Chapter 9 Chemical Equilibrium 9 5 Changing Equilibrium
Chapter 9 Chemical Equilibrium 9. 5 Changing Equilibrium Conditions: Le Châtelier’s Principle General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1
Le Châtelier’s Principle Le Châtelier’s principle states that § any change in equilibrium conditions upsets the equilibrium of the system § a system at equilibrium under stress will shift to relieve the stress § there will be a change in the rate of the forward or reverse reaction to return the system to equilibrium General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2
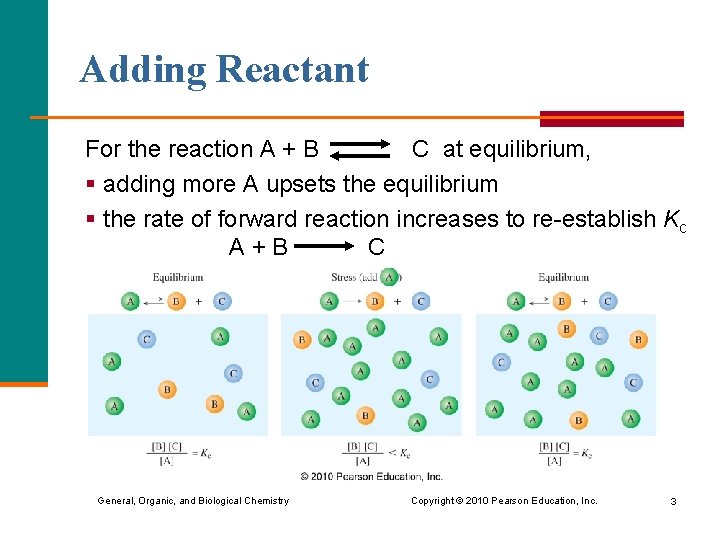
Adding Reactant For the reaction A + B C at equilibrium, § adding more A upsets the equilibrium § the rate of forward reaction increases to re-establish Kc A+B C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3
Effect of Adding Reactant Consider the following reaction at equilibrium. H 2(g) + F 2(g) 2 HF(g) § If more reactant (H 2 or F 2) is added, there is an increase in the number of collisions. § The rate of the forward reaction increases and forms more HF product until new equilibrium concentrations equal Kc again. § The effect of adding a reactant shifts the equilibrium toward the products. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4
