Chapter 9 Electron Configuration and Periodic Trends 9






- Slides: 27

Chapter 9 Electron Configuration and Periodic Trends 9. 6 Periodic Trends of the Elements 1 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

Valence Electrons The valence electrons • Determine the chemical properties of the elements. • Are the electrons in the s and p sublevels in the highest energy level. • Are related to the Group number of the element. Example: Phosphorus has 5 valence electrons P Group 5 A(15) 2 1 s 22 p 6 3 s 23 p 3

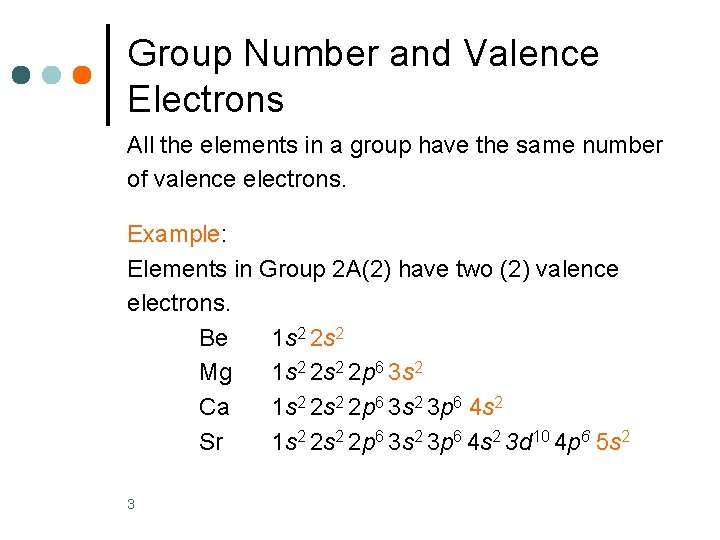
Group Number and Valence Electrons All the elements in a group have the same number of valence electrons. Example: Elements in Group 2 A(2) have two (2) valence electrons. Be 1 s 2 2 s 2 Mg 1 s 2 2 p 6 3 s 2 Ca 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 Sr 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 3

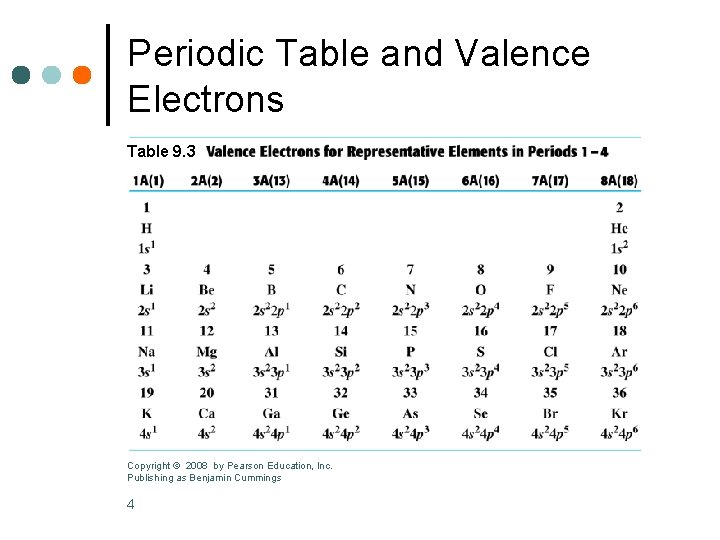
Periodic Table and Valence Electrons Table 9. 3 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 4

