Chapter 9 Chemical Names and Formulas Anything in










- Slides: 21

Chapter 9 Chemical Names and Formulas Anything in black letters = write it in your notes (‘knowts’)

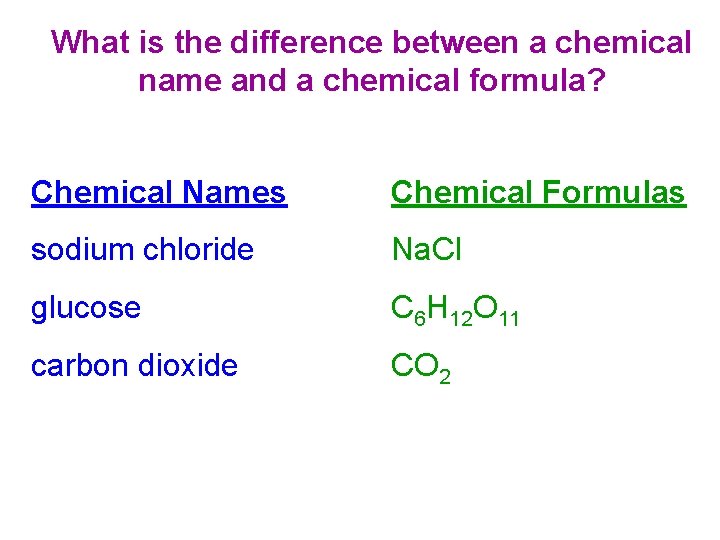
What is the difference between a chemical name and a chemical formula? Chemical Names Chemical Formulas sodium chloride Na. Cl glucose C 6 H 12 O 11 carbon dioxide CO 2

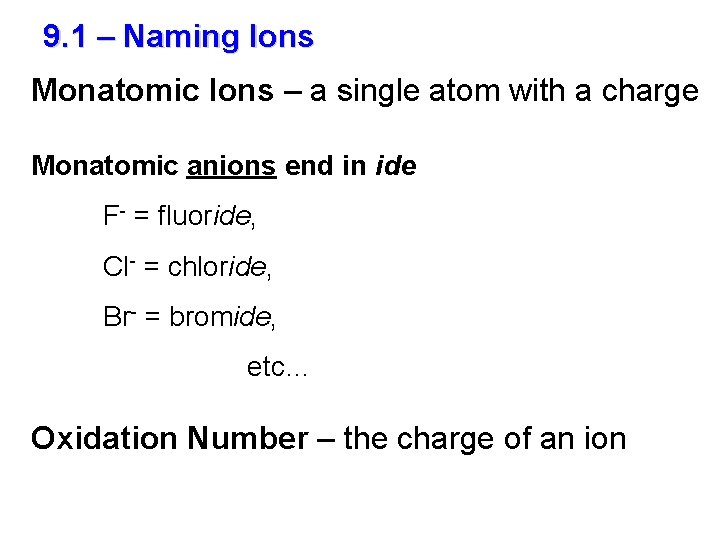
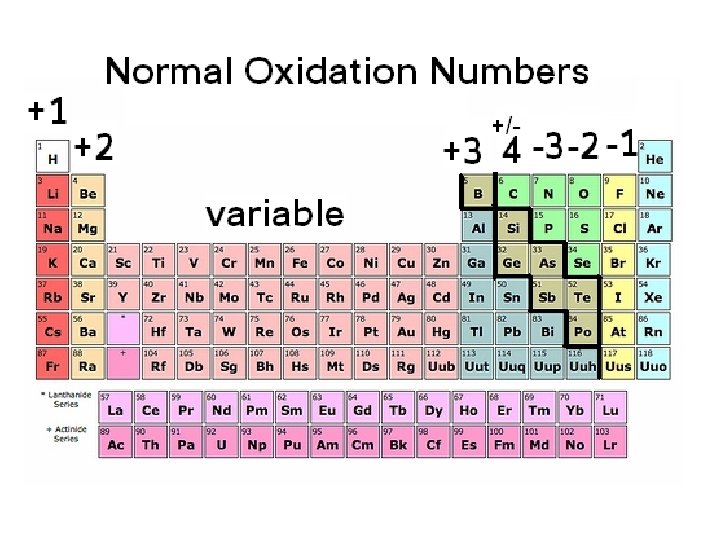
9. 1 – Naming Ions Monatomic Ions – a single atom with a charge Monatomic anions end in ide F- = fluoride, Cl- = chloride, Br- = bromide, etc… Oxidation Number – the charge of an ion