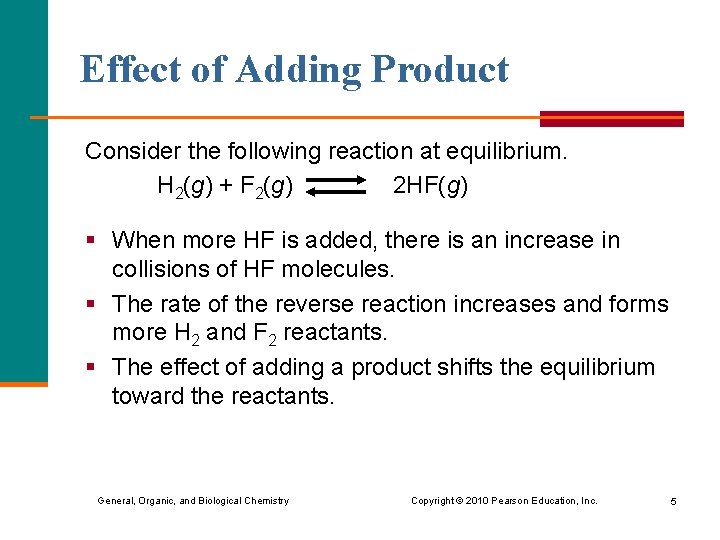
Effect of Adding Product Consider the following reaction at equilibrium. H 2(g) + F 2(g) 2 HF(g) § When more HF is added, there is an increase in collisions of HF molecules. § The rate of the reverse reaction increases and forms more H 2 and F 2 reactants. § The effect of adding a product shifts the equilibrium toward the reactants. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5
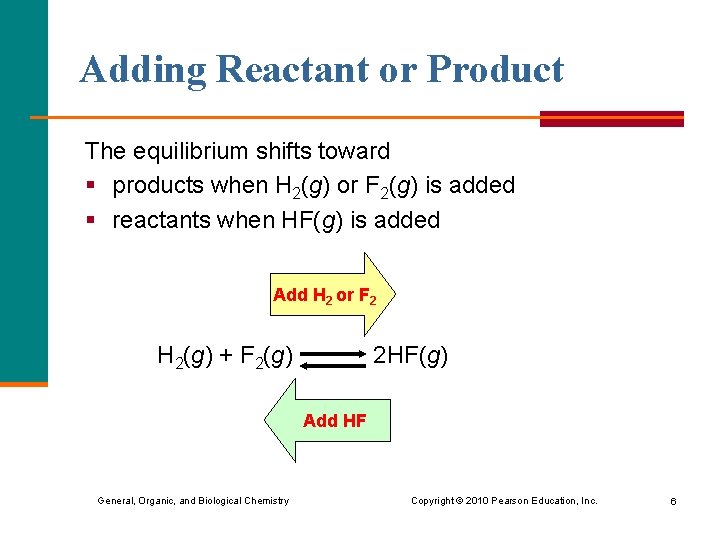
Adding Reactant or Product The equilibrium shifts toward § products when H 2(g) or F 2(g) is added § reactants when HF(g) is added Add H 2 or F 2 H 2(g) + F 2(g) 2 HF(g) Add HF General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6
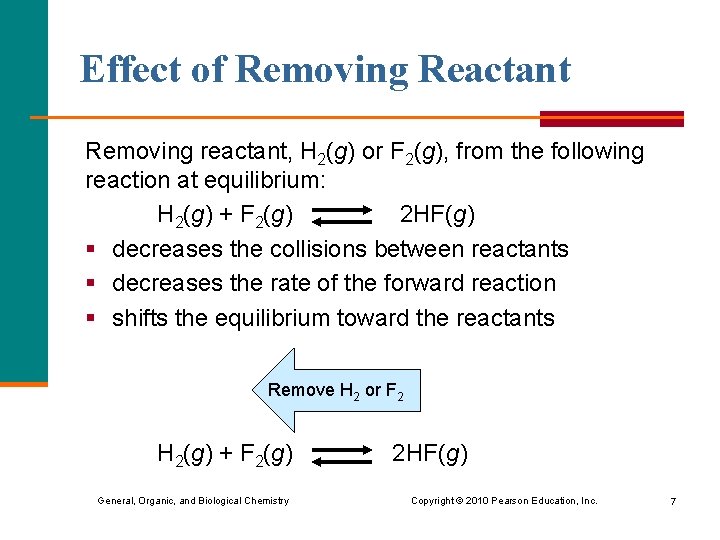
Effect of Removing Reactant Removing reactant, H 2(g) or F 2(g), from the following reaction at equilibrium: H 2(g) + F 2(g) 2 HF(g) § decreases the collisions between reactants § decreases the rate of the forward reaction § shifts the equilibrium toward the reactants Remove H 2 or F 2 H 2(g) + F 2(g) General, Organic, and Biological Chemistry 2 HF(g) Copyright © 2010 Pearson Education, Inc. 7
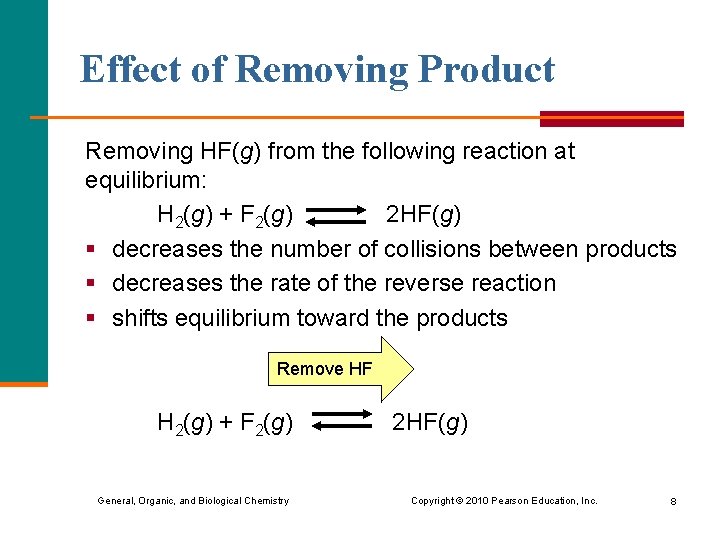