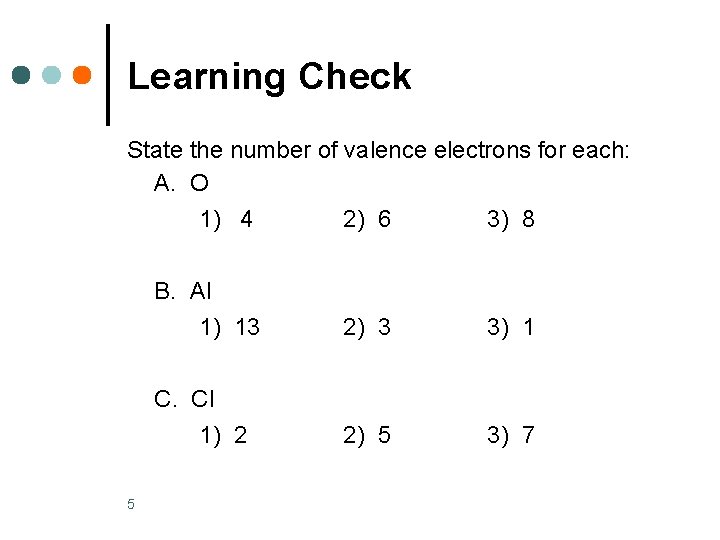
Learning Check State the number of valence electrons for each: A. O 5 1) 4 2) 6 3) 8 B. Al 1) 13 2) 3 3) 1 C. Cl 1) 2 2) 5 3) 7

Solution State the number of valence electrons for each. A. O 2) 6 B. Al 2) 3 C. Cl 3) 7 6

Learning Check State the number of valence electrons for each. A. Calcium 1) 1 2) 2 3) 3 B. Group 6 A (16) 1) 2 2) 4 3) 6 C. Tin 1) 2 2) 4 3) 14 7

Solution State the number of valence electrons for each. A. Calcium 2) 2 B. Group 6 A (16) 3) 6 C. Tin 2) 4 8

Learning Check State the number of valence electrons for each. A. 1 s 2 2 p 6 3 s 2 3 p 3 B. 1 s 2 2 p 6 3 s 2 3 p 64 s 2 3 d 104 p 4 C. 1 s 22 p 5 9

Solution State the number of valence electrons for each. A. 1 s 2 2 p 6 3 s 2 3 p 3 5 B. 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 4 6 C. 1 s 22 p 5 7 10

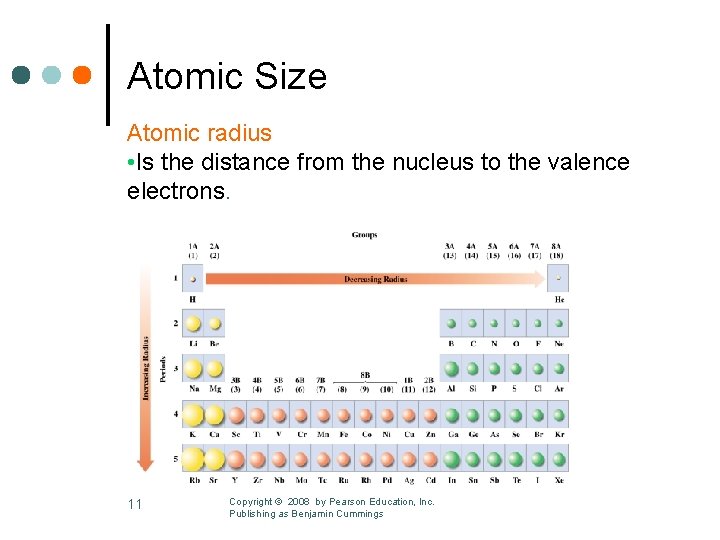
Atomic Size Atomic radius • Is the distance from the nucleus to the valence electrons. 11 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

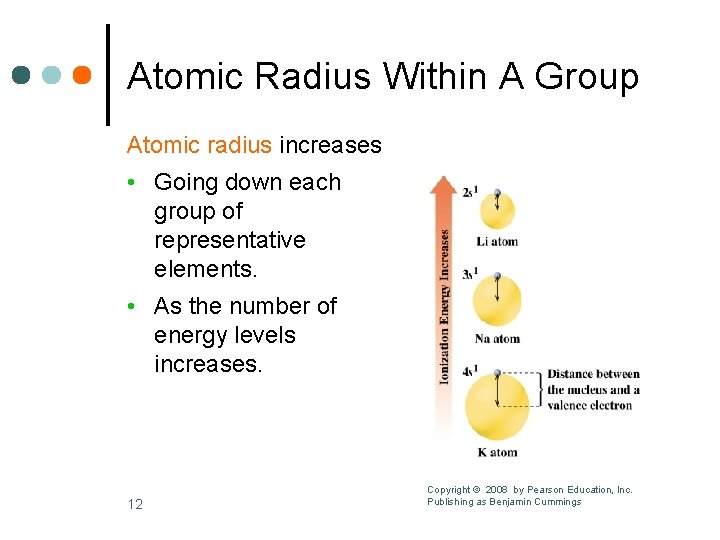
Atomic Radius Within A Group Atomic radius increases • Going down each group of representative elements. • As the number of energy levels increases. 12 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

