Chapter 9 Chemical Bonding Theories Valence Bond Theory
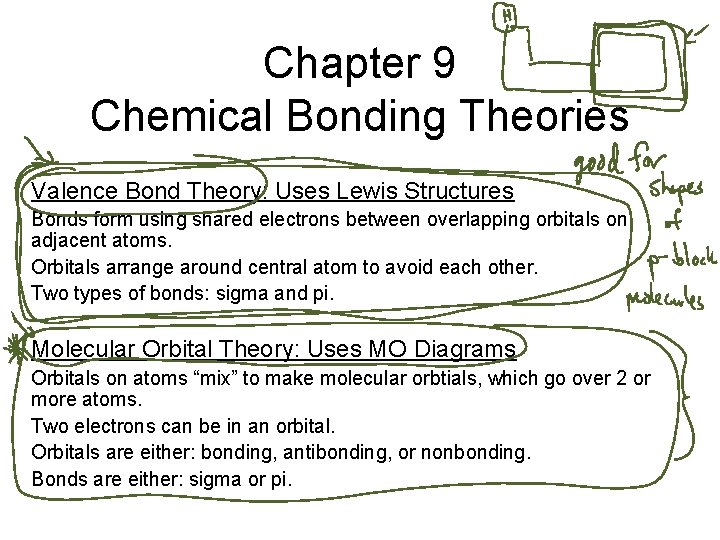
Chapter 9 Chemical Bonding Theories Valence Bond Theory: Uses Lewis Structures Bonds form using shared electrons between overlapping orbitals on adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. Molecular Orbital Theory: Uses MO Diagrams Orbitals on atoms “mix” to make molecular orbtials, which go over 2 or more atoms. Two electrons can be in an orbital. Orbitals are either: bonding, antibonding, or nonbonding. Bonds are either: sigma or pi.
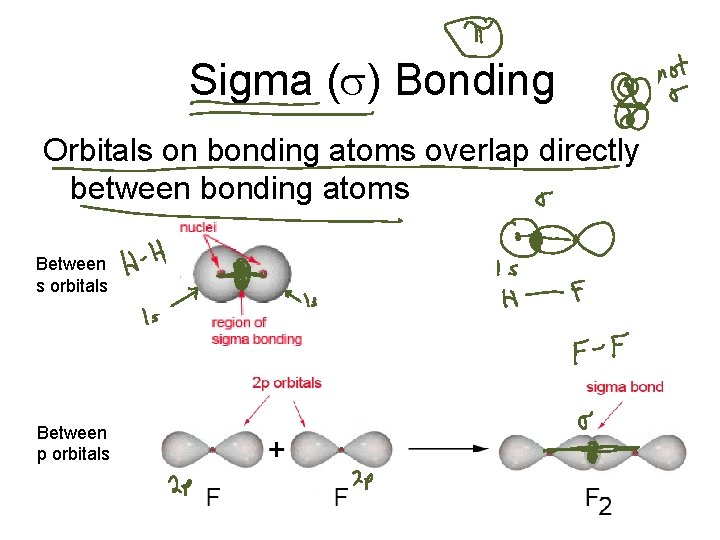
Sigma (s) Bonding Orbitals on bonding atoms overlap directly between bonding atoms Between s orbitals Between p orbitals
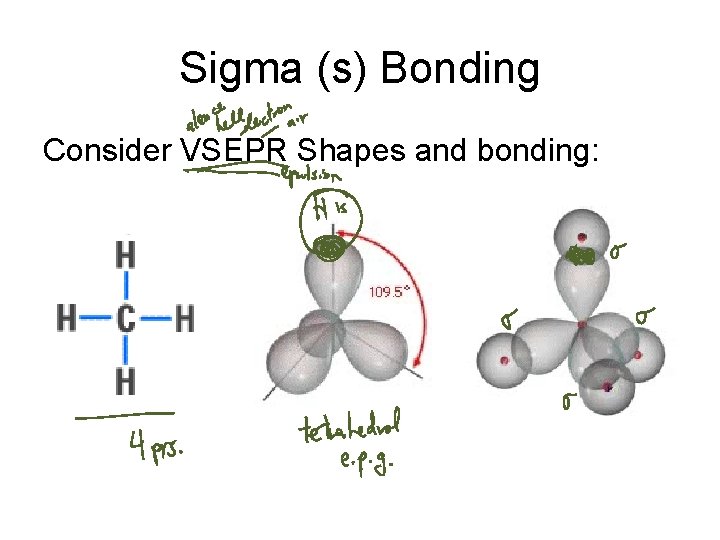
Sigma (s) Bonding Consider VSEPR Shapes and bonding:
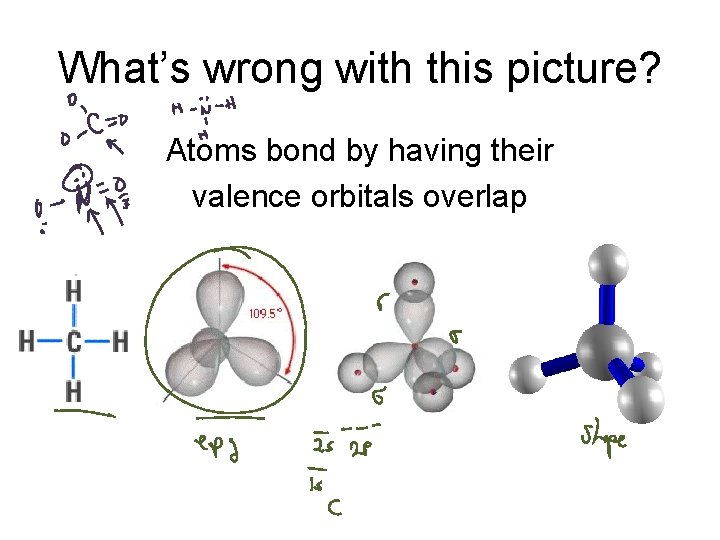
What’s wrong with this picture? Atoms bond by having their valence orbitals overlap

Enter question text. . . 1. 1 2. 2 3. 3
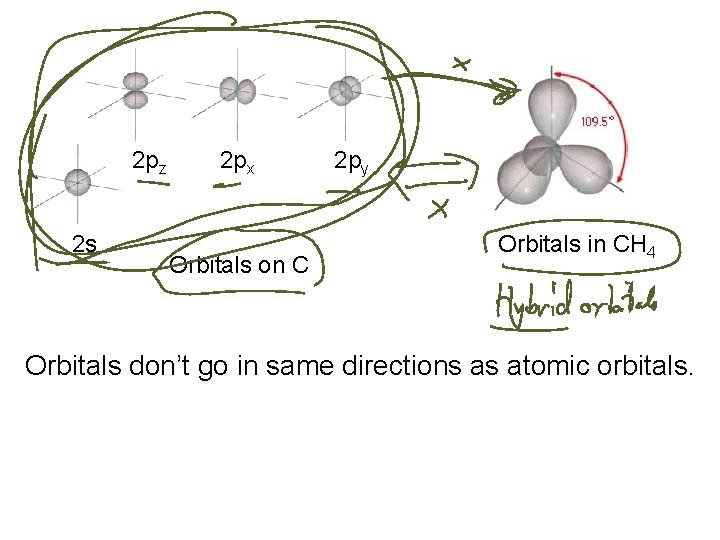
2 pz 2 s 2 px Orbitals on C 2 py Orbitals in CH 4 Orbitals don’t go in same directions as atomic orbitals.
