Chapter 9 Chemical Equilibrium 9 2 Chemical Equilibrium
Chapter 9 Chemical Equilibrium 9. 2 Chemical Equilibrium General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1
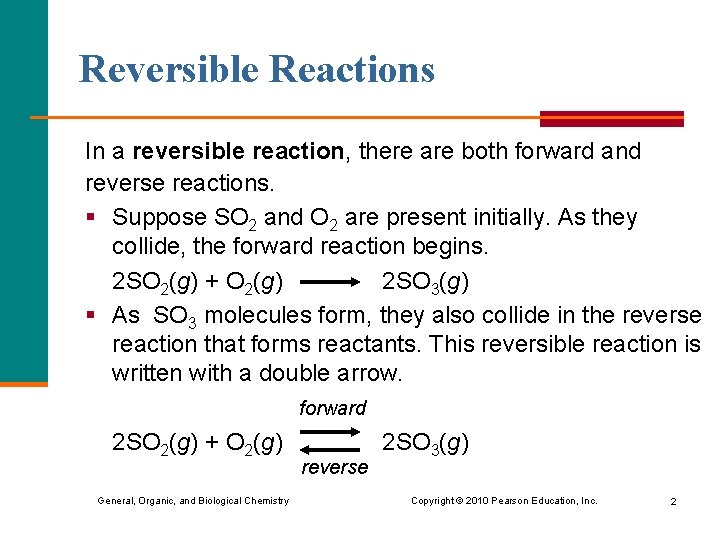
Reversible Reactions In a reversible reaction, there are both forward and reverse reactions. § Suppose SO 2 and O 2 are present initially. As they collide, the forward reaction begins. 2 SO 2(g) + O 2(g) 2 SO 3(g) § As SO 3 molecules form, they also collide in the reverse reaction that forms reactants. This reversible reaction is written with a double arrow. forward 2 SO 2(g) + O 2(g) 2 SO 3(g) reverse General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2
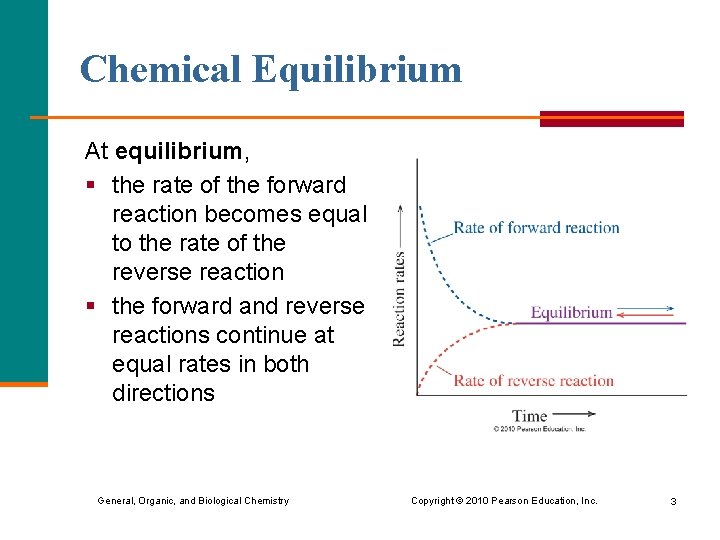
Chemical Equilibrium At equilibrium, § the rate of the forward reaction becomes equal to the rate of the reverse reaction § the forward and reverse reactions continue at equal rates in both directions General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

