Chapter 9 Cellular Respiration Harvesting Chemical Energy Energy


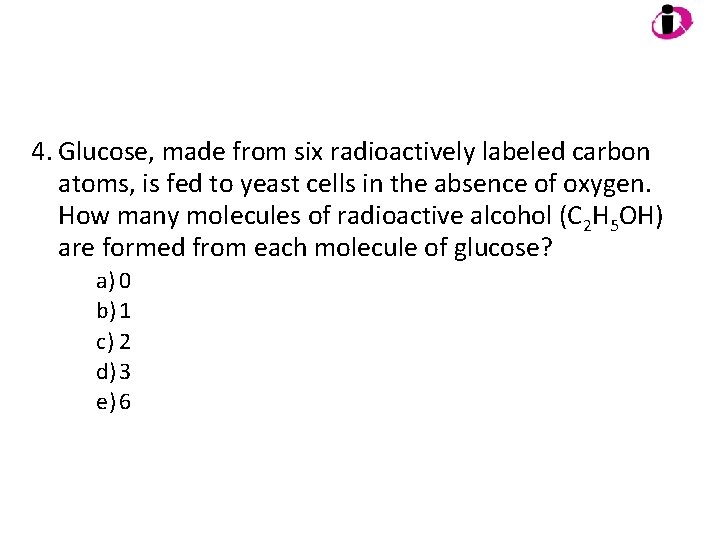
- Slides: 39

Chapter 9 Cellular Respiration: Harvesting Chemical Energy

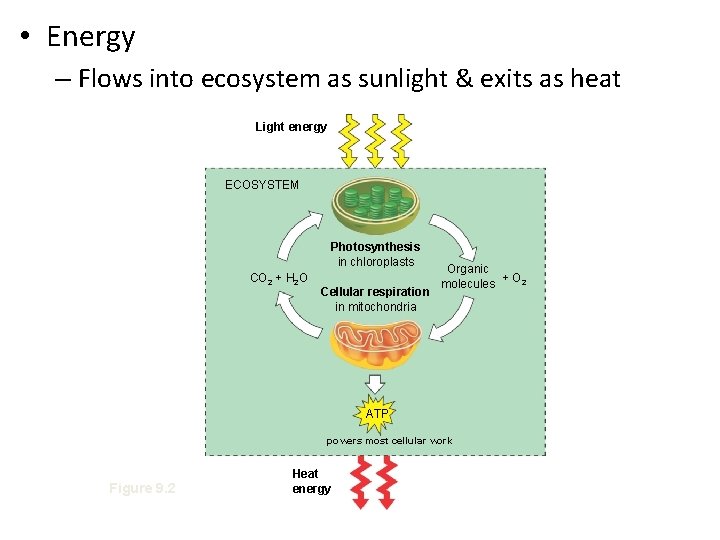
• Energy – Flows into ecosystem as sunlight & exits as heat Light energy ECOSYSTEM Photosynthesis in chloroplasts CO 2 + H 2 O Cellular respiration in mitochondria Organic + O 2 molecules ATP powers most cellular work Figure 9. 2 Heat energy

