Chapter 9 Covalent Bonding Orbitals What role do

Chapter 9 Covalent Bonding: Orbitals What role do orbitals play in bonding?
Hybridization • Assumption: bonding happens between valence electrons of atoms - wrong. • Hybridization (mixing) of orbitals occurrs during bonding – When one s and three p orbitals are hybridized, an “sp 3” orbital is formed (sp 3 hybridization). This happens with equivalent tetrahedral atomic orbitals. – sp 3 Hybridization Video

sp 2 Hybridization • sp 3 hybridized molecules have angles of 109. 5°, so in molecules where trigonal planar arrangement (one plane, 120°) is required, sp 3 hybridization can not be used. • These are formed with one 2 s and two 2 p orbitals, so it’s called sp 2 hybridization. This leaves behind one 2 p orbital (perpendicular to the plane). • sp 2 Hybridization Video
Bond Types • Sigma ( ) vs. Pi ( ) • When bonds (sharing electrons) form where the pair is shared in an area centered on a line running between atoms, it is a sigma bond (lobes point towards each other) • This happens in single bonds…and in double/triple bonds (hold on)

Pi Bonds • When electron pairs occupy the space above and below a line joining the atoms • When orbitals are parallel • Double bonds consist of one sigma and one pi bond • Triple bonds consist of one sigma and two pi bonds • *Think “hot dog”

sp Hybridization • When there is a linear (180°) structure, sp 3 and sp 2 hybridization will not work • Involves one s and one p orbital being hybridized
Localized Electron Model • STEPS: 1. Draw the Lewis structure 2. Determine the arrangement of electron pairs using VSEPR 3. Specify the hybrid orbitals needed • • Example: Predict the hybridization of each atom and describe the molecular structure of BF 4 Answer: boron is sp 3, fluorine is sp 3, molecular structure is tetrahedral

Examples… • CO – Answer: each atom is sp hybridized, bonds present are a sigma and two pis, lone pairs are sp orbitals
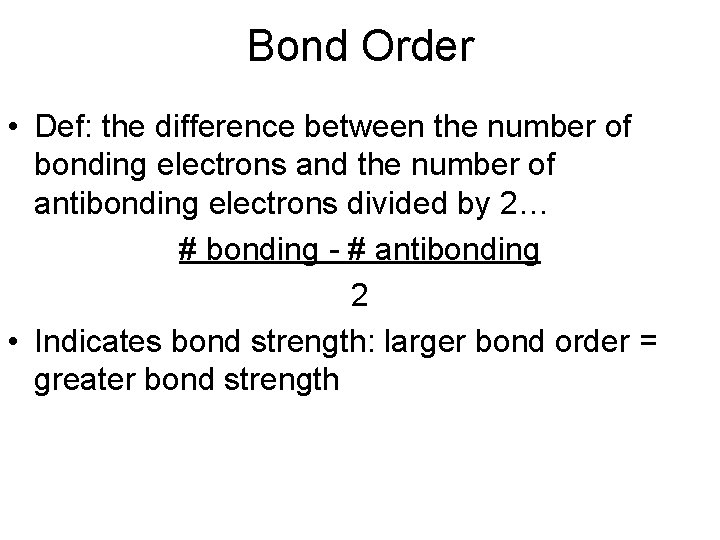
Bond Order • Def: the difference between the number of bonding electrons and the number of antibonding electrons divided by 2… # bonding - # antibonding 2 • Indicates bond strength: larger bond order = greater bond strength
- Slides: 9