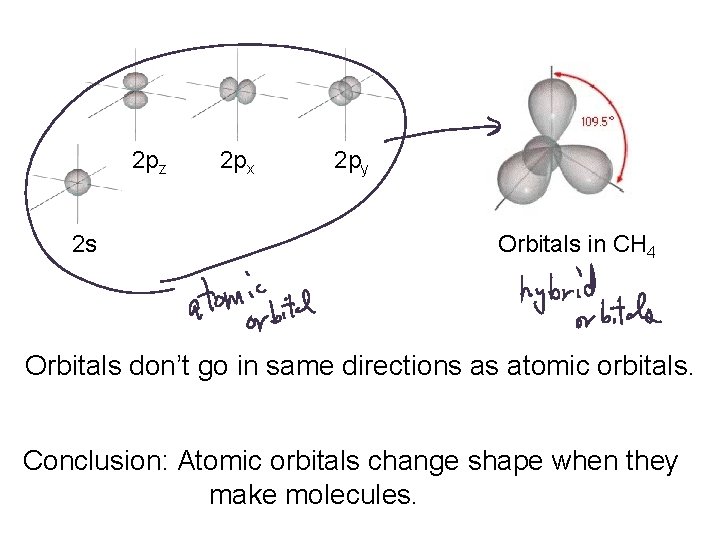
2 pz 2 s 2 px 2 py Orbitals in CH 4 Orbitals don’t go in same directions as atomic orbitals. Conclusion: Atomic orbitals change shape when they make molecules.
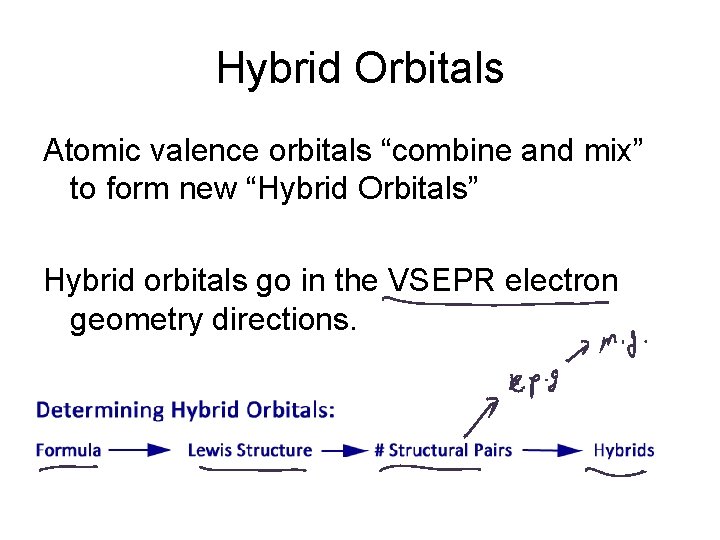
Hybrid Orbitals Atomic valence orbitals “combine and mix” to form new “Hybrid Orbitals” Hybrid orbitals go in the VSEPR electron geometry directions.
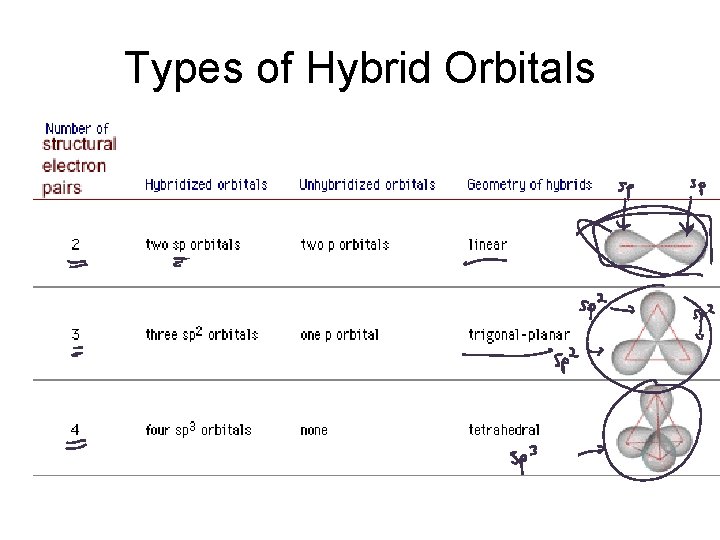