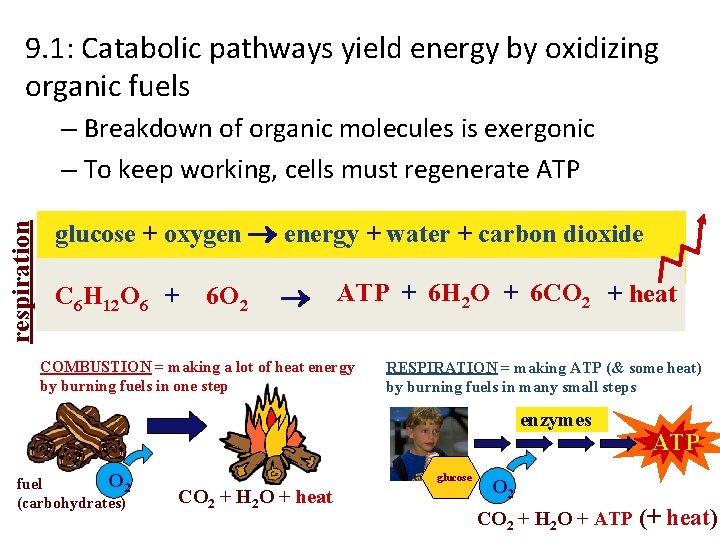
9. 1: Catabolic pathways yield energy by oxidizing organic fuels respiration – Breakdown of organic molecules is exergonic – To keep working, cells must regenerate ATP glucose + oxygen energy + water + carbon dioxide C 6 H 12 O 6 + 6 O 2 ATP + 6 H 2 O + 6 CO 2 + heat COMBUSTION = making a lot of heat energy by burning fuels in one step RESPIRATION = making ATP (& some heat) by burning fuels in many small steps enzymes O 2 fuel (carbohydrates) glucose CO 2 + H 2 O + heat ATP O 2 CO 2 + H 2 O + ATP (+ heat)

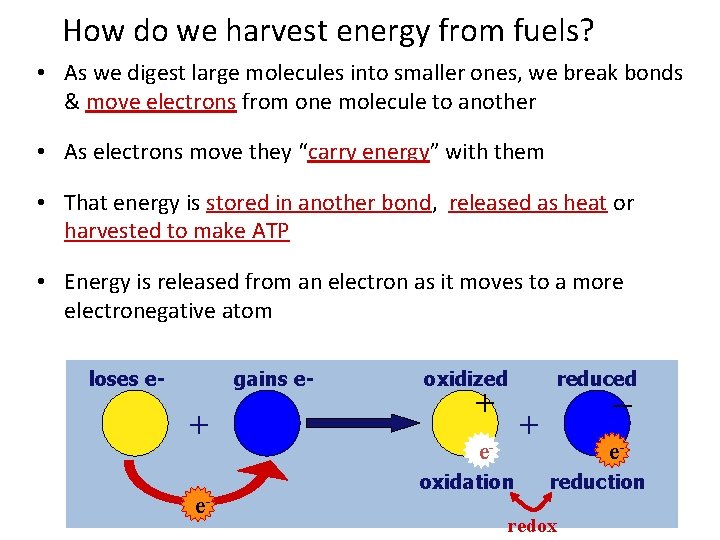
How do we harvest energy from fuels? • As we digest large molecules into smaller ones, we break bonds & move electrons from one molecule to another • As electrons move they “carry energy” with them • That energy is stored in another bond, released as heat or harvested to make ATP • Energy is released from an electron as it moves to a more electronegative atom loses e- gains e- + e- oxidized + reduced – + eoxidation ereduction redox

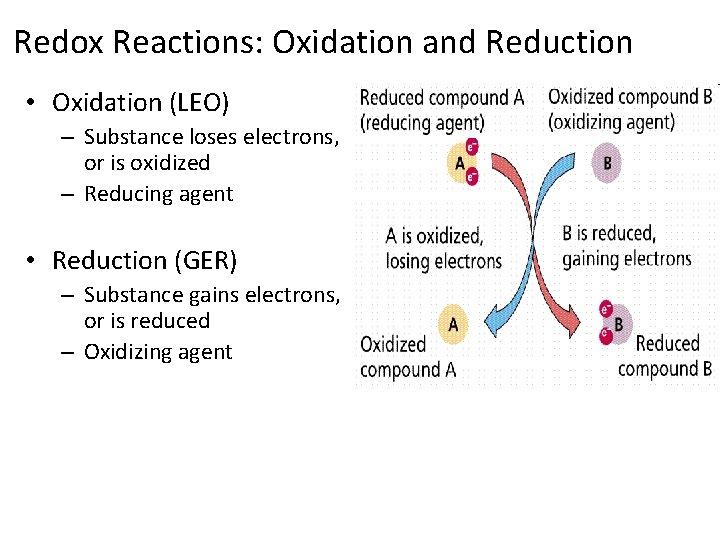
Redox Reactions: Oxidation and Reduction • Oxidation (LEO) – Substance loses electrons, or is oxidized – Reducing agent • Reduction (GER) – Substance gains electrons, or is reduced – Oxidizing agent