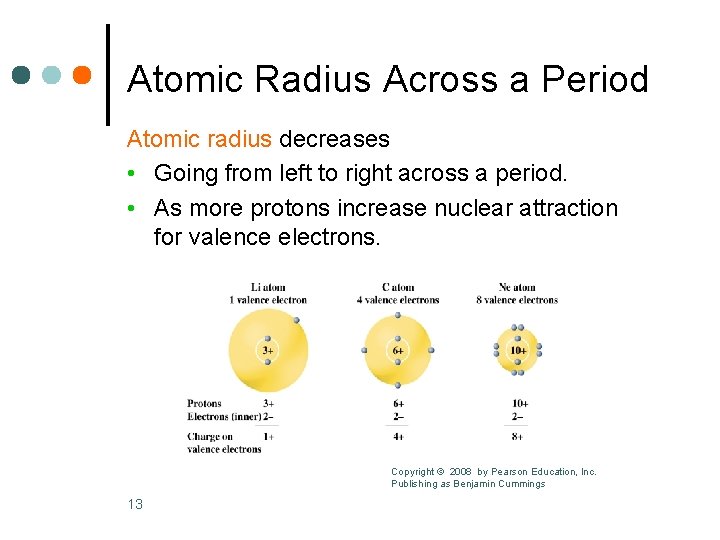
Atomic Radius Across a Period Atomic radius decreases • Going from left to right across a period. • As more protons increase nuclear attraction for valence electrons. Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 13

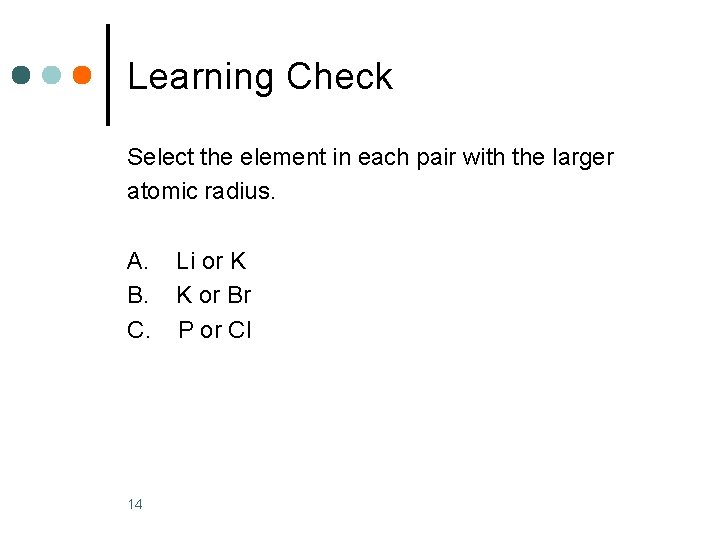
Learning Check Select the element in each pair with the larger atomic radius. A. B. C. 14 Li or K K or Br P or Cl

Solution Select the element in each pair with the larger atomic radius. A. K B. K C. P 15

