Chapter 9 Classifying Chemical Reactions Types of Reactions


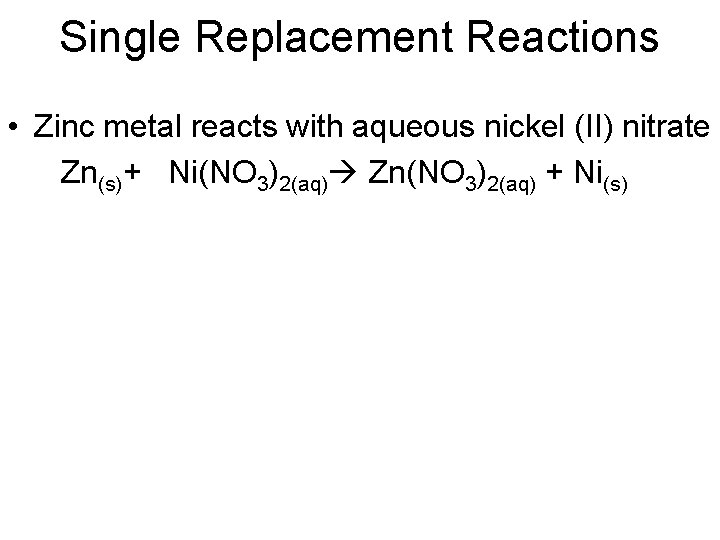













- Slides: 34

Chapter 9 Classifying Chemical Reactions

Types of Reactions • We will consider five types of reactions : 1. 2. 3. 4. 5. Single displacement reactions Double displacement reactions Decomposition reactions Synthesis reactions Combustion reactions

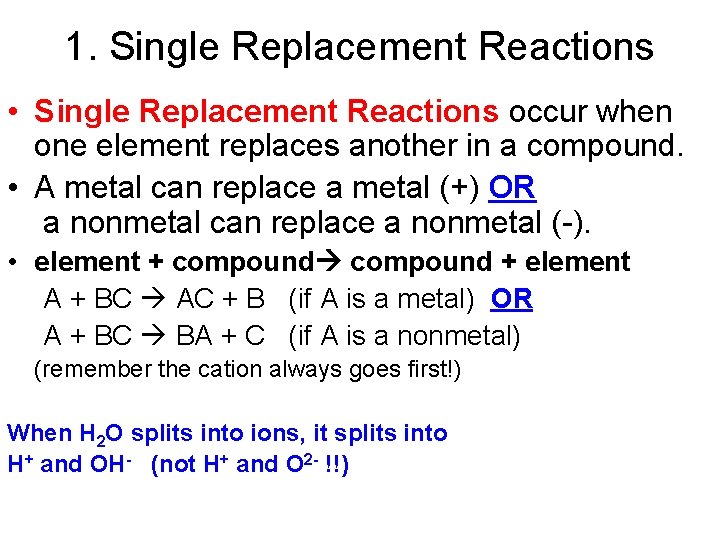
1. Single Replacement Reactions • Single Replacement Reactions occur when one element replaces another in a compound. • A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). • element + compound + element A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (remember the cation always goes first!) When H 2 O splits into ions, it splits into H+ and OH- (not H+ and O 2 - !!)

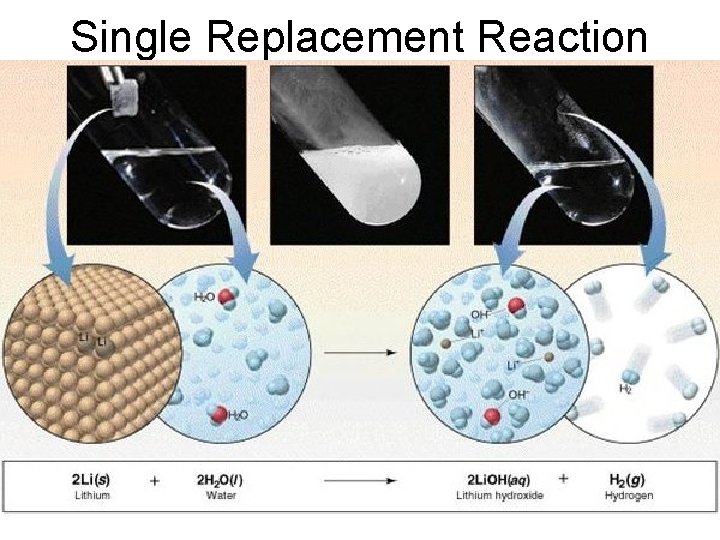
Single Replacement • A small piece of lithium metal is added to water.