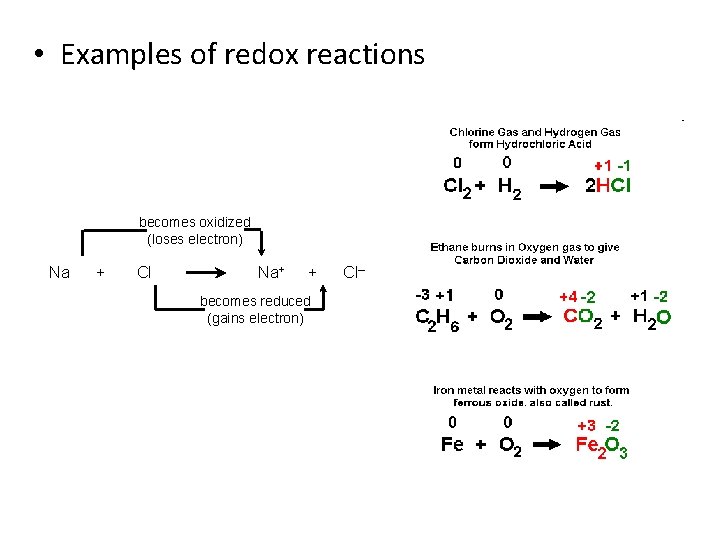
• Examples of redox reactions becomes oxidized (loses electron) Na + Cl Na + + becomes reduced (gains electron) Cl–

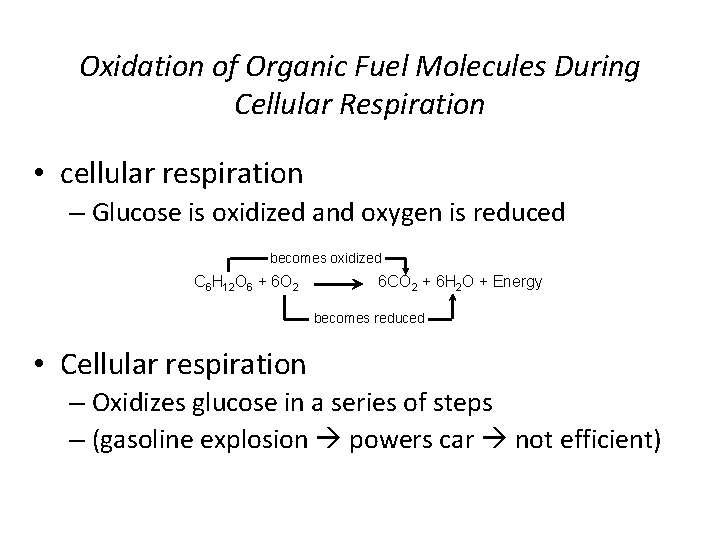
Oxidation of Organic Fuel Molecules During Cellular Respiration • cellular respiration – Glucose is oxidized and oxygen is reduced becomes oxidized C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + Energy becomes reduced • Cellular respiration – Oxidizes glucose in a series of steps – (gasoline explosion powers car not efficient)