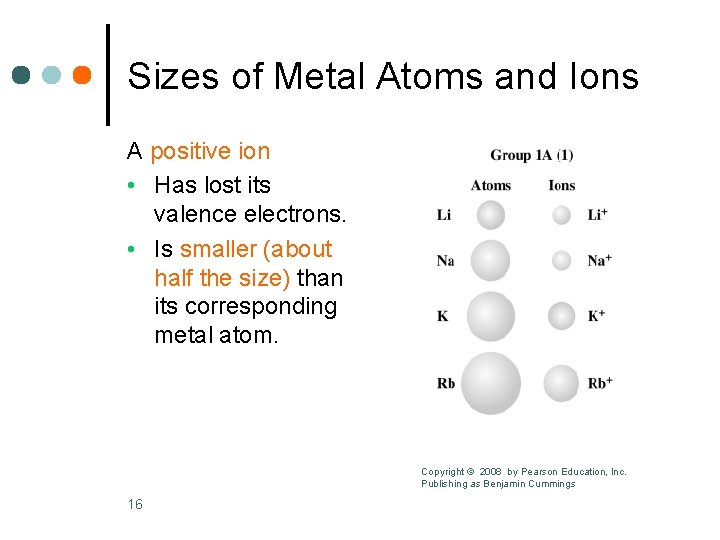
Sizes of Metal Atoms and Ions A positive ion • Has lost its valence electrons. • Is smaller (about half the size) than its corresponding metal atom. Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 16

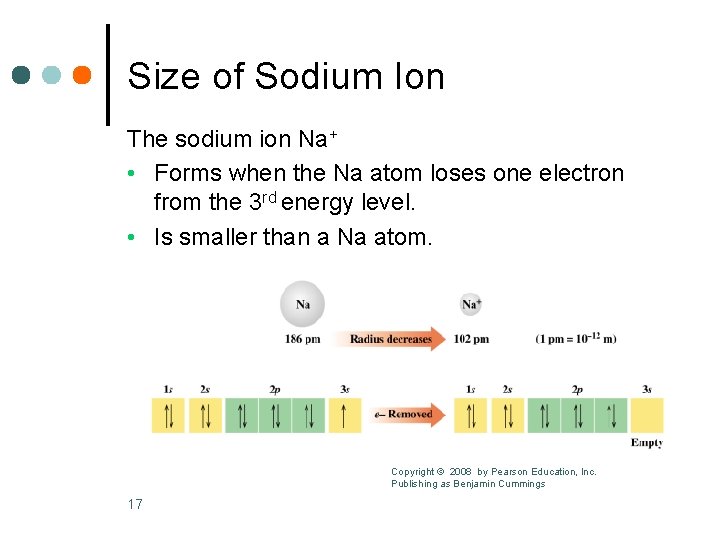
Size of Sodium Ion The sodium ion Na+ • Forms when the Na atom loses one electron from the 3 rd energy level. • Is smaller than a Na atom. Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 17

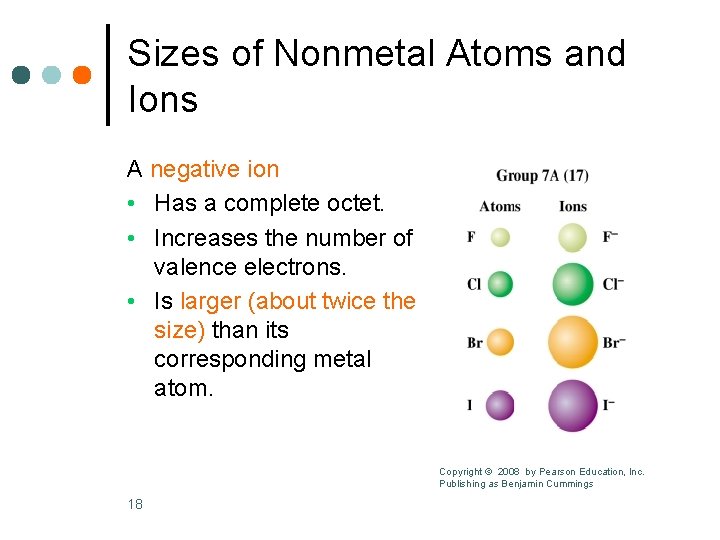
Sizes of Nonmetal Atoms and Ions A negative ion • Has a complete octet. • Increases the number of valence electrons. • Is larger (about twice the size) than its corresponding metal atom. Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 18