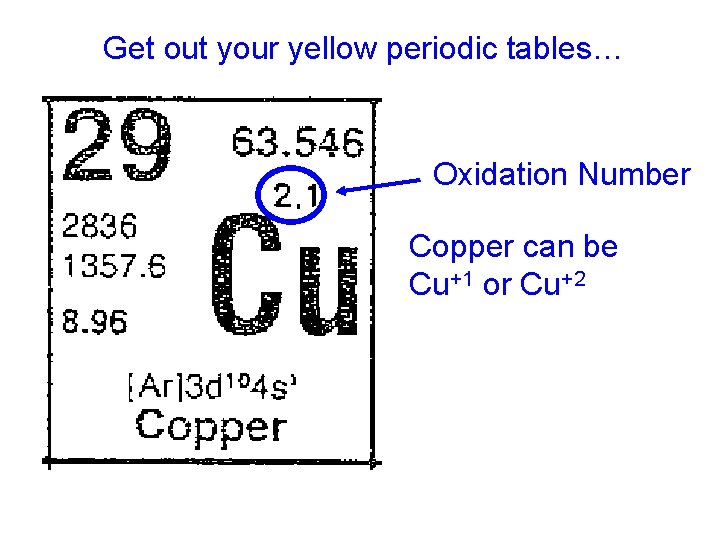
Get out your yellow periodic tables… Oxidation Number Copper can be Cu+1 or Cu+2

Roman numerals are used to show the oxidation number of a variable cation. Copper (I) = Cu+ Copper (II) = Cu 2+ Iron (II) = Fe 2+ Iron (III) = Fe 3+

Classical names can also be used. Copper (I) – Cu+ = cuprous Copper (II) – Cu 2+ = cupric Iron (II) – Fe 2+ = ferrous Iron (III) – Fe 3+ = ferric

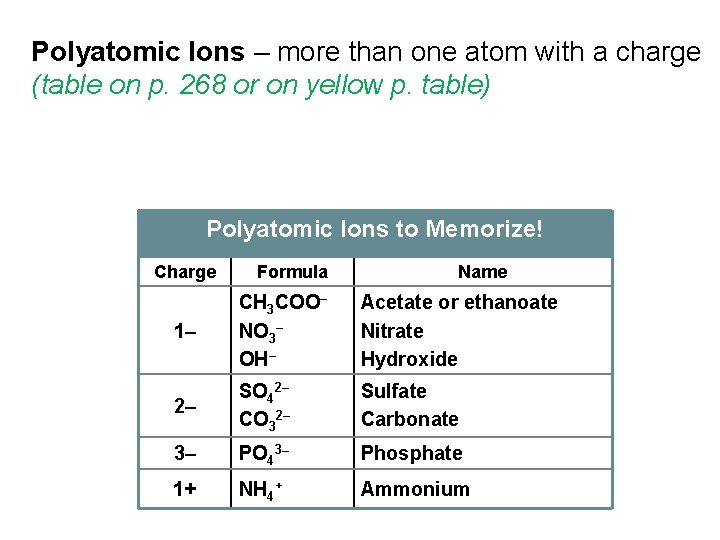
Polyatomic Ions – more than one atom with a charge (table on p. 268 or on yellow p. table) Polyatomic Ions to Memorize! Charge Formula Name 1– CH 3 COO– NO 3– OH– Acetate or ethanoate Nitrate Hydroxide 2– SO 42– CO 32– Sulfate Carbonate 3– PO 43– Phosphate 1+ NH 4+ Ammonium