Chemical Equilibrium (continued) When equilibrium is reached, § there is no further change in the amounts of reactant and product General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4
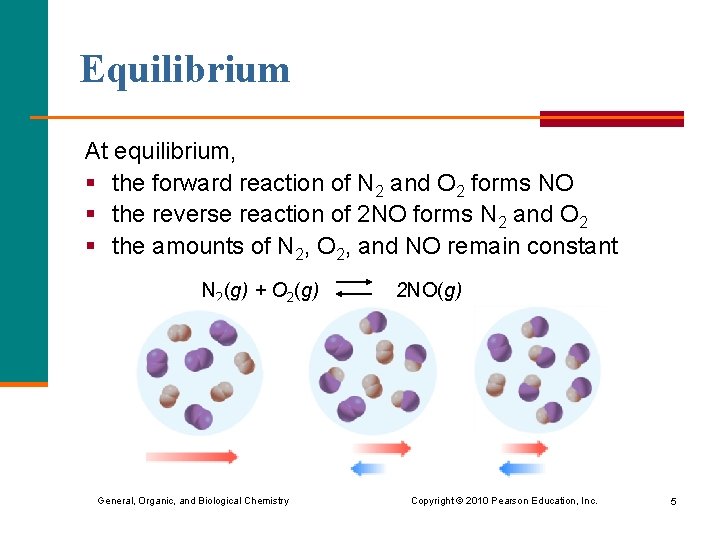
Equilibrium At equilibrium, § the forward reaction of N 2 and O 2 forms NO § the reverse reaction of 2 NO forms N 2 and O 2 § the amounts of N 2, O 2, and NO remain constant N 2(g) + O 2(g) 2 NO(g) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5
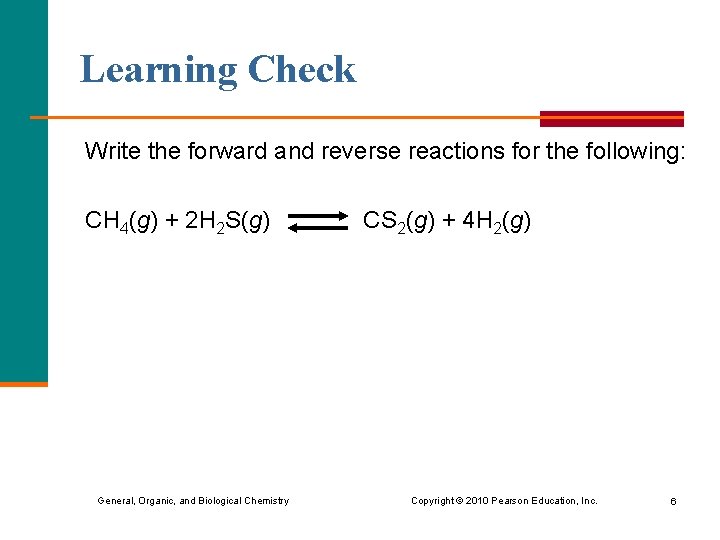
Learning Check Write the forward and reverse reactions for the following: CH 4(g) + 2 H 2 S(g) CS 2(g) + 4 H 2(g) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6
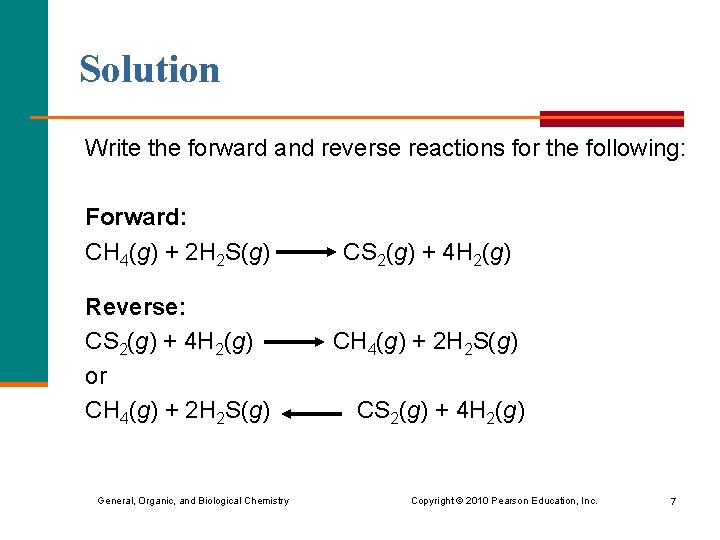
Solution Write the forward and reverse reactions for the following: Forward: CH 4(g) + 2 H 2 S(g) CS 2(g) + 4 H 2(g) Reverse: CS 2(g) + 4 H 2(g) CH 4(g) + 2 H 2 S(g) or CH 4(g) + 2 H 2 S(g) CS 2(g) + 4 H 2(g) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7
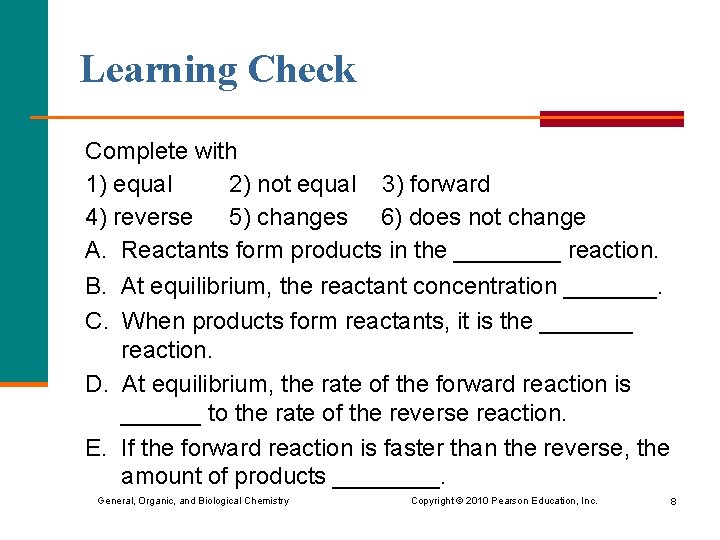