Single Replacement Reaction

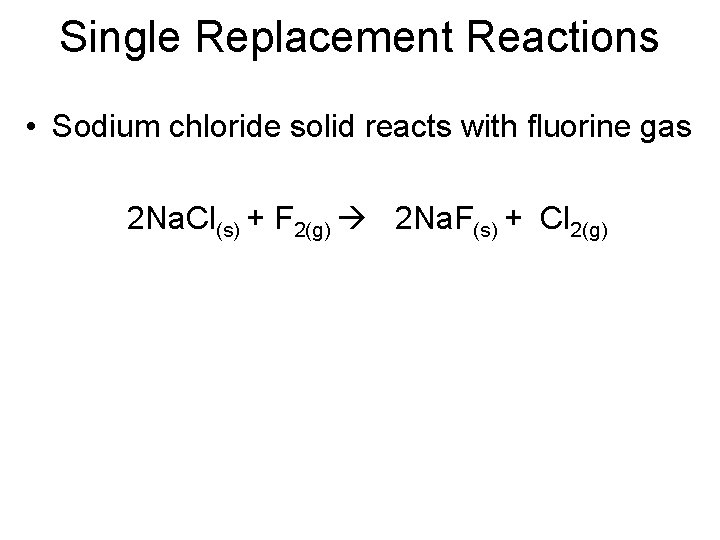
Single Replacement Reactions • Sodium chloride solid reacts with fluorine gas 2 Na. Cl(s) + F 2(g) 2 Na. F(s) + Cl 2(g)
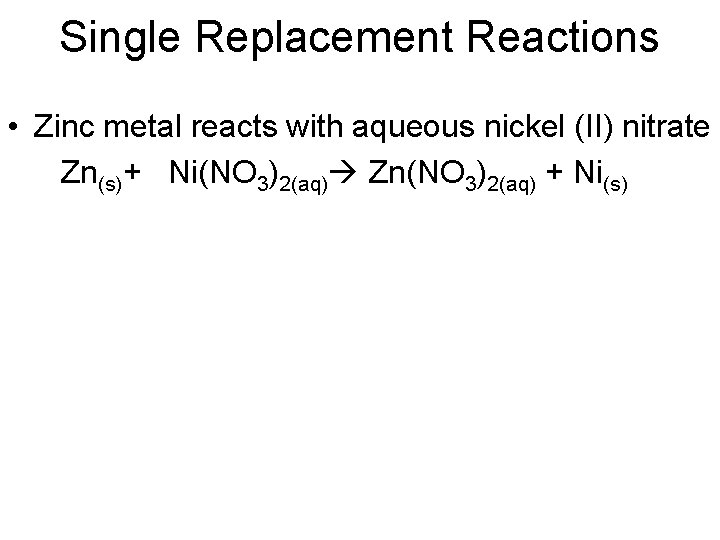
Single Replacement Reactions • Zinc metal reacts with aqueous nickel (II) nitrate Zn(s)+ Ni(NO 3)2(aq) Zn(NO 3)2(aq) + Ni(s)

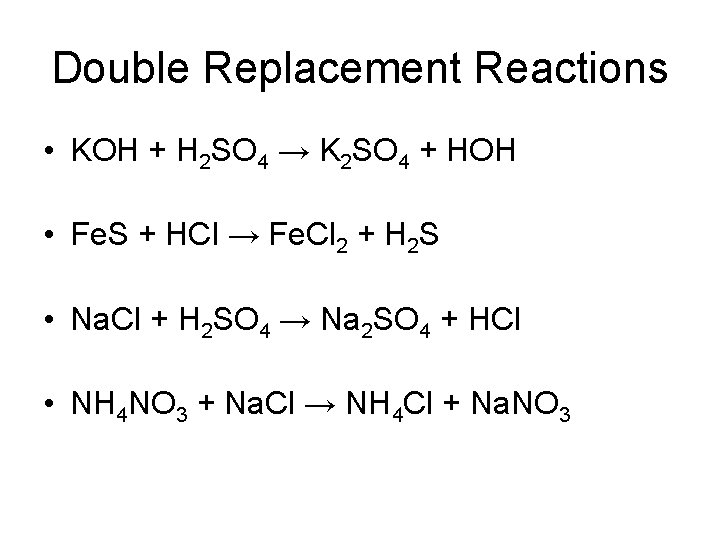
2. Double Replacement Reactions • Double Replacement Reactions occur when the cations in two compounds switch places. • compound + compound • AB + CD AD + CB