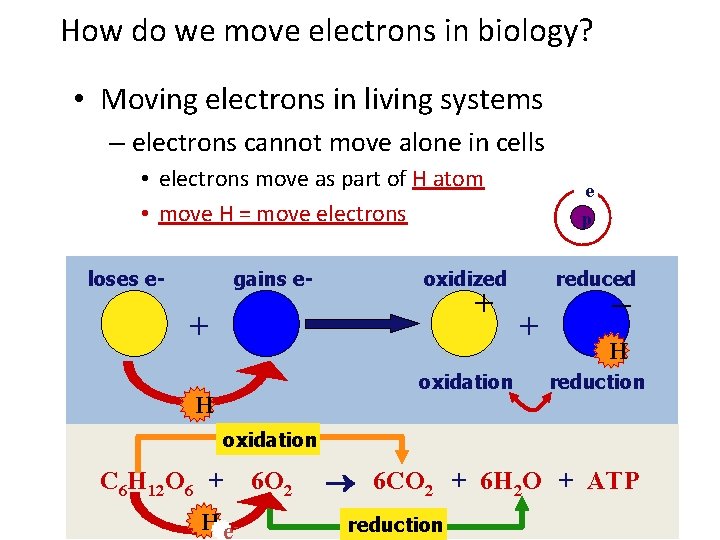
How do we move electrons in biology? • Moving electrons in living systems – electrons cannot move alone in cells • electrons move as part of H atom • move H = move electrons loses e- gains e- oxidized + + oxidation H e p reduced + – H reduction oxidation C 6 H 12 O 6 + H e- 6 O 2 6 CO 2 + 6 H 2 O + ATP reduction

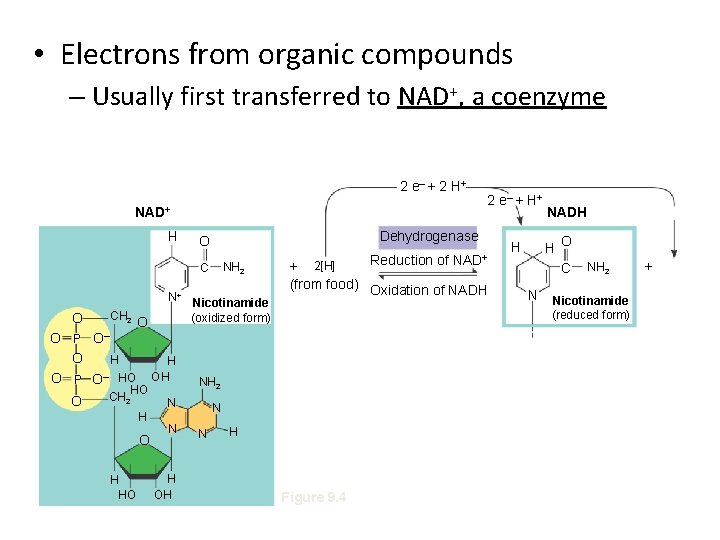
• Electrons from organic compounds – Usually first transferred to NAD+, a coenzyme 2 e– + 2 H + NAD+ Dehydrogenase O NH 2 H C N+ CH 2 O O– O P O HO O O HO CH 2 H O H HO H OH Nicotinamide (oxidized form) N H OH Reduction of NAD+ + 2[H] (from food) Oxidation of NADH NH 2 N N N 2 e– + H Figure 9. 4 NADH H O C H N NH 2 Nicotinamide (reduced form) +

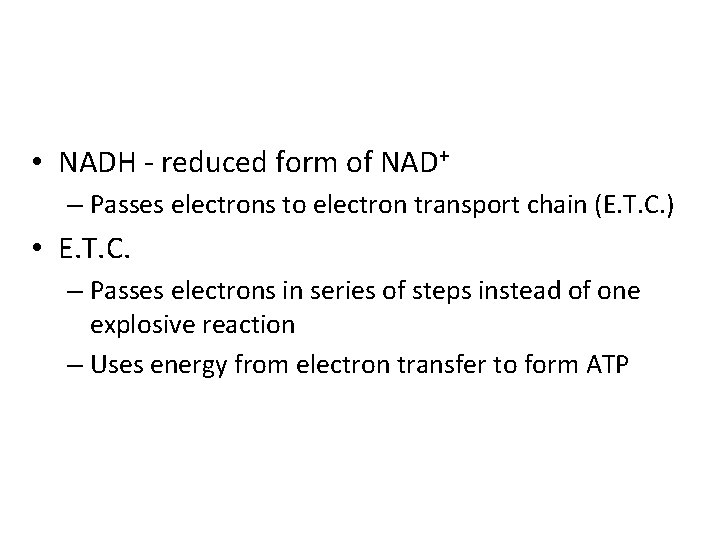
• NADH - reduced form of NAD+ – Passes electrons to electron transport chain (E. T. C. ) • E. T. C. – Passes electrons in series of steps instead of one explosive reaction – Uses energy from electron transfer to form ATP

