Chapter 9 Chemical Equilibrium 9 4 Using Equilibrium
Chapter 9 Chemical Equilibrium 9. 4 Using Equilibrium Constants General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1
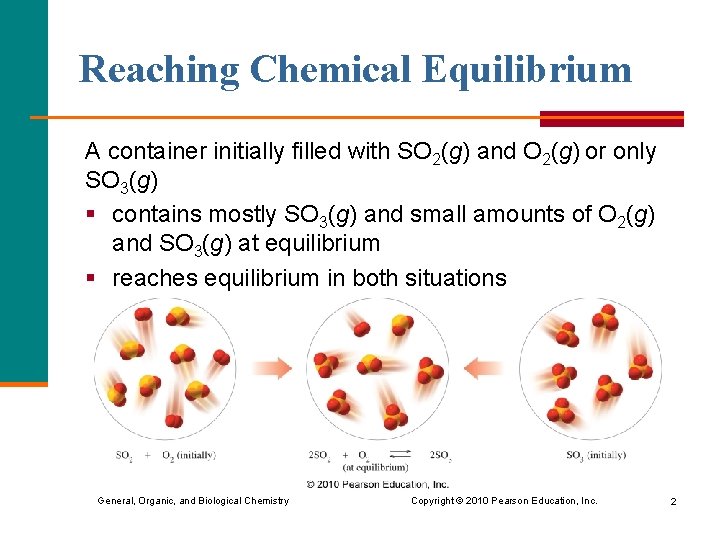
Reaching Chemical Equilibrium A container initially filled with SO 2(g) and O 2(g) or only SO 3(g) § contains mostly SO 3(g) and small amounts of O 2(g) and SO 3(g) at equilibrium § reaches equilibrium in both situations General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2
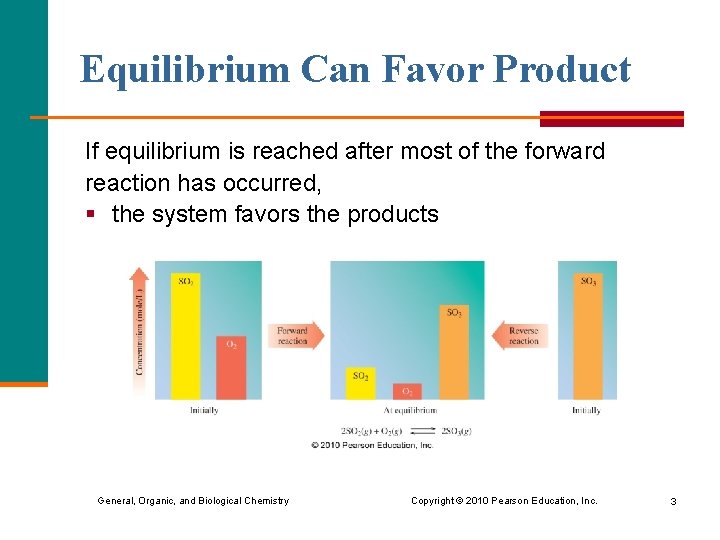
Equilibrium Can Favor Product If equilibrium is reached after most of the forward reaction has occurred, § the system favors the products General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3
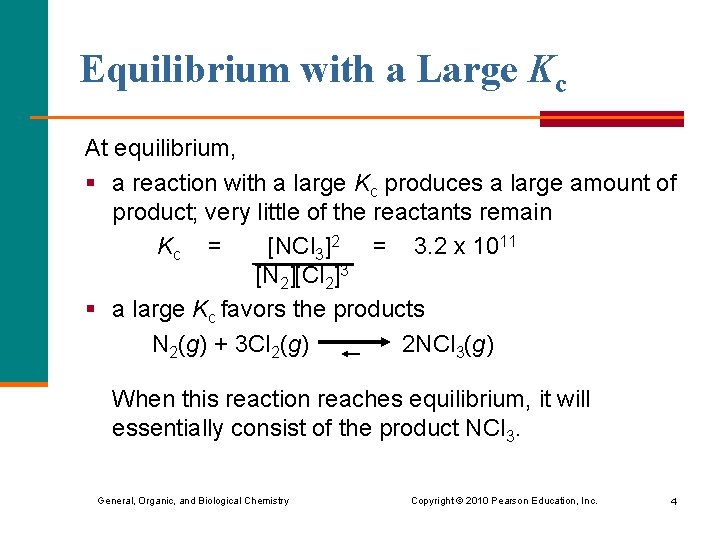
Equilibrium with a Large Kc At equilibrium, § a reaction with a large Kc produces a large amount of product; very little of the reactants remain Kc = [NCl 3]2 = 3. 