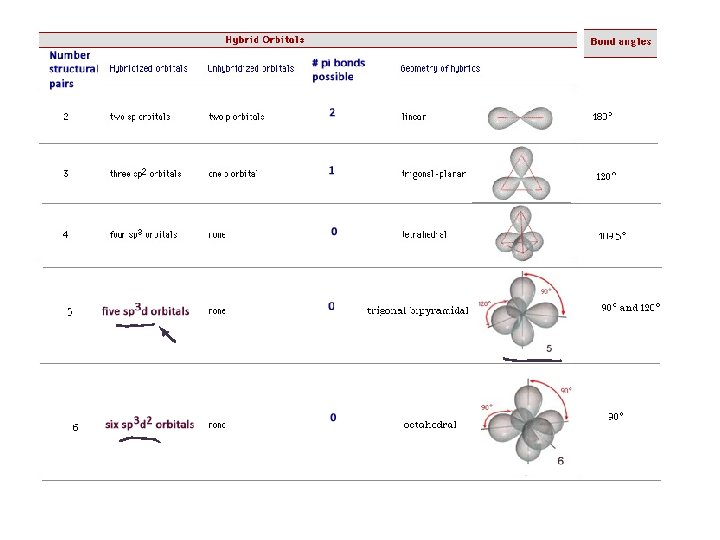
Types of Hybrid Orbitals
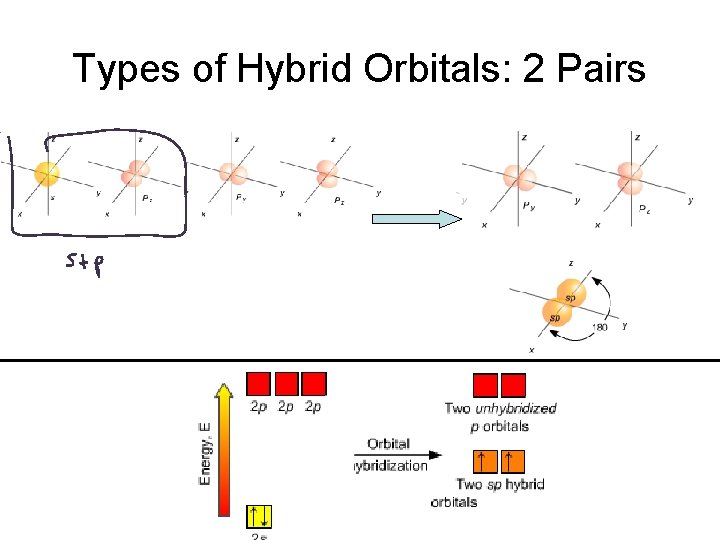
Types of Hybrid Orbitals: 2 Pairs
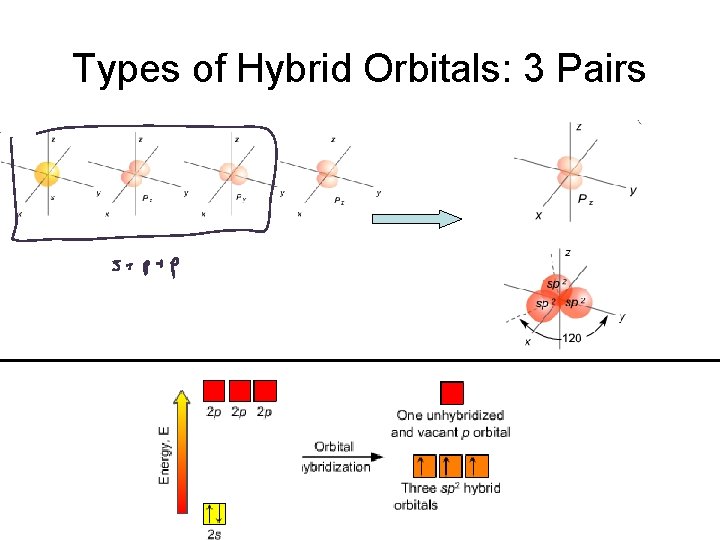
Types of Hybrid Orbitals: 3 Pairs
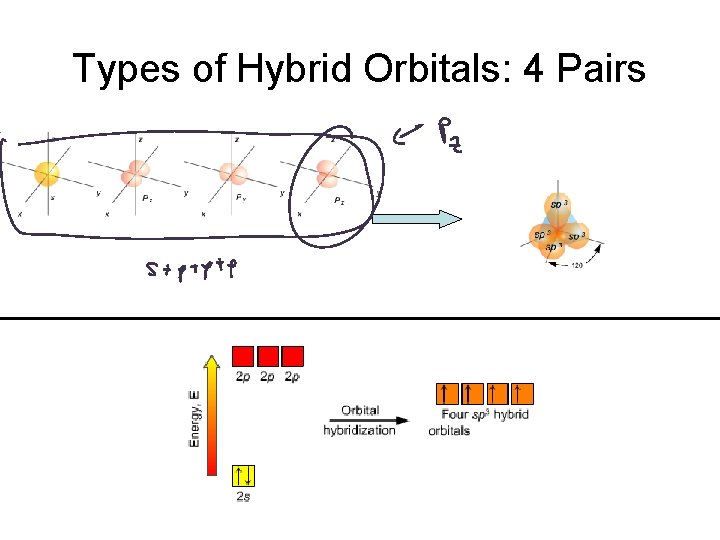
Types of Hybrid Orbitals: 4 Pairs
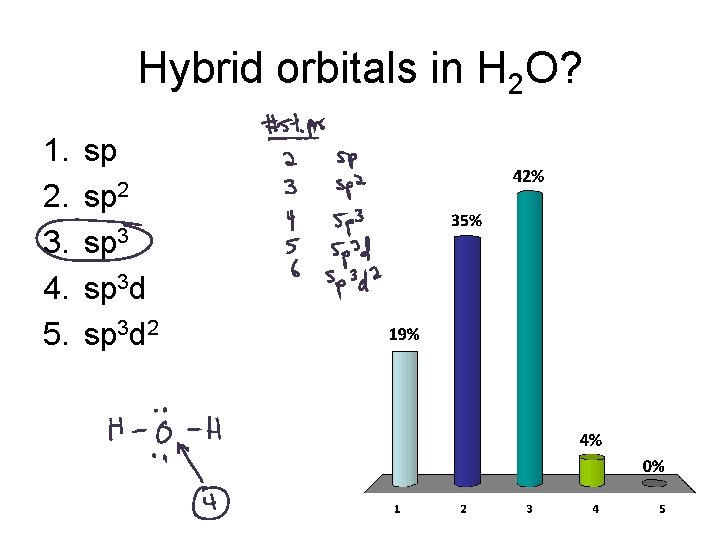
Hybrid orbitals in H 2 O? 1. 2. 3. 4. 5. sp sp 2 sp 3 d 2
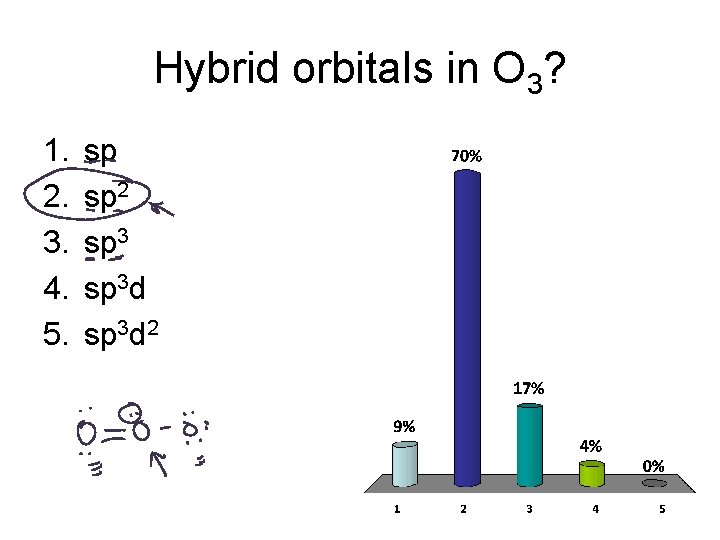