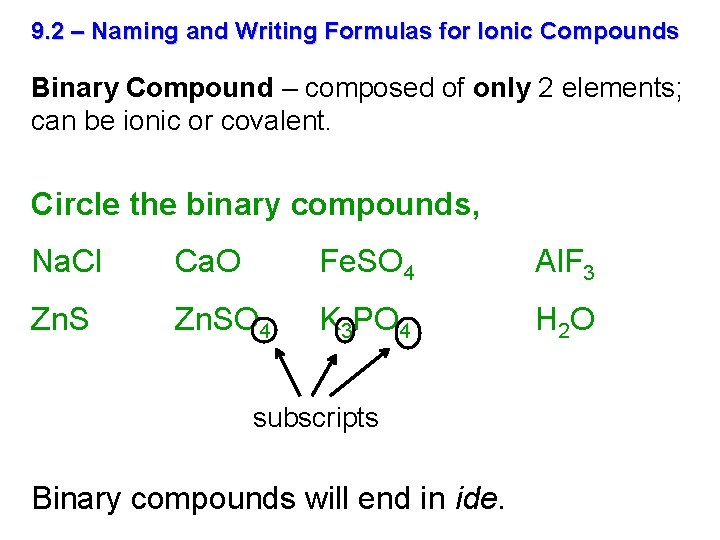
9. 2 – Naming and Writing Formulas for Ionic Compounds Binary Compound – composed of only 2 elements; can be ionic or covalent. Circle the binary compounds, Na. Cl Ca. O Fe. SO 4 Al. F 3 Zn. SO 4 K 3 PO 4 H 2 O subscripts Binary compounds will end in ide.

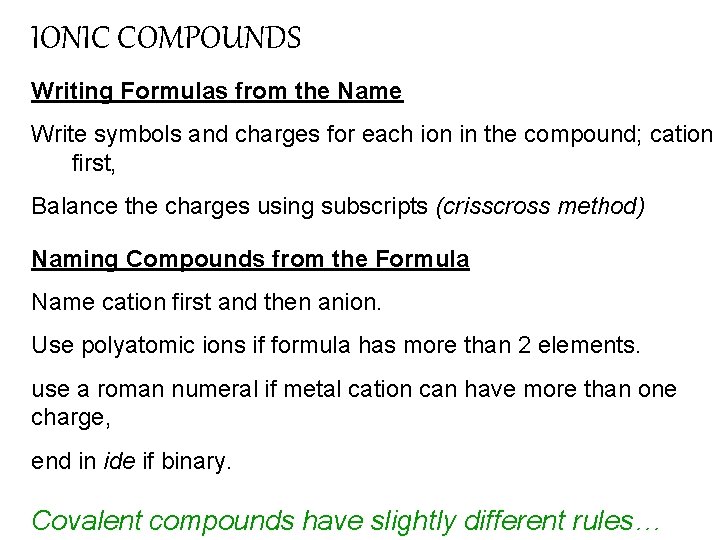
IONIC COMPOUNDS Writing Formulas from the Name Write symbols and charges for each ion in the compound; cation first, Balance the charges using subscripts (crisscross method) Naming Compounds from the Formula Name cation first and then anion. Use polyatomic ions if formula has more than 2 elements. use a roman numeral if metal cation can have more than one charge, end in ide if binary. Covalent compounds have slightly different rules…

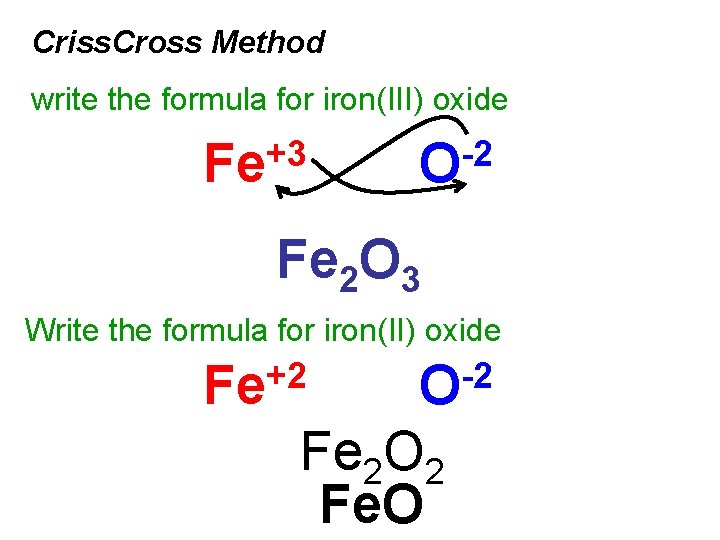
Criss. Cross Method write the formula for iron(III) oxide +3 Fe -2 O Fe 2 O 3 Write the formula for iron(II) oxide +2 Fe -2 O Fe 2 O 2 Fe. O

Write formulas for these compounds, calcium chloride potassium sulfate barium nitrate calcium fluoride copper(II) oxide ammonium phosphate