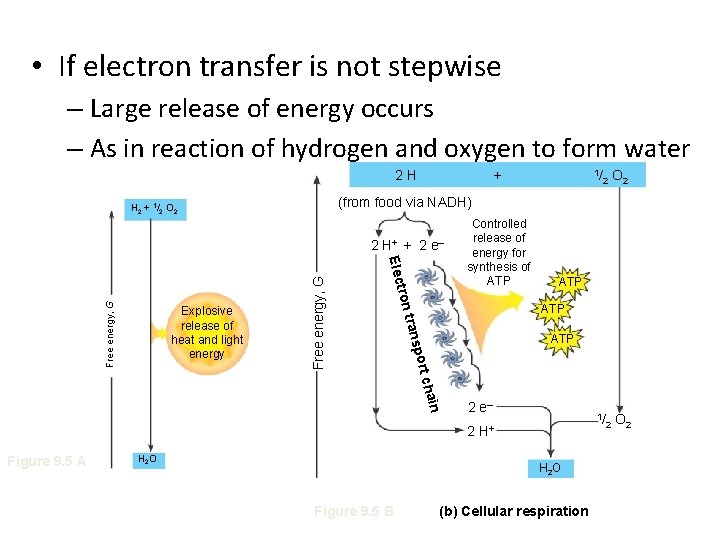
• If electron transfer is not stepwise – Large release of energy occurs – As in reaction of hydrogen and oxygen to form water 2 H + Controlled release of energy for synthesis of ATP O 2 1/ O 2 ATP port n chai 2 e– 2 H 2 O ATP trans Free energy, G tron Elec Free energy, G 2 H+ + 2 e – Figure 9. 5 A 2 (from food via NADH) H 2 + 1/2 O 2 Explosive release of heat and light energy 1/ H+ H 2 O Figure 9. 5 B (b) Cellular respiration 2

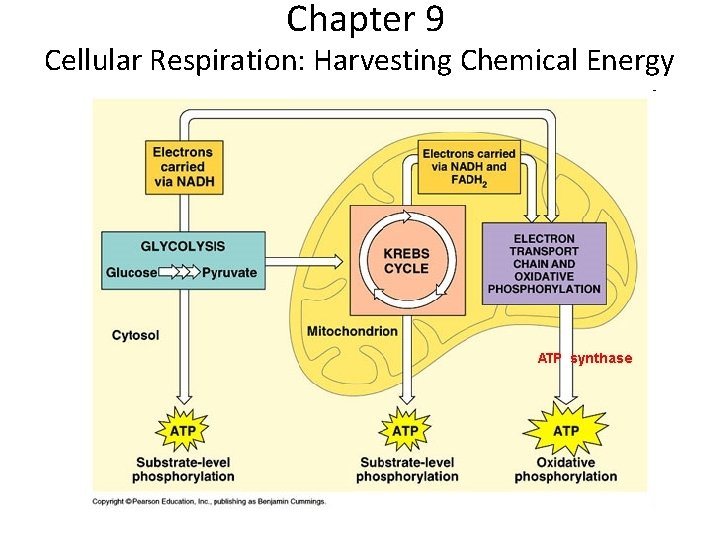
Stages of Cellular Respiration: A Preview 1. Glycolysis: Breaks down glucose into 2 pyruvates 2. Citric acid cycle: Completes breakdown of glucose 3. Oxidative phosphorylation - Driven by E. T. C, makes ATP What do I need to know? - Location in cell What goes in What comes out General summary of each step