2 x 1011 [N 2][Cl 2]3 § a large Kc favors the products N 2(g) + 3 Cl 2(g) 2 NCl 3(g) When this reaction reaches equilibrium, it will essentially consist of the product NCl 3. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4
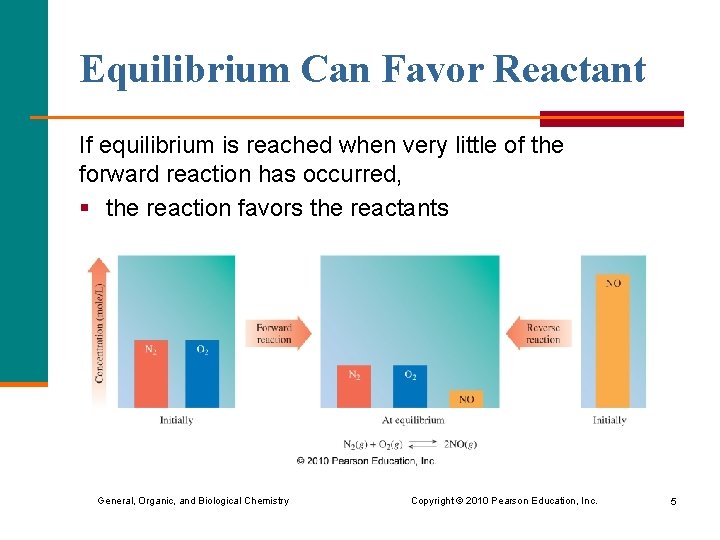
Equilibrium Can Favor Reactant If equilibrium is reached when very little of the forward reaction has occurred, § the reaction favors the reactants General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5
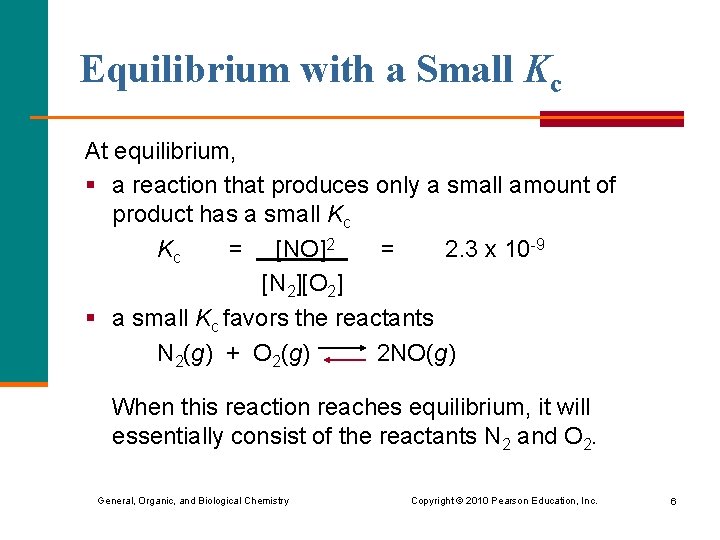
Equilibrium with a Small Kc At equilibrium, § a reaction that produces only a small amount of product has a small Kc Kc = [NO]2 = 2. 3 x 10 -9 [N 2][O 2] § a small Kc favors the reactants N 2(g) + O 2(g) 2 NO(g) When this reaction reaches equilibrium, it will essentially consist of the reactants N 2 and O 2. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6
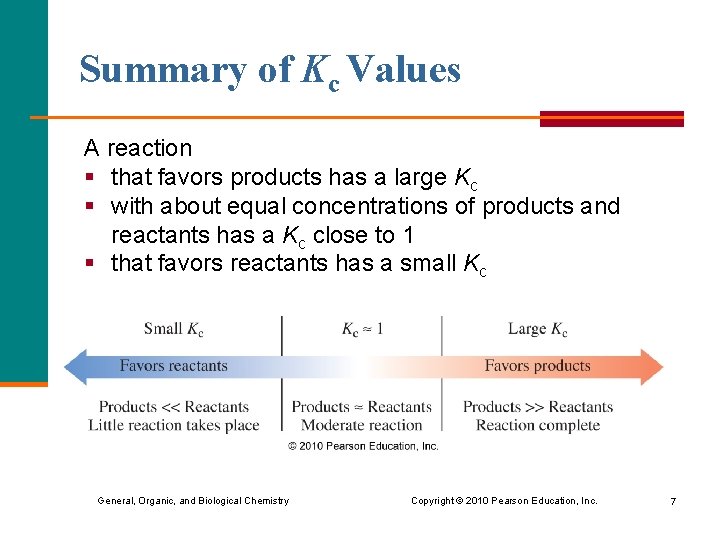