Effect of Removing Product Removing HF(g) from the following reaction at equilibrium: H 2(g) + F 2(g) 2 HF(g) § decreases the number of collisions between products § decreases the rate of the reverse reaction § shifts equilibrium toward the products Remove HF H 2(g) + F 2(g) General, Organic, and Biological Chemistry 2 HF(g) Copyright © 2010 Pearson Education, Inc. 8
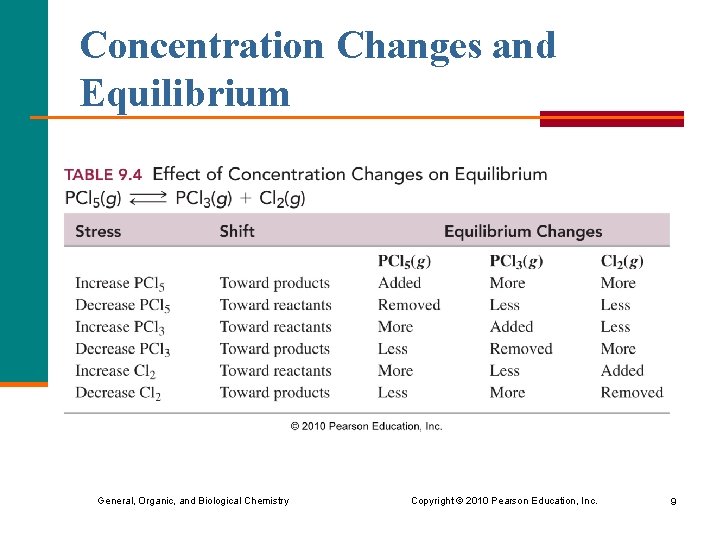
Concentration Changes and Equilibrium General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

Effect of a Catalyst Adding a catalyst § lowers the activation energy of the forward reaction § increases the rate of the forward reaction § lowers the activation energy of the reverse reaction § increases the rate of the reverse reaction § decreases the time to reach equilibrium § has no effect on the equilibrium General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10
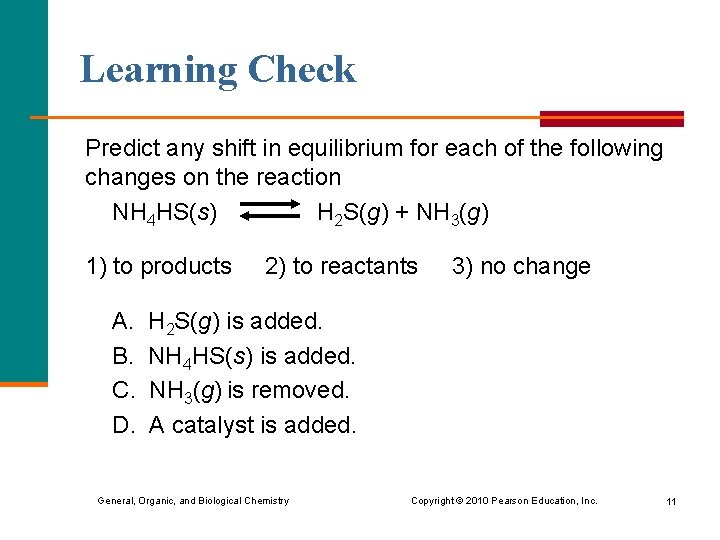
Learning Check Predict any shift in equilibrium for each of the following changes on the reaction NH 4 HS(s) H 2 S(g) + NH 3(g) 1) to products A. B. C. D. 2) to reactants 3) no change H 2 S(g) is added. NH 4 HS(s) is added. NH 3(g) is removed. A catalyst is added. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11