Double Replacement Reactions • KOH + H 2 SO 4 → K 2 SO 4 + HOH • Fe. S + HCl → Fe. Cl 2 + H 2 S • Na. Cl + H 2 SO 4 → Na 2 SO 4 + HCl • NH 4 NO 3 + Na. Cl → NH 4 Cl + Na. NO 3

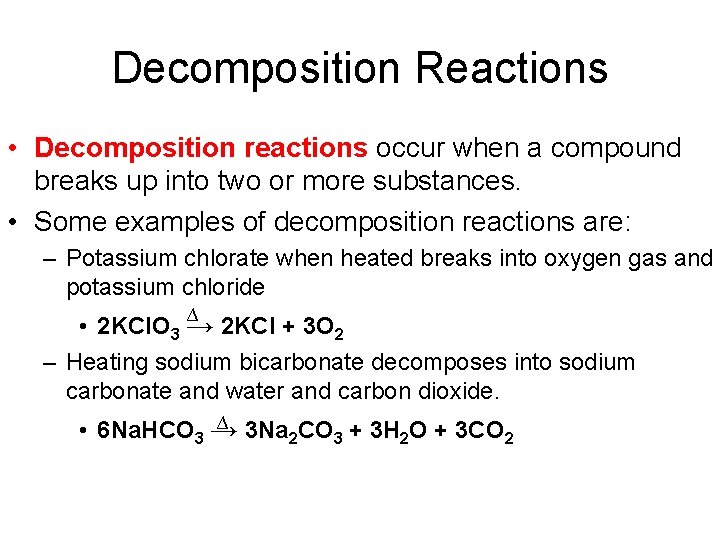
Decomposition Reactions • Decomposition reactions occur when a compound breaks up into two or more substances. • Some examples of decomposition reactions are: – Potassium chlorate when heated breaks into oxygen gas and potassium chloride ∆ • 2 KCl. O 3 → 2 KCl + 3 O 2 – Heating