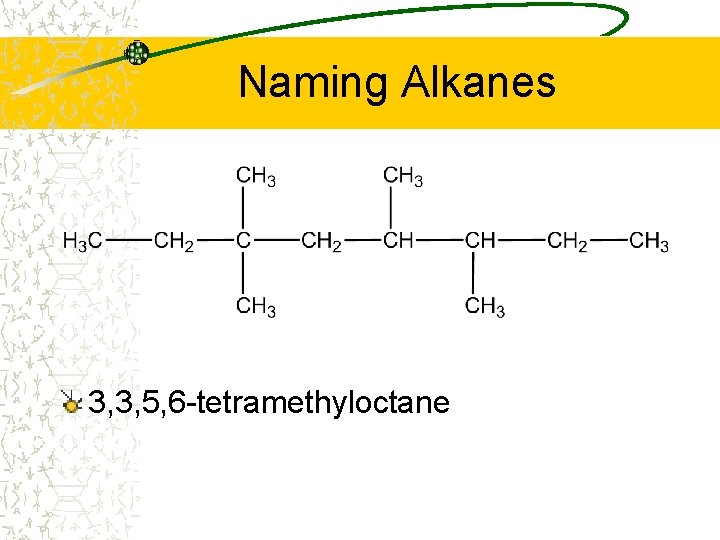
Naming Alkanes 3, 3, 5, 6 -tetramethyloctane
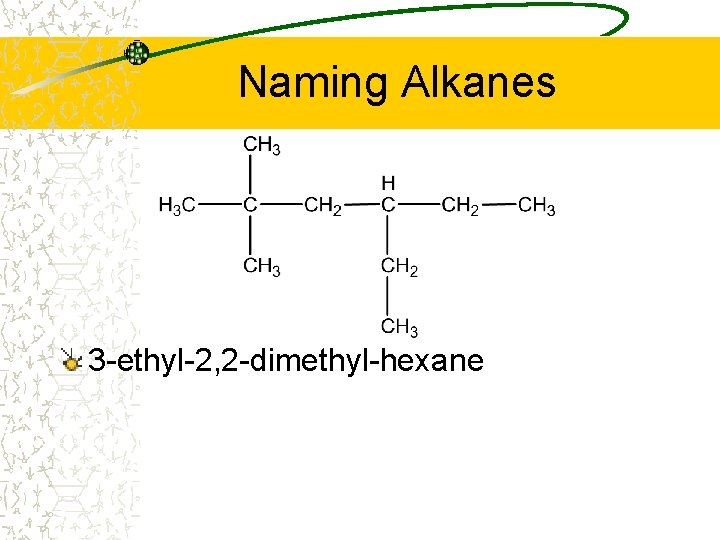
Naming Alkanes 3 -ethyl-2, 2 -dimethyl-hexane
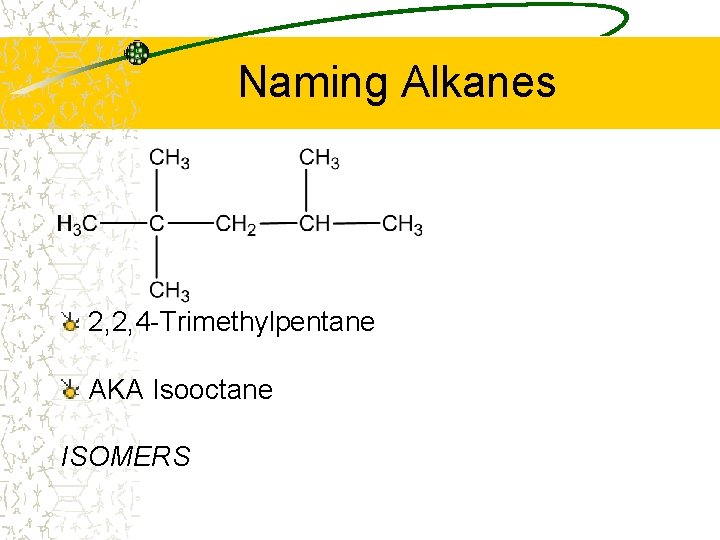
Naming Alkanes 2, 2, 4 -Trimethylpentane AKA Isooctane ISOMERS
- Slides: 19