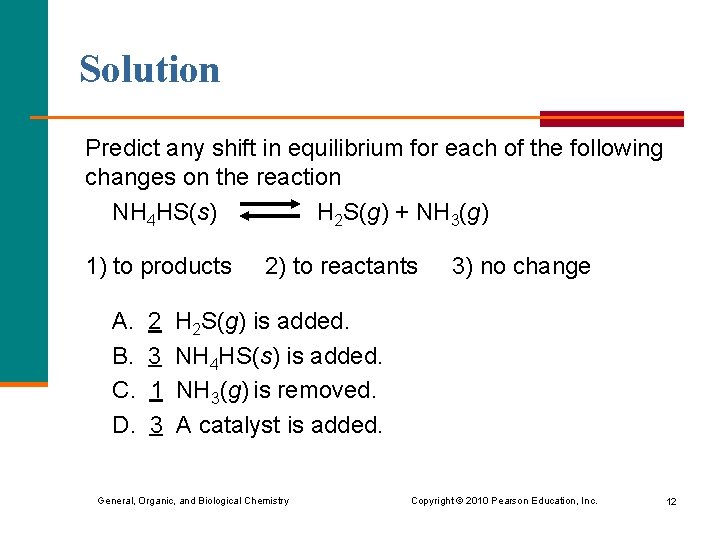
Solution Predict any shift in equilibrium for each of the following changes on the reaction NH 4 HS(s) H 2 S(g) + NH 3(g) 1) to products A. B. C. D. 2 3 1 3 2) to reactants 3) no change H 2 S(g) is added. NH 4 HS(s) is added. NH 3(g) is removed. A catalyst is added. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12
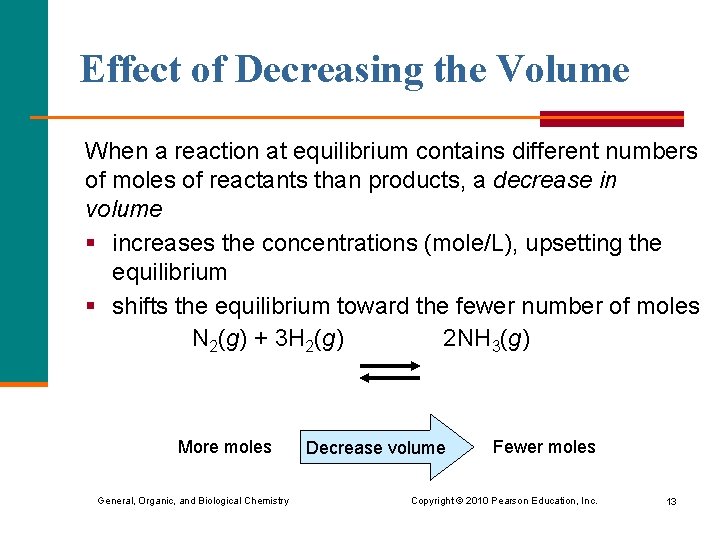
Effect of Decreasing the Volume When a reaction at equilibrium contains different numbers of moles of reactants than products, a decrease in volume § increases the concentrations (mole/L), upsetting the equilibrium § shifts the equilibrium toward the fewer number of moles N 2(g) + 3 H 2(g) 2 NH 3(g) More moles General, Organic, and Biological Chemistry Decrease volume Fewer moles Copyright © 2010 Pearson Education, Inc. 13

Volume Decrease and Equilibrium A volume decrease § shifts the equilibrium toward the side (A) with the smaller number of moles General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14
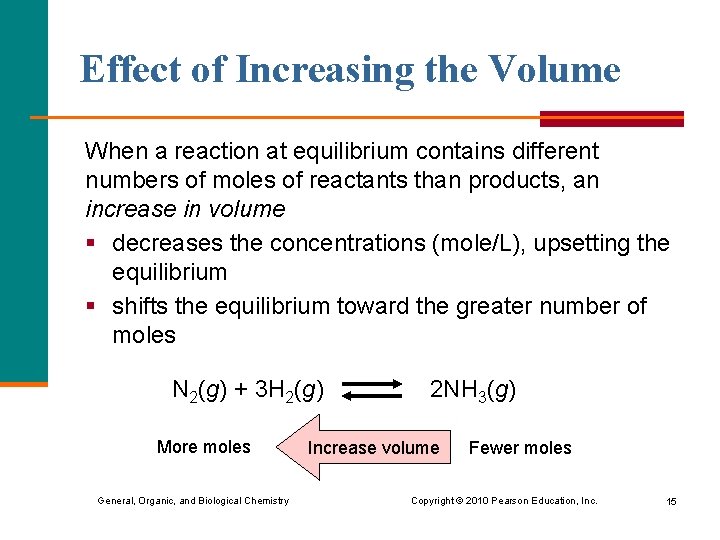