sodium bicarbonate decomposes into sodium carbonate and water and carbon dioxide. ∆ • 6 Na. HCO 3 → 3 Na 2 CO 3 + 3 H 2 O + 3 CO 2

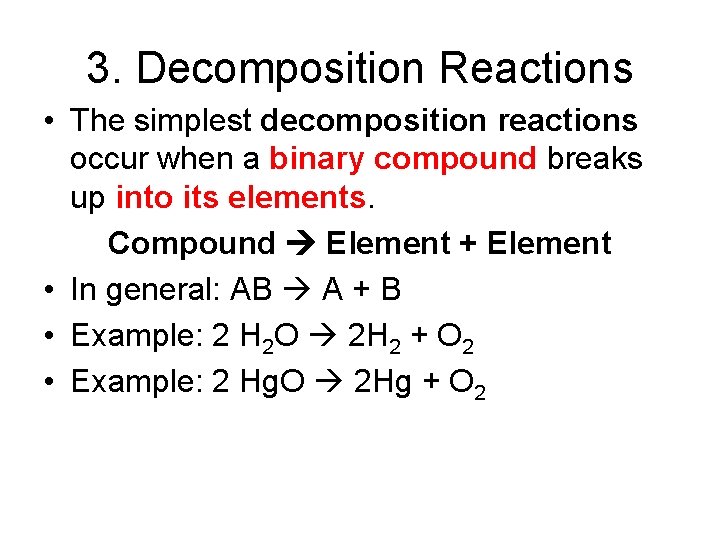
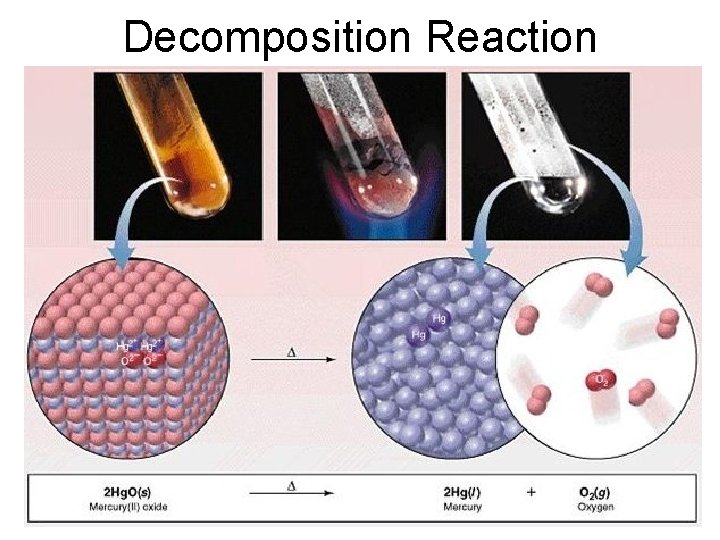
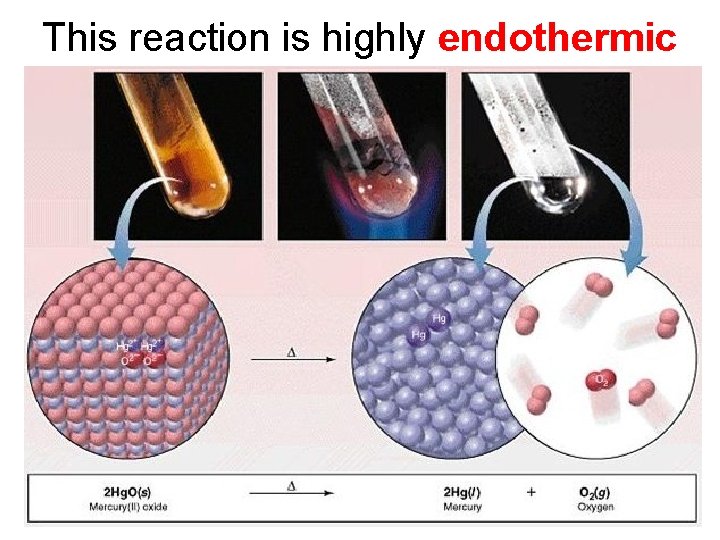
3. Decomposition Reactions • The simplest decomposition reactions occur when a binary compound breaks up into its elements. Compound Element + Element • In general: AB A + B • Example: 2 H 2 O 2 H 2 + O 2 • Example: 2 Hg. O 2 Hg + O 2