Summary of Kc Values A reaction § that favors products has a large Kc § with about equal concentrations of products and reactants has a Kc close to 1 § that favors reactants has a small Kc General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7
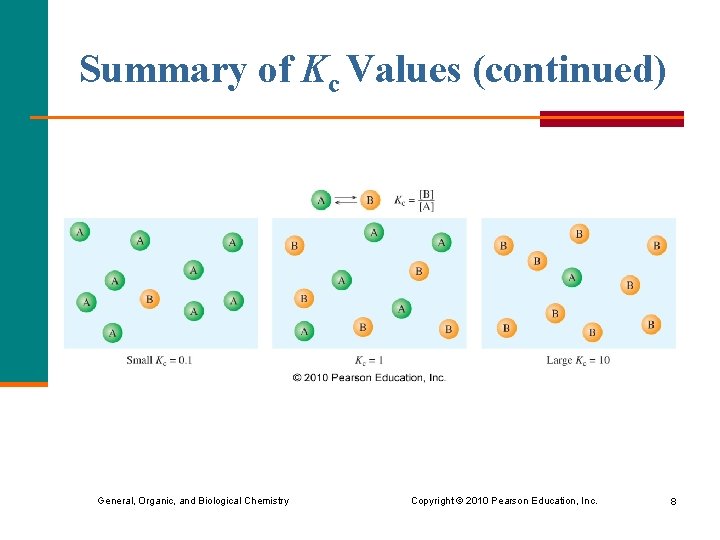
Summary of Kc Values (continued) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8
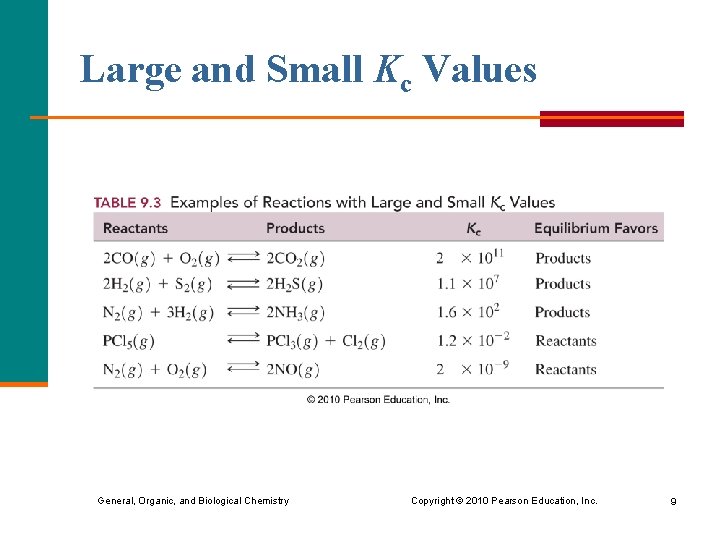
Large and Small Kc Values General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9
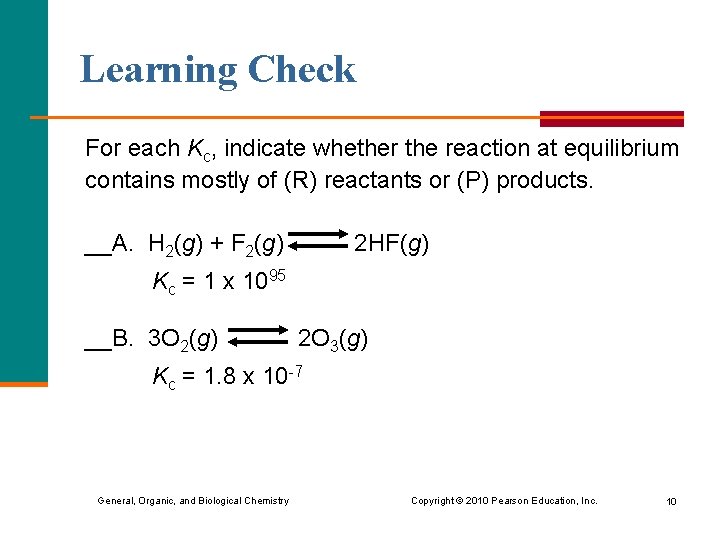
Learning Check For each Kc, indicate whether the reaction at equilibrium contains mostly of (R) reactants or (P) products. __A. H 2(g) + F 2(g) 2 HF(g) Kc = 1 x 1095 __B. 3 O 2(g) 2 O 3(g) Kc = 1. 8 x 10 -7 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10
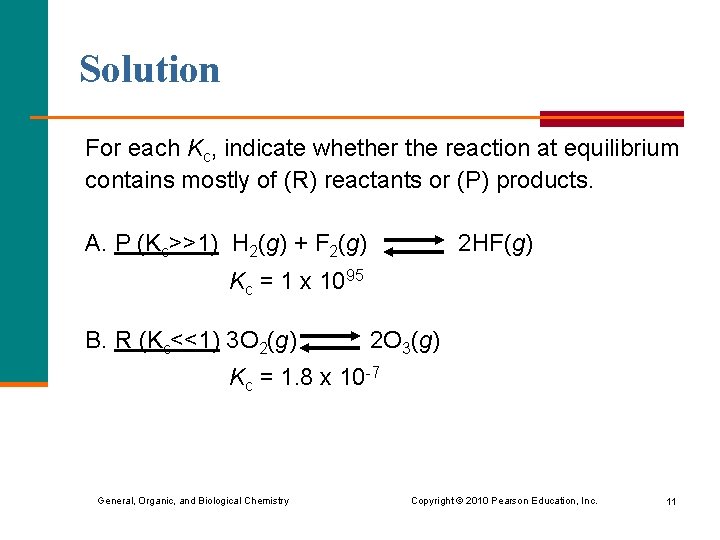
Solution For each Kc, indicate whether the reaction at equilibrium contains mostly of (R) reactants or (P) products. A. P (Kc>>1) H 2(g) + F 2(g) 2 HF(g) Kc = 1 x 1095 B. R (Kc<<1) 3 O 2(g) 2 O 3(g) Kc = 1. 8 x 10 -7 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11