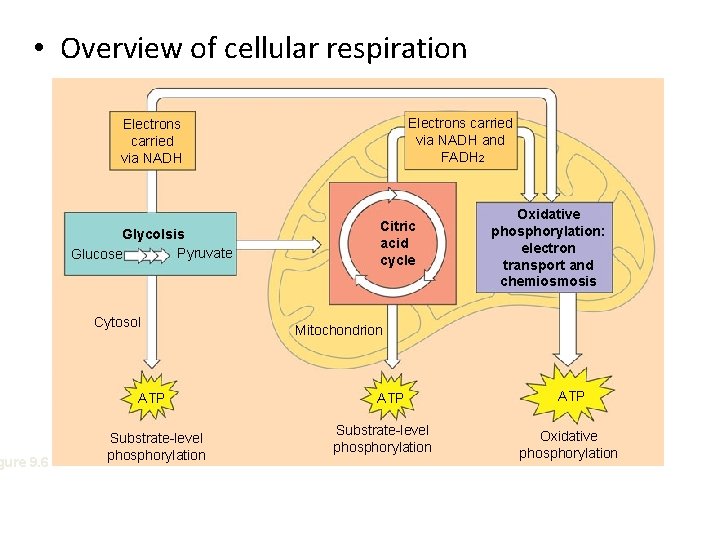
• Overview of cellular respiration gure 9. 6 Electrons carried via NADH and FADH 2 Electrons carried via NADH Glycolsis Pyruvate Glucose Cytosol ATP Substrate-level phosphorylation Citric acid cycle Oxidative phosphorylation: electron transport and chemiosmosis Mitochondrion ATP Substrate-level phosphorylation ATP Oxidative phosphorylation

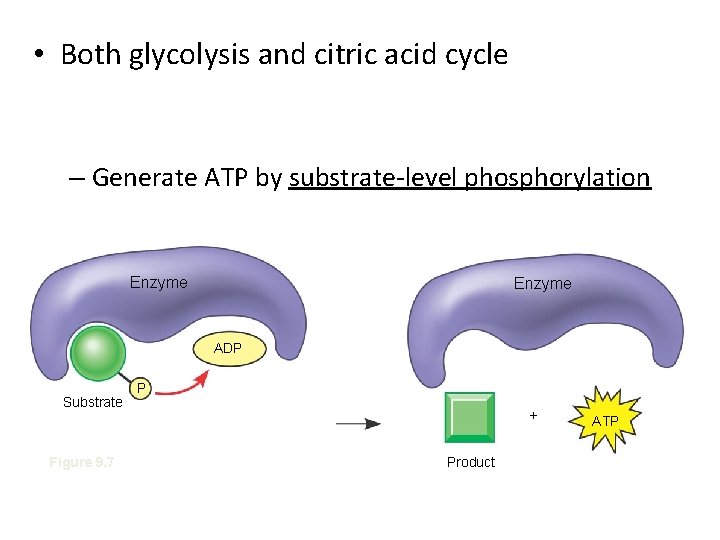
• Both glycolysis and citric acid cycle – Generate ATP by substrate-level phosphorylation Enzyme ADP Substrate Figure 9. 7 P + Product ATP

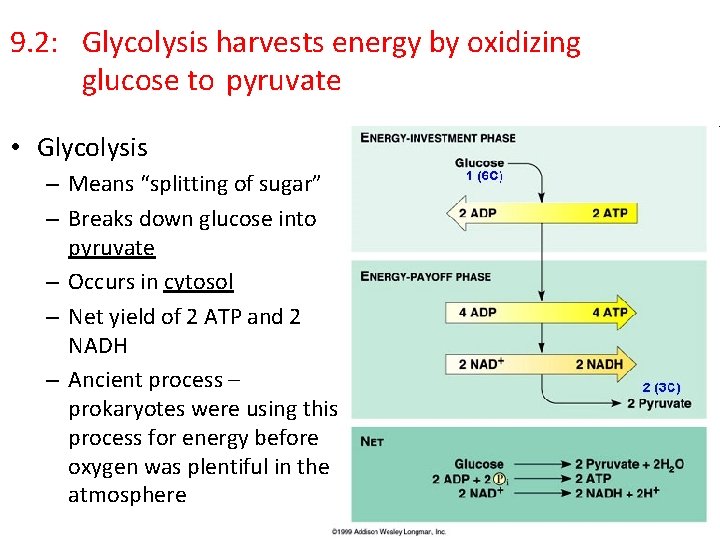
9. 2: Glycolysis harvests energy by oxidizing glucose to pyruvate • Glycolysis – Means “splitting of sugar” – Breaks down glucose into pyruvate – Occurs in cytosol – Net yield of 2 ATP and 2 NADH – Ancient process – prokaryotes were using this process for energy before oxygen was plentiful in the atmosphere