Decomposition Reaction

This reaction is highly endothermic

Energy Changes • Many decomposition reactions involve large changes in energy (they are highly endothermic or highly exothermic).

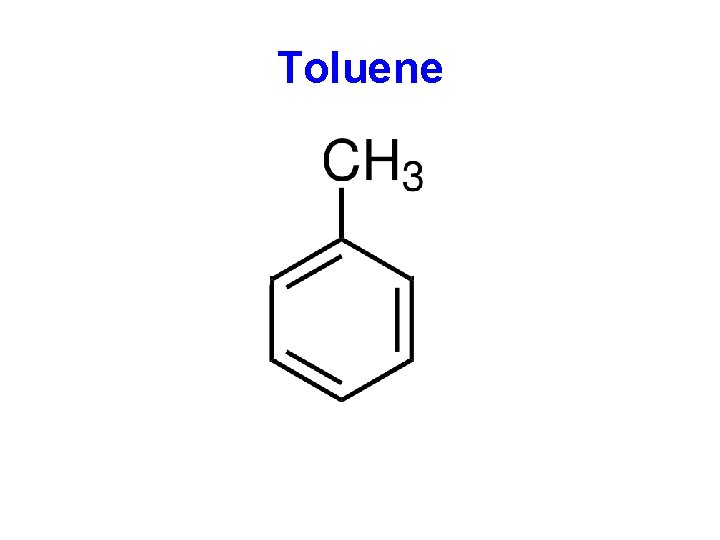
Toluene A A A


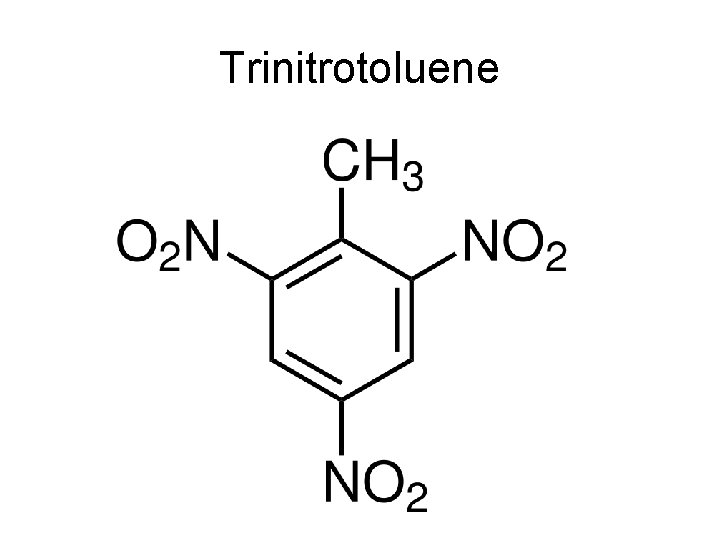
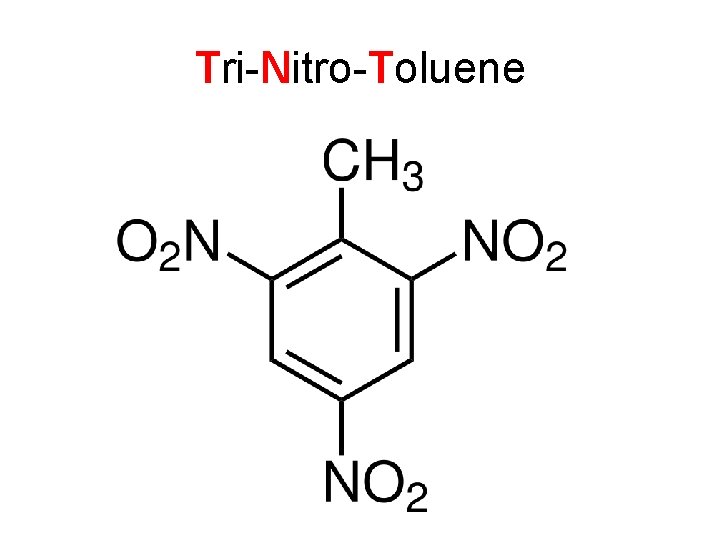
Trinitrotoluene

Tri-Nitro-Toluene

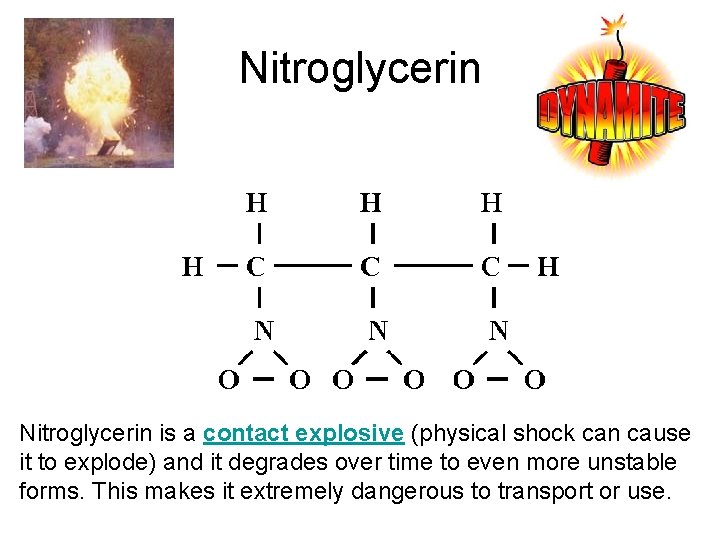
Nitroglycerin is a contact explosive (physical shock can cause it to explode) and it degrades over time to even more unstable forms. This makes it extremely dangerous to transport or use.

Alfred Nobel • Nobel found that when nitroglycerin was added to an absorbent inert substance it became safer. • He patented this in 1867 as dynamite.


Alfred Nobel “The Merchant of Death is Dead”

Nobel Prizes • Nobel signed his last will and testament and set aside the bulk of his estate to establish the Nobel Prizes.