Learning Check Complete with 1) equal 2) not equal 3) forward 4) reverse 5) changes 6) does not change A. Reactants form products in the ____ reaction. B. At equilibrium, the reactant concentration _______. C. When products form reactants, it is the _______ reaction. D. At equilibrium, the rate of the forward reaction is ______ to the rate of the reverse reaction. E. If the forward reaction is faster than the reverse, the amount of products ____. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8
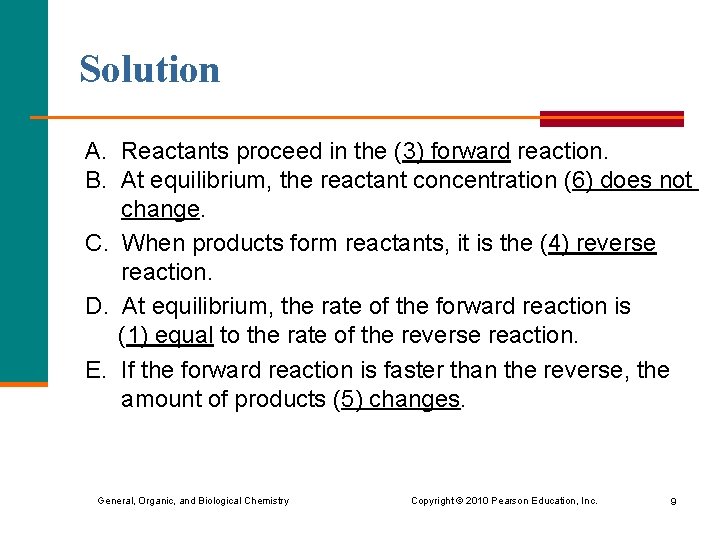
Solution A. Reactants proceed in the (3) forward reaction. B. At equilibrium, the reactant concentration (6) does not change. C. When products form reactants, it is the (4) reverse reaction. D. At equilibrium, the rate of the forward reaction is (1) equal to the rate of the reverse reaction. E. If the forward reaction is faster than the reverse, the amount of products (5) changes. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9
- Slides: 9