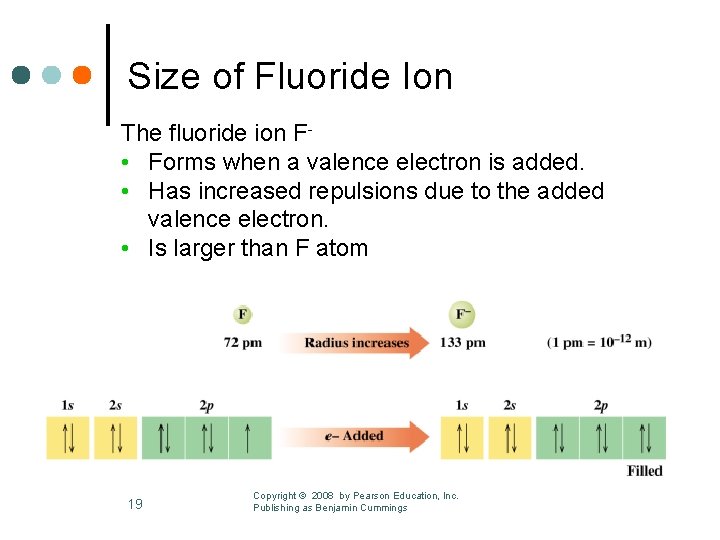
Size of Fluoride Ion The fluoride ion F • Forms when a valence electron is added. • Has increased repulsions due to the added valence electron. • Is larger than F atom 19 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

Learning Check 1. Which is larger in each of the following? A. K or K+ B. Al or Al 3+ C. S 2 - or S 2. Which is smaller in each of the following? A. N 3 - or N B. Cl or Cl. C. Sr 2+ or Sr 20

Solution 1. Which is larger in each of the following? A. K > K+ B. Al > Al 3+ C. S 2 - > S 2. Which is smaller in each of the following? A. N < N 3 B. Cl < Cl. C. Sr 2+< Sr 21

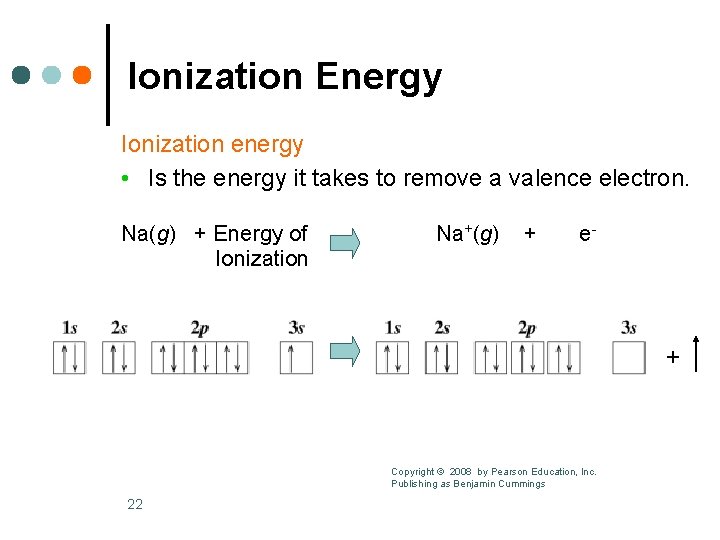
Ionization Energy Ionization energy • Is the energy it takes to remove a valence electron. Na(g) + Energy of Ionization Na+(g) + e- + Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 22