Effect of Increasing the Volume When a reaction at equilibrium contains different numbers of moles of reactants than products, an increase in volume § decreases the concentrations (mole/L), upsetting the equilibrium § shifts the equilibrium toward the greater number of moles N 2(g) + 3 H 2(g) More moles General, Organic, and Biological Chemistry 2 NH 3(g) Increase volume Fewer moles Copyright © 2010 Pearson Education, Inc. 15

Volume Increase and Equilibrium A volume increase § shifts the equilibrium toward the side (B and C) with the greater number of moles General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16
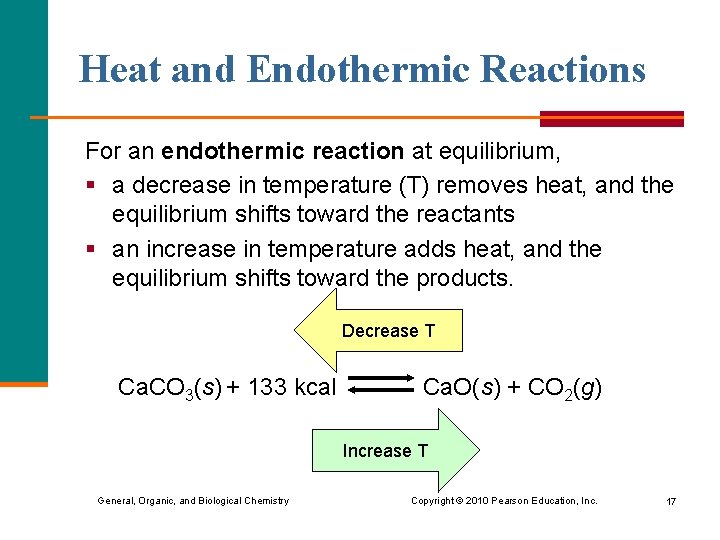
Heat and Endothermic Reactions For an endothermic reaction at equilibrium, § a decrease in temperature (T) removes heat, and the equilibrium shifts toward the reactants § an increase in temperature adds heat, and the equilibrium shifts toward the products. Decrease T Ca. CO 3(s) + 133 kcal Ca. O(s) + CO 2(g) Increase T General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17
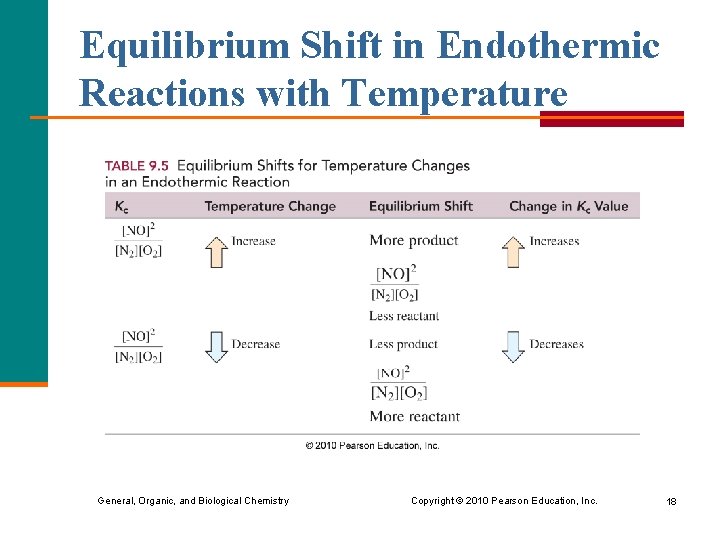
Equilibrium Shift in Endothermic Reactions with Temperature General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18
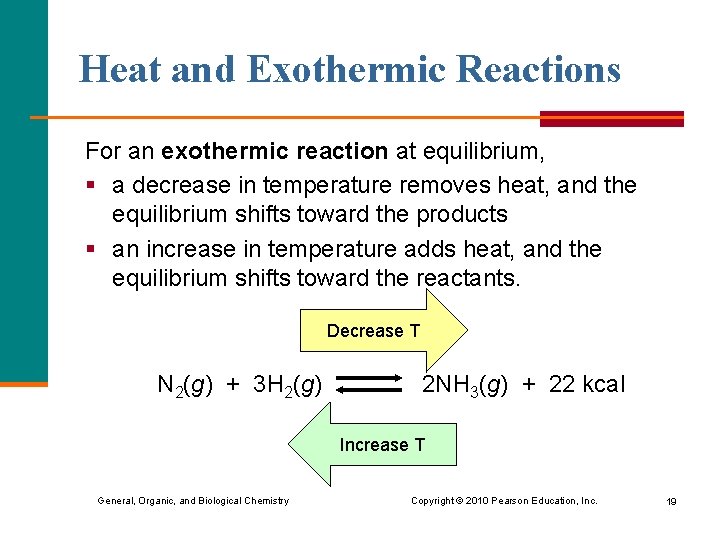