Hybrid orbitals in O 3? 1. 2. 3. 4. 5. sp sp 2 sp 3 d 2
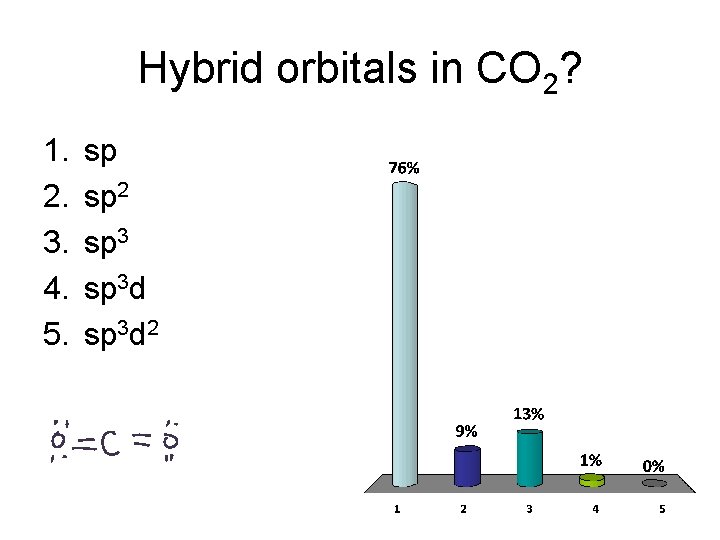
Hybrid orbitals in CO 2? 1. 2. 3. 4. 5. sp sp 2 sp 3 d 2
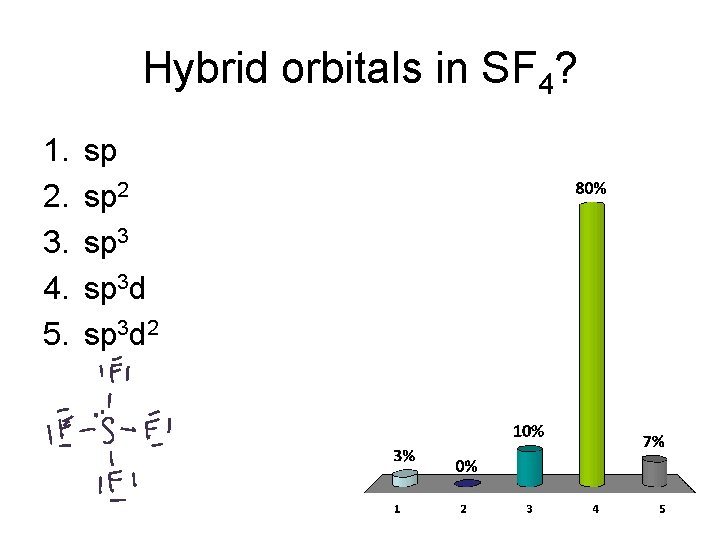
Hybrid orbitals in SF 4? 1. 2. 3. 4. 5. sp sp 2 sp 3 d 2
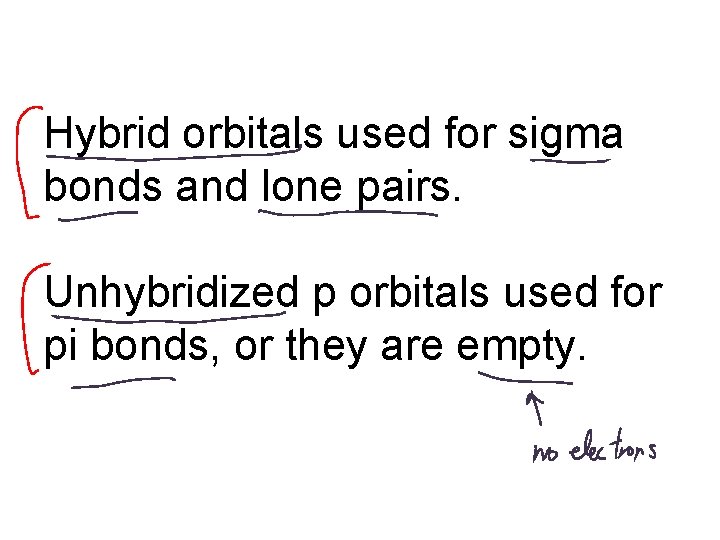
Hybrid orbitals used for sigma bonds and lone pairs. Unhybridized p orbitals used for pi bonds, or they are empty.
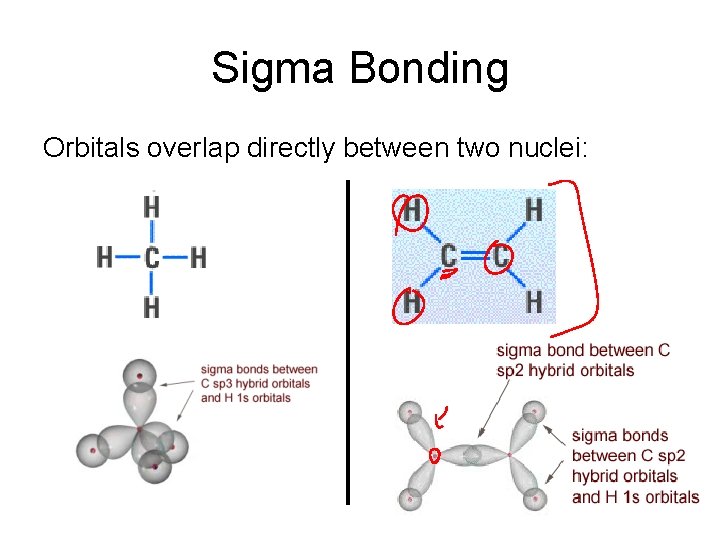
Sigma Bonding Orbitals overlap directly between two nuclei:
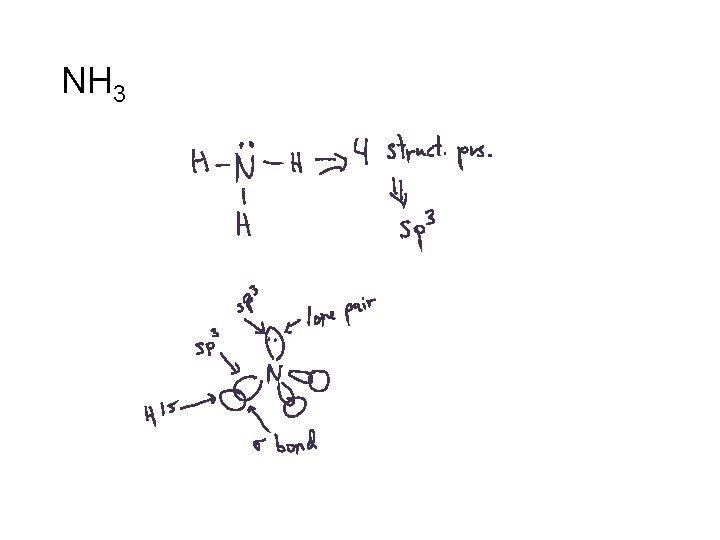
NH 3
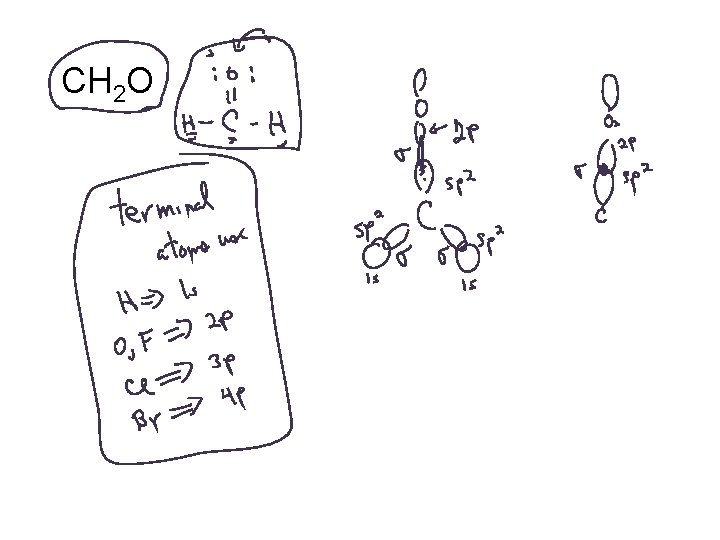
CH 2 O

Sigma vs. Pi Bonding Sigma Bonding involves hybrid orbitals and/or H 1 s orbitals. Pi bonding involves unhybridized p orbitals.
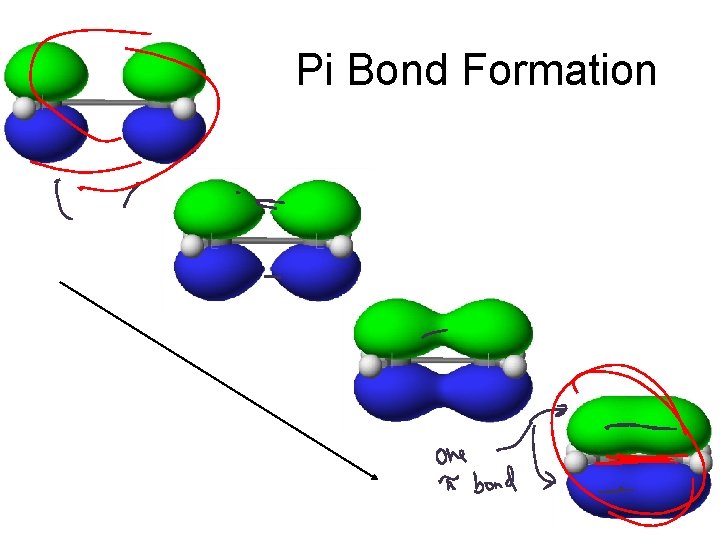
Pi Bond Formation
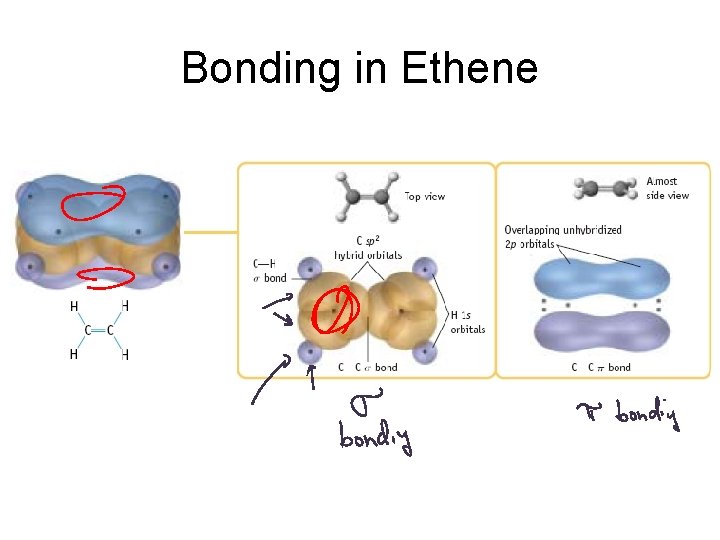
Bonding in Ethene
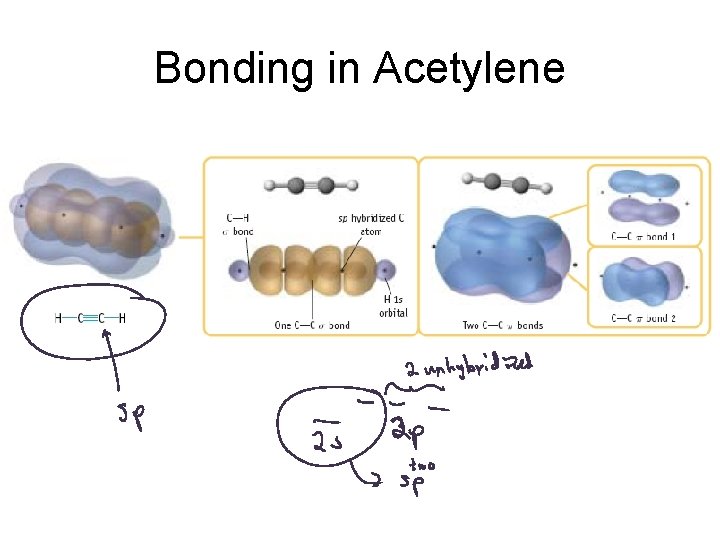
Bonding in Acetylene
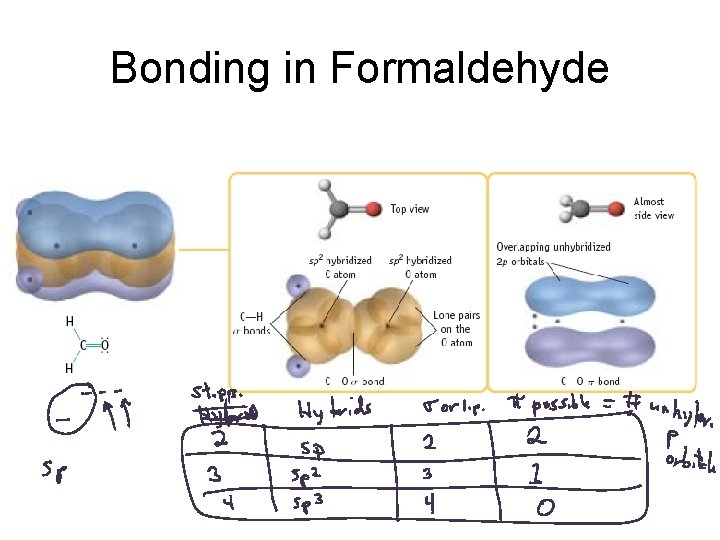
Bonding in Formaldehyde
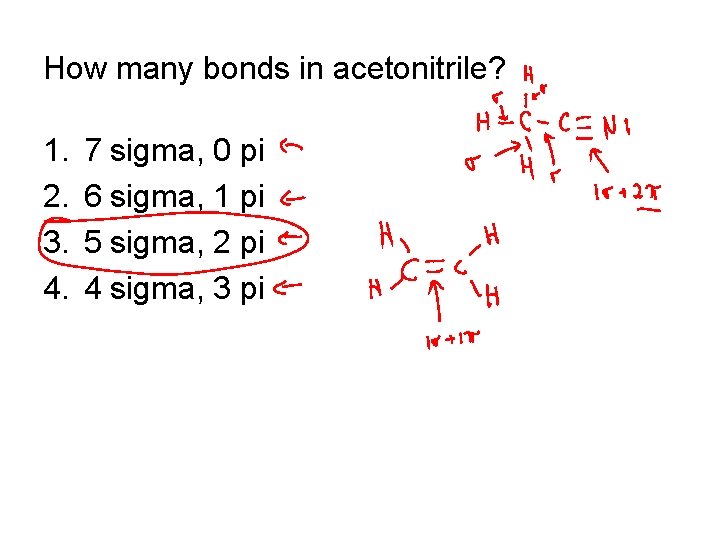
How many bonds in acetonitrile? 1. 2. 3. 4. 7 sigma, 0 pi 6 sigma, 1 pi 5 sigma, 2 pi 4 sigma, 3 pi
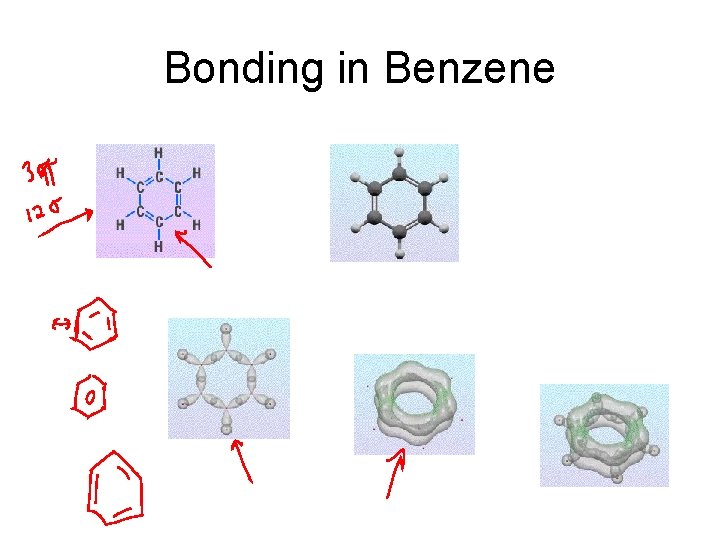
Bonding in Benzene
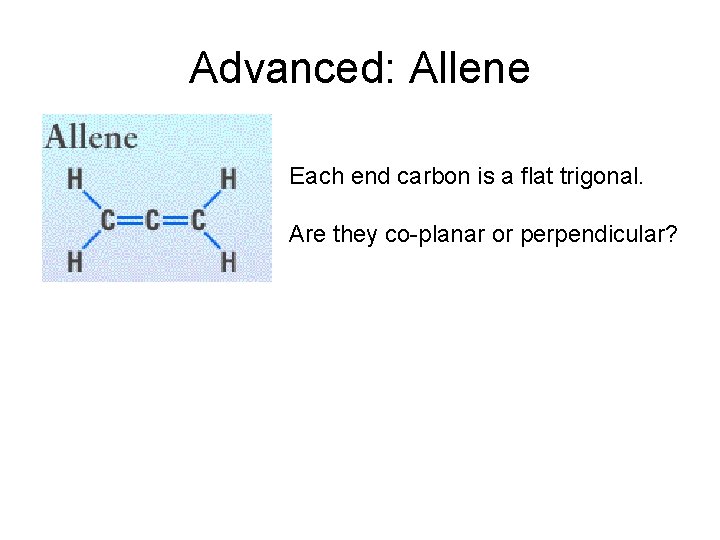
Advanced: Allene Each end carbon is a flat trigonal. Are they co-planar or perpendicular?
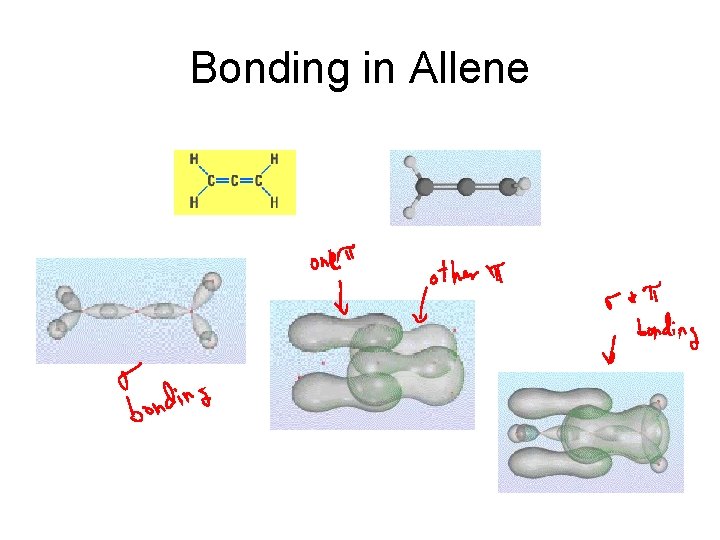
Bonding in Allene
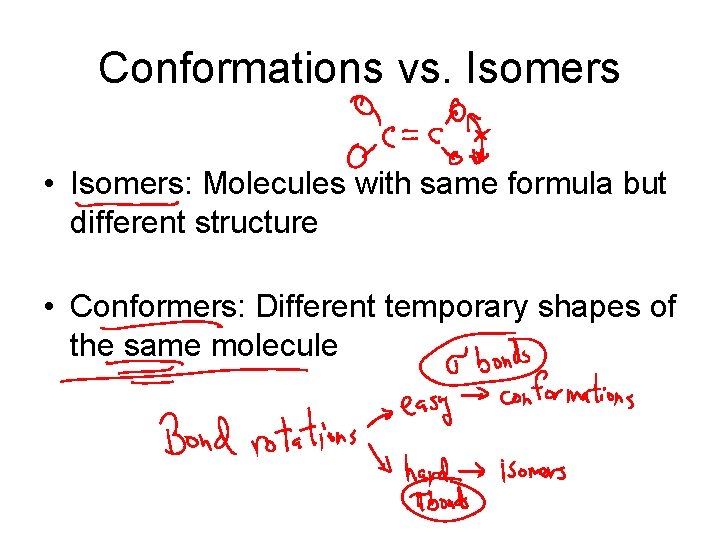
Conformations vs. Isomers • Isomers: Molecules with same formula but different structure • Conformers: Different temporary shapes of the same molecule
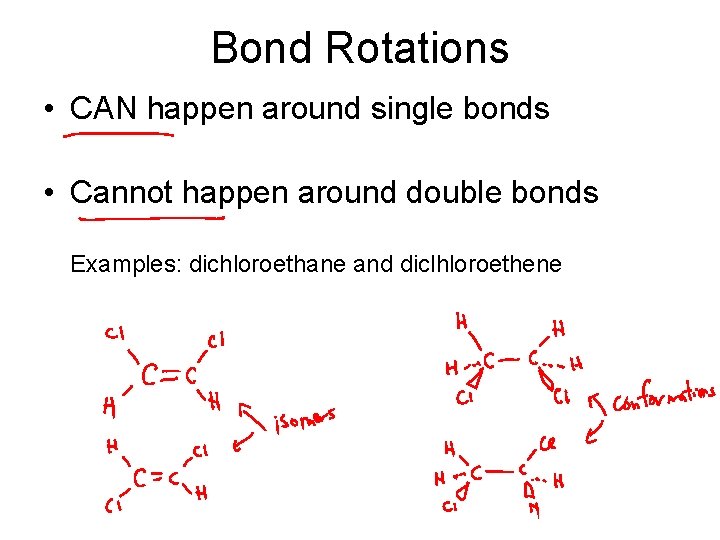
Bond Rotations • CAN happen around single bonds • Cannot happen around double bonds Examples: dichloroethane and diclhloroethene
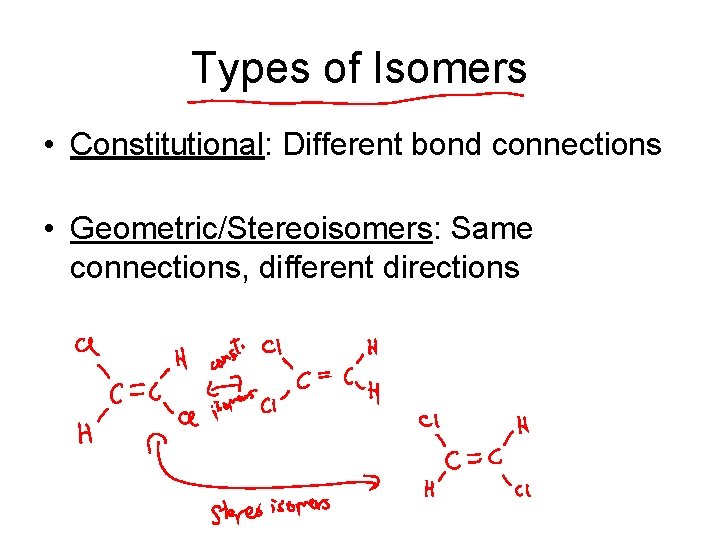
Types of Isomers • Constitutional: Different bond connections • Geometric/Stereoisomers: Same connections, different directions
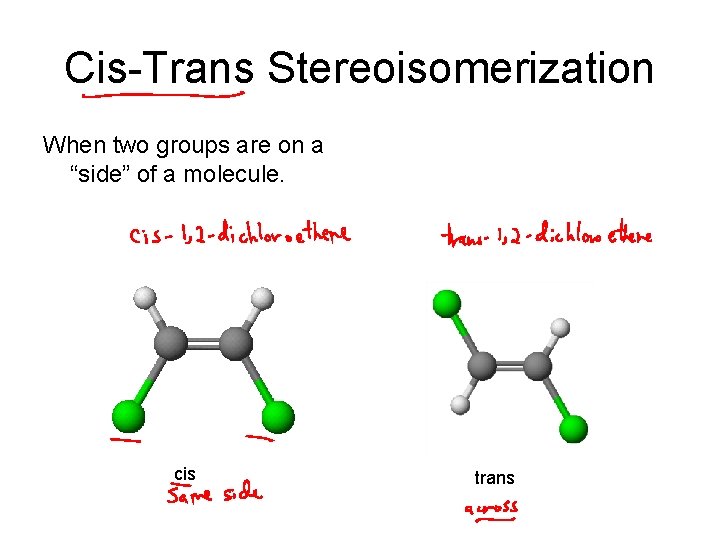
Cis-Trans Stereoisomerization When two groups are on a “side” of a molecule. cis trans
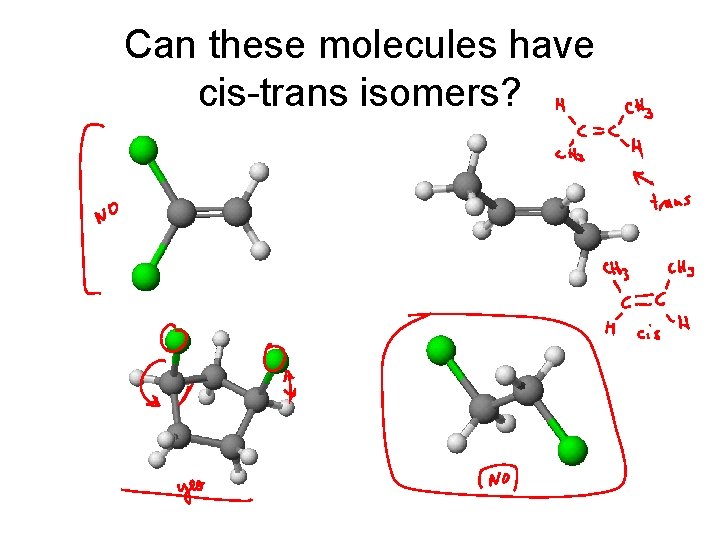
Can these molecules have cis-trans isomers?
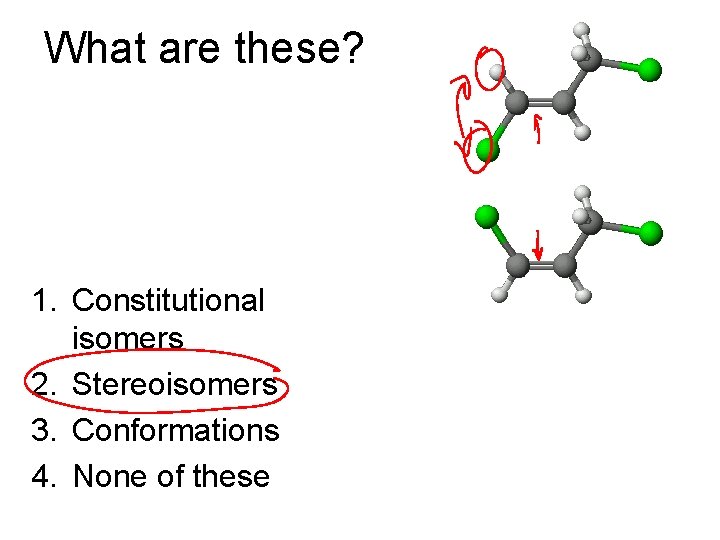
What are these? 1. Constitutional isomers 2. Stereoisomers 3. Conformations 4. None of these
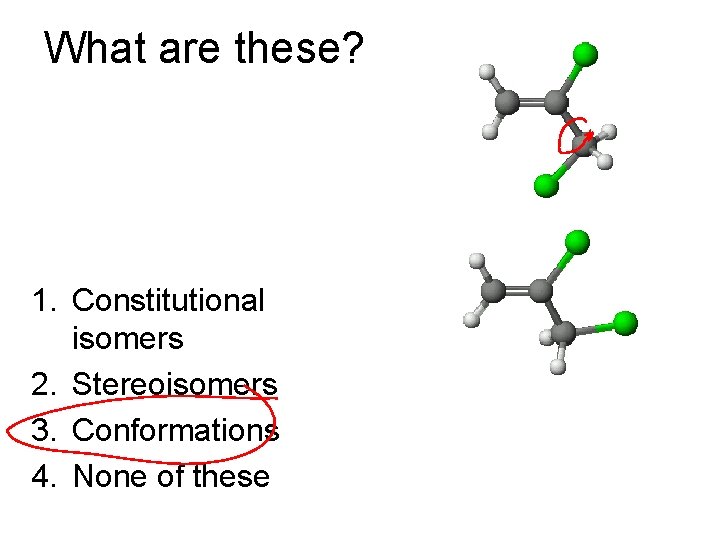
What are these? 1. Constitutional isomers 2. Stereoisomers 3. Conformations 4. None of these
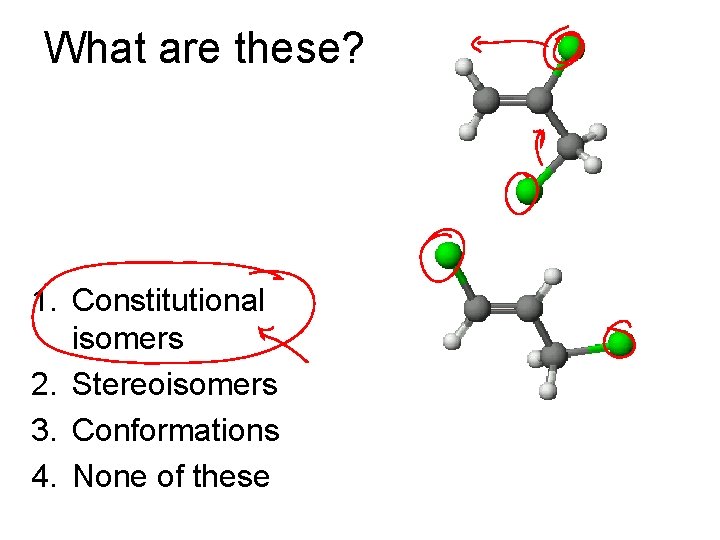
What are these? 1. Constitutional isomers 2. Stereoisomers 3. Conformations 4. None of these

A message from Mom.
- Slides: 39