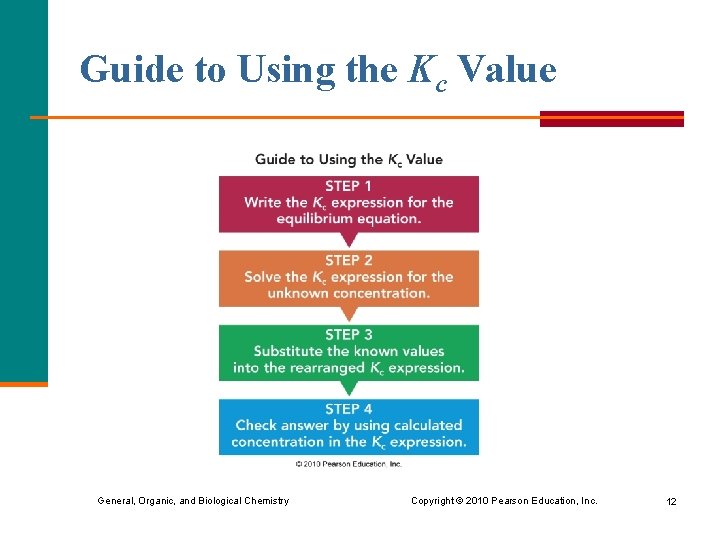
Guide to Using the Kc Value General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12
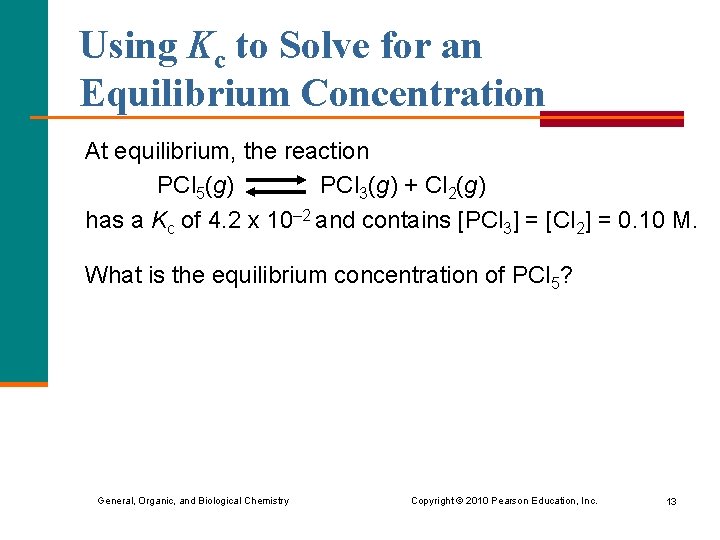
Using Kc to Solve for an Equilibrium Concentration At equilibrium, the reaction PCl 5(g) PCl 3(g) + Cl 2(g) has a Kc of 4. 2 x 10– 2 and contains [PCl 3] = [Cl 2] = 0. 10 M. What is the equilibrium concentration of PCl 5? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13
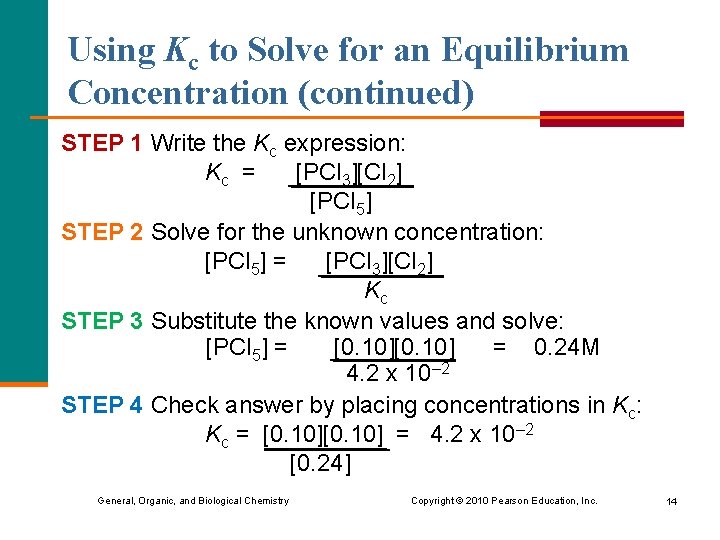
Using Kc to Solve for an Equilibrium Concentration (continued) STEP 1 Write the Kc expression: Kc = [PCl 3][Cl 2] [PCl 5] STEP 2 Solve for the unknown concentration: [PCl 5] = [PCl 3][Cl 2] Kc STEP 3 Substitute the known values and solve: [PCl 5] = [0. 10] = 0. 24 M 4. 2 x 10– 2 STEP 4 Check answer by placing concentrations in Kc: Kc = [0. 10] = 4. 2 x 10– 2 [0. 24] General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14