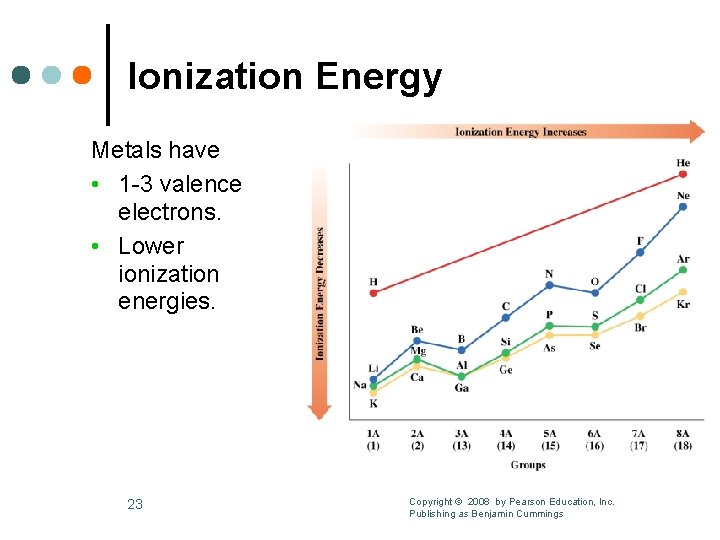
Ionization Energy Metals have • 1 -3 valence electrons. • Lower ionization energies. 23 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

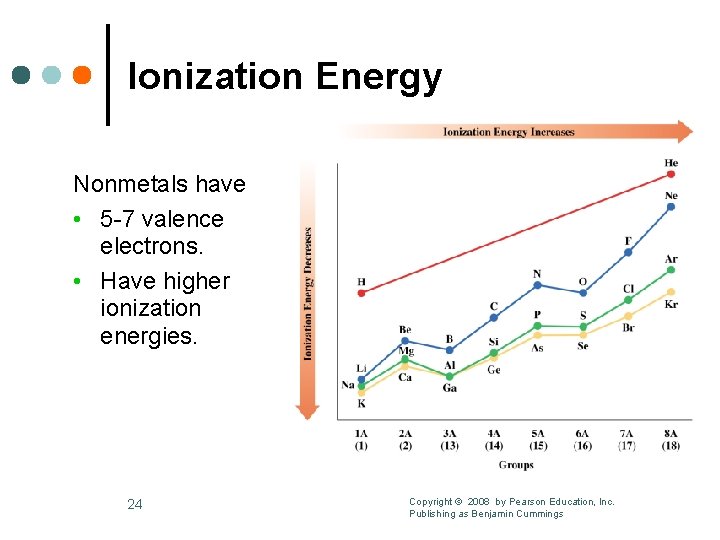
Ionization Energy Nonmetals have • 5 -7 valence electrons. • Have higher ionization energies. 24 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

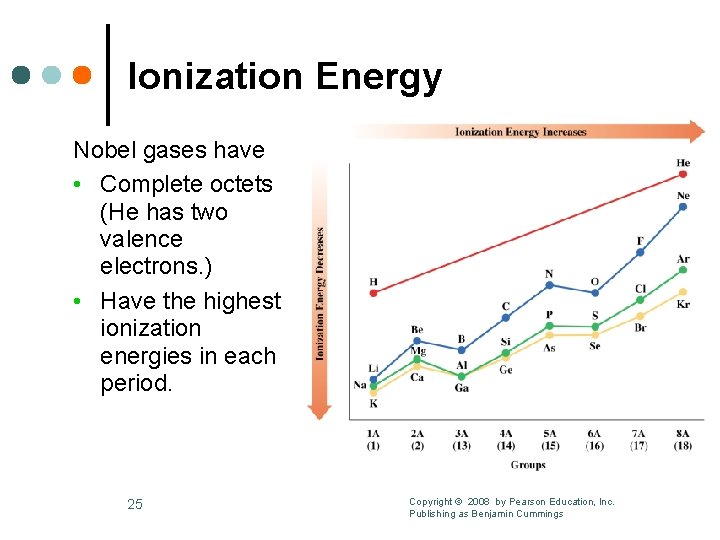
Ionization Energy Nobel gases have • Complete octets (He has two valence electrons. ) • Have the highest ionization energies in each period. 25 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

Learning Check Select the element in each pair with the higher Ionization energy. A. B. C. 26 Li or K K or Br P or Cl

Solution Select the element in each pair with the higher Ionization energy. A. B. C. 27 Li Br Cl