Heat and Exothermic Reactions For an exothermic reaction at equilibrium, § a decrease in temperature removes heat, and the equilibrium shifts toward the products § an increase in temperature adds heat, and the equilibrium shifts toward the reactants. Decrease T N 2(g) + 3 H 2(g) 2 NH 3(g) + 22 kcal Increase T General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

Equilibrium Shifts with Temperature in Exothermic Reactions General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20
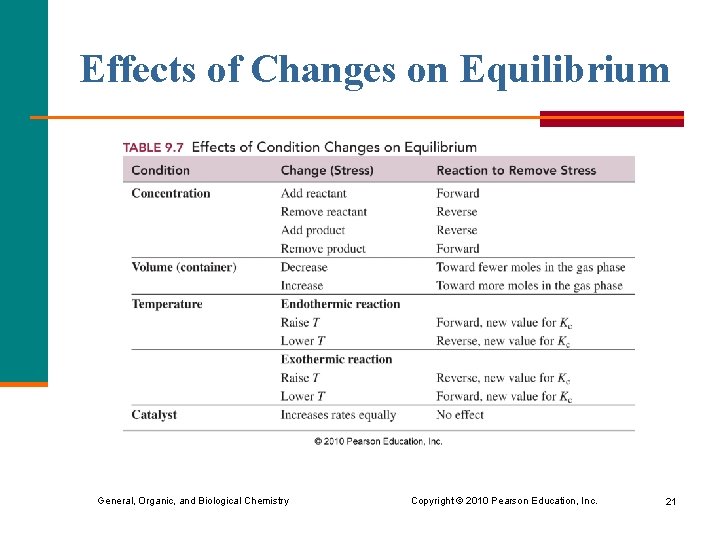
Effects of Changes on Equilibrium General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 21

Learning Check Indicate if each change on a reaction at equilibrium shifts 2 NO 2(g) + heat 2 NO(g) + O 2(g) 1) towards products 2) towards reactants 3) no change A. B. C. D. E. adding NO(g) adding N 2(g) raising the temperature removing O 2(g) increasing the volume General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 22
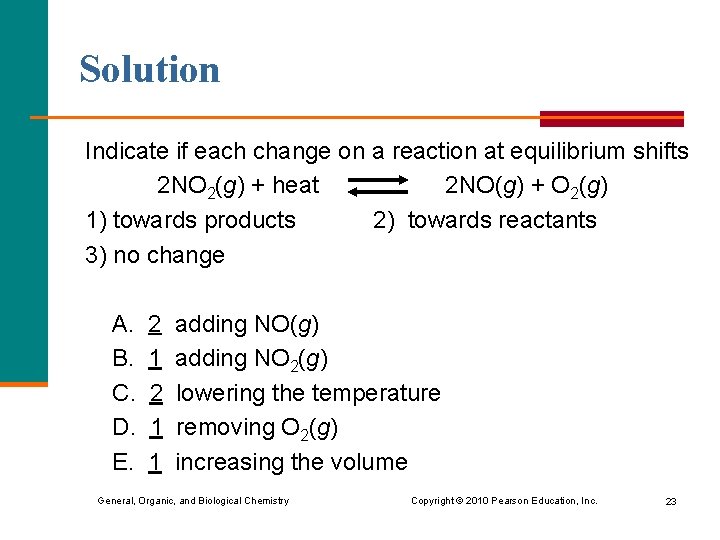
Solution Indicate if each change on a reaction at equilibrium shifts 2 NO 2(g) + heat 2 NO(g) + O 2(g) 1) towards products 2) towards reactants 3) no change A. B. C. D. E. 2 1 1 adding NO(g) adding NO 2(g) lowering the temperature removing O 2(g) increasing the volume General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 23
- Slides: 23