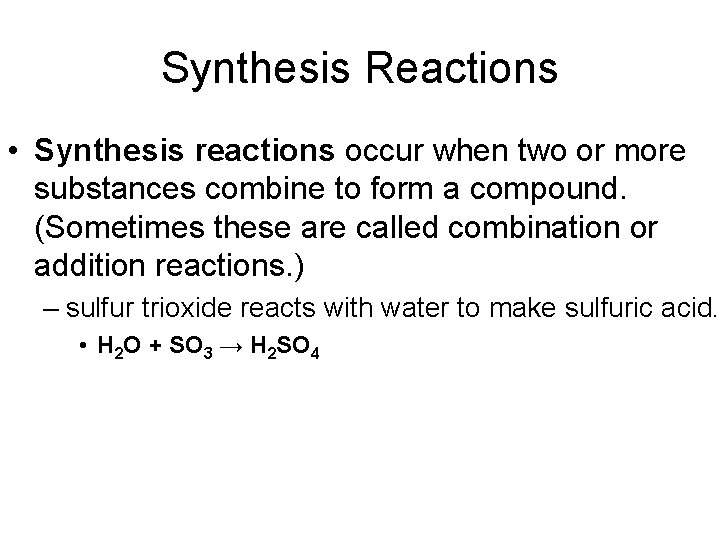
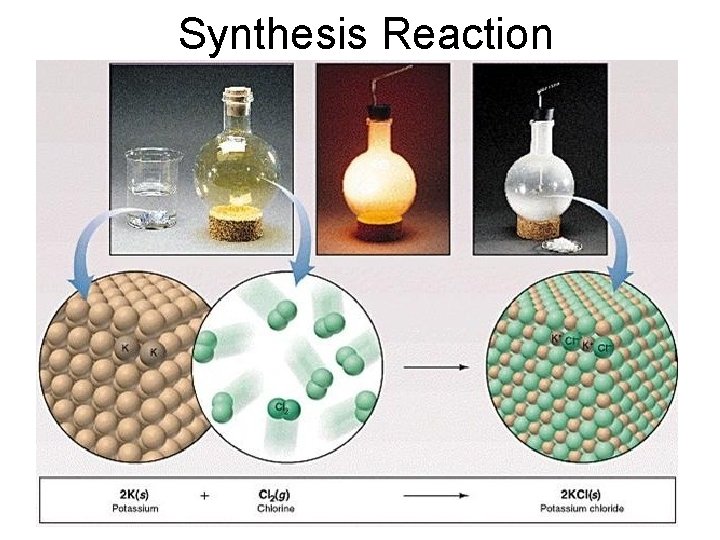
Synthesis Reactions • Synthesis reactions occur when two or more substances combine to form a compound. (Sometimes these are called combination or addition reactions. ) – sulfur trioxide reacts with water to make sulfuric acid. • H 2 O + SO 3 → H 2 SO 4

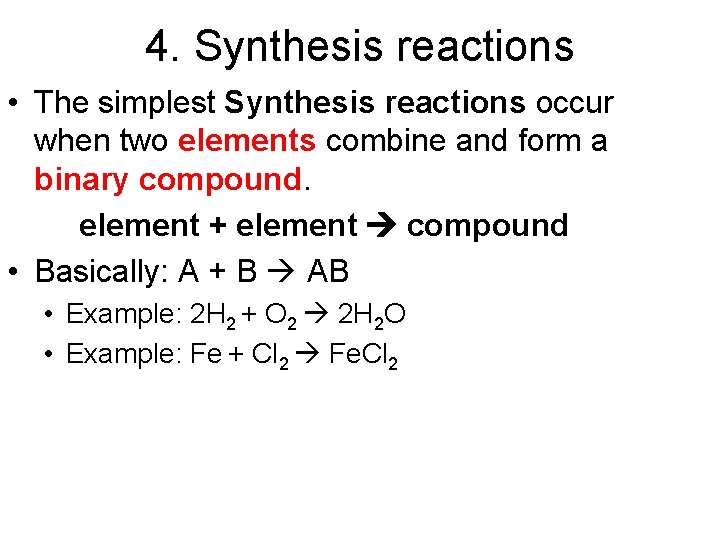
4. Synthesis reactions • The simplest Synthesis reactions occur when two elements combine and form a binary compound. element + element compound • Basically: A + B AB • Example: 2 H 2 + O 2 2 H 2 O • Example: Fe + Cl 2 Fe. Cl 2

Synthesis Reaction

5. Combustion Reactions • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. • This is also called burning!!! • The products of combustion are carbon dioxide and water.

Combustion Reactions • Example: - Cx. Hy + O 2 CO 2 + H 2 O • Combustion is used to heat homes and run automobiles (example: octane in gasoline, is C 8 H 18). • Combustion also got you to school today.

Cellular Respiration C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O

Combustion Reactions • Cx. Hy + O 2 CO 2 + H 2 O • Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by-products like carbon monoxide)

Complete vs. Incomplete Combustion • Determined by the amount of oxygen. • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion.

Combustion Reactions Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.

Gas Lighting and CO Poisoning

Homework • Reaction Type and Balancing Worksheet
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Types of chemical reactions redox
Types of chemical reactions redox Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions Chemical reaction types
Chemical reaction types 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Five chemical changes
Five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions 5 type of reactions
5 type of reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Fatoumata dembele chef
Fatoumata dembele chef Combustion chemical reaction
Combustion chemical reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations What is an active metal
What is an active metal Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Chemical formulas and chemical compounds chapter 7 review
Chemical formulas and chemical compounds chapter 7 review An example of redox reaction
An example of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers