Chapter 9 Chemical Reactions Recognizing Reactions by Pattern

Chapter 9 Chemical Reactions Recognizing Reactions by Pattern (Chapter 9. 1): Combination Reactions Decomposition Reactions Single Replacement Reactions (Displacement) Double Replacement Reactions (Exchange) Combustion Reactions

Classification by Reaction Type Combustion Reactions – reaction with oxygen to produce heat & light C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g) Acid-Base Reactions (Neutralization) – give a salt & water H 2 SO 4(aq) + KOH(aq) Precipitation Reactions – an insoluble compound is produced Zn. Cl 2(aq) + Ag. NO 3(aq) Oxidation-Reduction Reactions – involve an exchange of electrons Zn(s) + Cu. SO 4(aq)
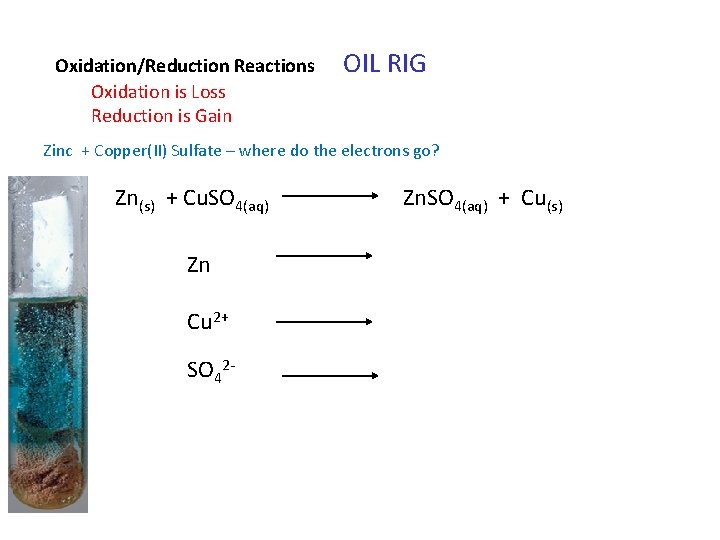
Oxidation/Reduction Reactions Oxidation is Loss Reduction is Gain OIL RIG Zinc + Copper(II) Sulfate – where do the electrons go? Zn(s) + Cu. SO 4(aq) Zn Cu 2+ SO 42 - Zn. SO 4(aq) + Cu(s)

Oxidation/Reduction Reactions Loss of Electrons = Oxidation Gain of Electrons = Reduction Zinc + Hydrochloric Acid – where do the electrons go? Zn(s) + 2 HCl(aq) Zn 2 H+ 2 Cl- Zn. Cl 2(aq) + H 2(g)

Single Replacement Reactions – Will a reaction take place?

Will a reaction take place? Classify as Combustion, Single Replacement, Double Replacement (precipitation or neutralization). Complete and Balance the equations. Classify 1. magnesium(s) + cadmium nitrate(aq) Single Replacement 2. hydrogen(g) + oxygen(g) Combination/Combustion 3. aluminum(s) + sulfuric acid(aq) 4. sodium hydroxide(aq) + sulfuric acid(aq) 5. potassium hydroxide(aq) + zinc chloride(aq) 6. Palladium(s) + cadmium nitrate(aq) 7. methane(g) + oxygen(g) 8. zinc nitrate(aq) + hydrogen sulfide(g) 9. mercury(II) sulfate(aq) + ammonium nitrate(aq) 10. iron(III) nitrate(aq) + sodium chromate(aq) 11. aluminum chloride(aq) + sodium nitrate(aq)

Collision Theory and Chemical Reactions Three conditions required for a reaction to occur: 1. Collision 2. Energy 3. Orientation

Energy Changes in Chemical Reactions: Breaking Bonds – Forming Bonds – CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

An Exothermic Reaction releases Energy Combustion of Methane: CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) + 211 kcal/mol Heat is released into surroundings Temperature increases Heat of Reaction is negative H = -211 kcal

An Endothermic Reaction absorbs Energy Decomposition of Ammonia: 22 kcal/mol + 2 NH 3(g) Heat is absorbed Temperature decreases Heat of Reaction is positive H = +22 kcal N 2(g) + 3 H 2(g)

Factors that Affect Reaction Rate: Physical Nature of Reactants: State, Particle Size Concentration Temperature Catalyst

Equilibrium Reactions: chemical or physical processes that do not go to completion. Physical Equilibrium— 1. sugar dissolving in water http: //www. youtube. com/watch? v=g 5 w. Ng_d. Ks. YY&list=PL 8 d. Puua. Lj. Xt. PHzz. Yu. Wy 6 f. YEa. X 9 m. QQ 8 o. Gr&index=28

2. The freezing point of water = the temperature at which H 2 O(l) is in equilibrium with H 2 O(s). H 2 O(l) H 2 O(s) rate of melting = rate of freezing 3. CO 2 dissolved in water CO 2(aq) CO 2(g) CO 2 (aq)

Molar concentration Chemical Equilibrium— consider the formation of ammonia N 2(g) + 3 H 2(g) 2 NH 3(g) time at Equilibrium: a) the concentrations of reactants & products is no longer changing. b) the rate of the forward rxn equals the rate of the reverse rxn.

The Equilibrium Constant Expression: for a chemical reaction a. A + b. B Keq = [C]c[D]d [A]a[B]b c. C + d. D Molar concentrations of Products Molar concentrations of Reactants N 2(g) + 3 H 2(g) 2 NH 3(g) H 2(g) + I 2(g) 2 HI(g)

Writing Equilibrium Constant Expressions: -include only substances whose concentrations can change. -note that the concentration of a liquid is constant -and a solid is not part of the solution -so, only substances that are gas (g) or dissolved in solution (aq) are included sucrose(s) sucrose(aq)

Write equilibrium constant expressions for the following: H 2(g) + F 2(g) Ag. Cl(s) 2 HF(g) Ag+(aq) + Cl-(aq) Mn. O 2(s) + 4 H+(aq) + 2 Cl-(aq) Cu 2+(aq) + 2 OH-(aq) Mn 2+(aq) + Cl 2(g) + 2 H 2 O(l) Cu(OH)2(s)

Write the equilibrium constant expression: H 2 CO 3(aq) H+(aq) + HCO 3 -(aq) Calculate Keq for the dissociation of carbonic acid, given: [H+] = 1. 1 x 10 -5 M [HCO 3 -] = 1. 1 x 10 -5 M [H 2 CO 3] = 2. 9 x 10 -4 M

What does the value of Keq mean? Keq > 100 Keq < 0. 01 Keq 0. 01 to 100 N 2(g) + 3 H 2(g) [N 2] = 0. 25 M [NH 3] = 0. 11 M [H 2] = 1. 91 M 2 NH 3(g) find Keq =

Calculate and interpret the equilibrium constant: a) 2 HI(g) H 2(g) + I 2(g) [HI] = 0. 84 M [H 2] = 3. 54 M [I 2] = 3. 54 M b) Co. Cl 42 -(aq) + 6 H 2 O(l) [Co. Cl 42 -] = 8. 5 x 10 -2 M [Co(H 2 O)62+] = 1. 5 x 10 -5 M [Cl-] = 1. 00 M Co(H 2 O)62+(aq) + 4 Cl-(aq)

Le. Chatelier’s Principle: if a stress is placed on a system at equilibrium, the system will respond by changing the rate of the forward or reverse reaction in such a way as to minimize the stress.

Consider 2 hockey players— The game begins with 500 pucks on johnnie’s side; And ends when the movement of pucks has reached equilibrium: Pjohn Ptom

The game begins with 500 pucks on johnnie’s side: Ptom Pjohn # of pucks 500 time at Equilibrium: Keq = Now, add 500 more pucks to johnnie’s side:

Chemical Equilibrium & Le. Chatelier’s Principle: Concentration Changes Stress: Add H 2 Remove H 2 Add HI Remove HI Result:

Chemical Equilibrium & Le. Chatelier’s Principle: Pressure Changes Stress: P P Result:

Chemical Equilibrium & Le. Chatelier’s Principle: Temperature Changes Stress: T T Result:

Write the equilibrium constant expression: Co. Cl 42 -(aq) + 6 H 2 O(l) Predict the result: ¯T [Cl-] P Co(H 2 O)62+(aq) + 4 Cl-(aq) + heat

Write the equilibrium constant expression: 52 kcal/mol + PCl 5(g) Predict the result: ¯T [Cl 2] [PCl 3] P + Catalyst Cl 2 (g) + PCl 3 (g)

Equilibrium in Chemical Systems: Production of Ammonia N 2(g) + 3 H 2(g) 2 NH 3(g) + 22 kcal/mol
- Slides: 29