Name these compounds, Mg. Cl 2 Al. F 3 Ca. O Fe. SO 4 Zn. S K 3 PO 4 Na. OH

ASSIGNMENT: Read 9. 1 & 9. 2 Practice Worksheet

9. 3 – Naming and Writing Formulas for Molecular Compounds Identify Compounds as Ionic or Covalent Naming Binary Molecular Compounds (prefixes) Writing Formulas for Molecular Compounds

So far, we have only discussed naming ionic compounds. Now we will discuss how to name covalent compounds. ionic compounds covalent compounds Fe. Cl 3 iron(III) chloride CO 2 carbon dioxide Na. Cl sodium chloride PF 3 phosphorus trifluoride K 3 PO 4 potassium phosphate CS 2 carbon disulfide Binary molecular compounds use prefixes to show the number of atoms.

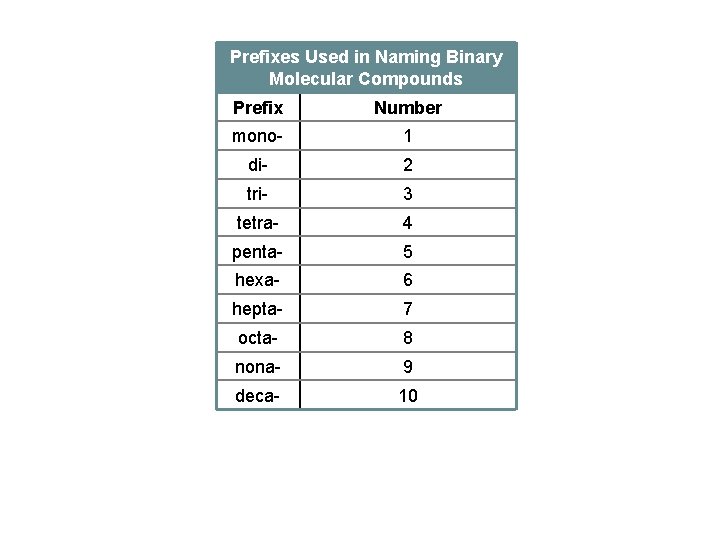
Prefixes Used in Naming Binary Molecular Compounds Prefix Number mono- 1 di- 2 tri- 3 tetra- 4 penta- 5 hexa- 6 hepta- 7 octa- 8 nona- 9 deca- 10

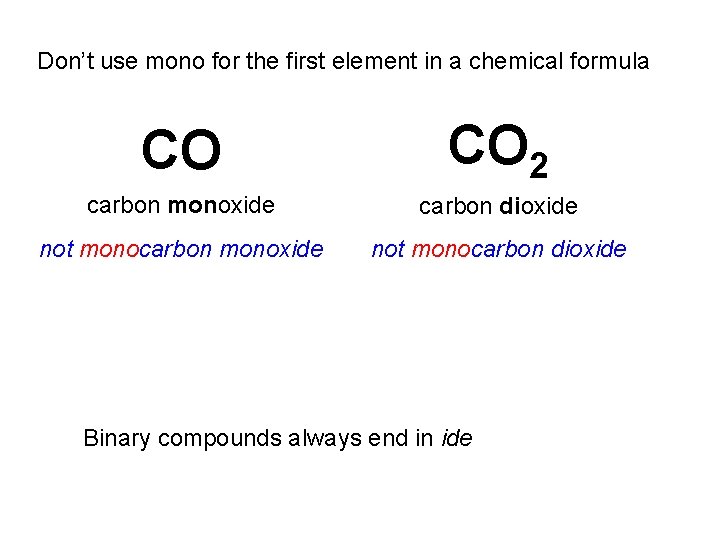
Don’t use mono for the first element in a chemical formula CO CO 2 carbon monoxide carbon dioxide not monocarbon monoxide not monocarbon dioxide Binary compounds always end in ide

Name these compounds, NCl 3 nitrogen trichloride BCl 3 boron trichloride N 2 H 4 dinitrogen tetrahydride NO 2 nitrogen dioxide N 2 O dinitrogen oxide SO 3 sulfur trioxide CO 2 carbon dioxide

Write formulas for these compounds, phosphorus pentachloride PCl 5 iodine heptafluoride IF 7 chlorine trifluoride Cl. F 3 carbon monoxide CO carbon disulfide CS 2 sulfur trioxide SO 3

ASSIGNMENT: Chapter 9 #27 -36 (page 283)