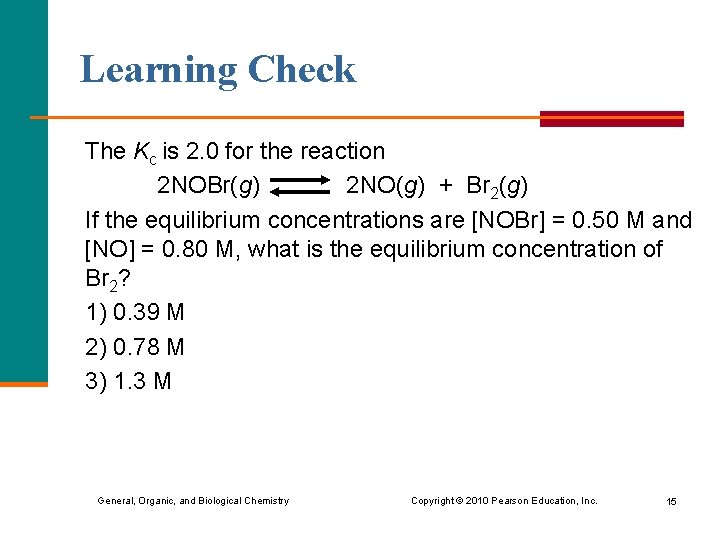
Learning Check The Kc is 2. 0 for the reaction 2 NOBr(g) 2 NO(g) + Br 2(g) If the equilibrium concentrations are [NOBr] = 0. 50 M and [NO] = 0. 80 M, what is the equilibrium concentration of Br 2? 1) 0. 39 M 2) 0. 78 M 3) 1. 3 M General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15
![Solution STEP 1 Write the Kc expression: Kc = [NO]2 [Br 2] [NOBr]2 STEP Solution STEP 1 Write the Kc expression: Kc = [NO]2 [Br 2] [NOBr]2 STEP](http://slidetodoc.com/presentation_image_h2/45c1dee92045de0d48d45e42d5c32019/image-16.jpg)
Solution STEP 1 Write the Kc expression: Kc = [NO]2 [Br 2] [NOBr]2 STEP 2 Solve for the unknown concentration: [Br 2] = [NOBr]2 Kc [NO]2 STEP 3 Substitute known values and solve: [Br 2] = [0. 50]2 2. 0 = 0. 78 M (2) [0. 80]2 STEP 4 Check answer by placing concentrations in Kc: Kc = [0. 80]2[0. 78] = 2. 0 2 [0. 50] General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16
- Slides: 16