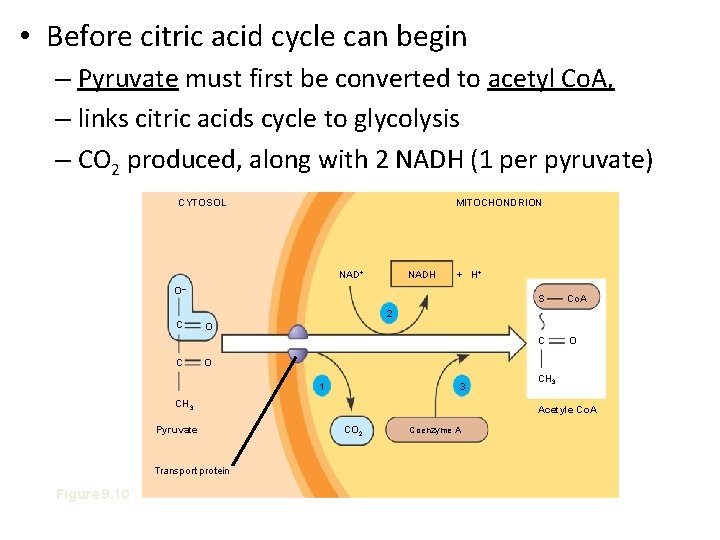
• Before citric acid cycle can begin – Pyruvate must first be converted to acetyl Co. A, – links citric acids cycle to glycolysis – CO 2 produced, along with 2 NADH (1 per pyruvate) CYTOSOL MITOCHONDRION NAD+ NADH + H+ O– S Co. A C O 2 C C O O 1 3 CH 3 Pyruvate Transport protein Figure 9. 10 CH 3 Acetyle Co. A CO 2 Coenzyme A

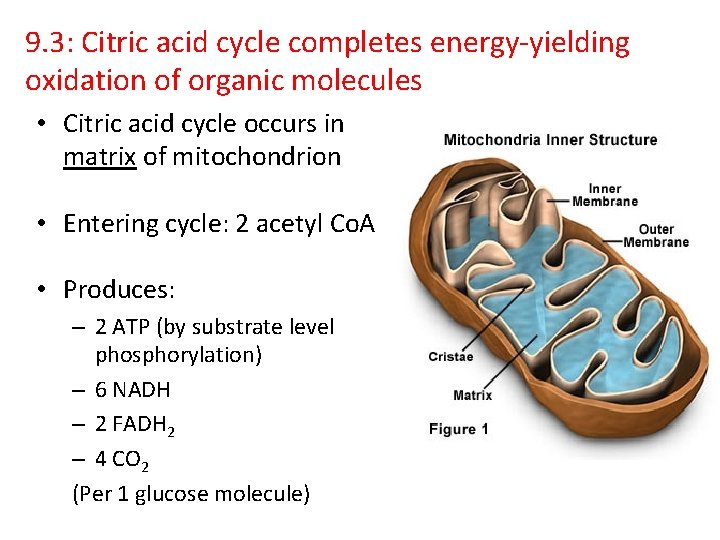
9. 3: Citric acid cycle completes energy-yielding oxidation of organic molecules • Citric acid cycle occurs in matrix of mitochondrion • Entering cycle: 2 acetyl Co. A • Produces: – 2 ATP (by substrate level phosphorylation) – 6 NADH – 2 FADH 2 – 4 CO 2 (Per 1 glucose molecule)

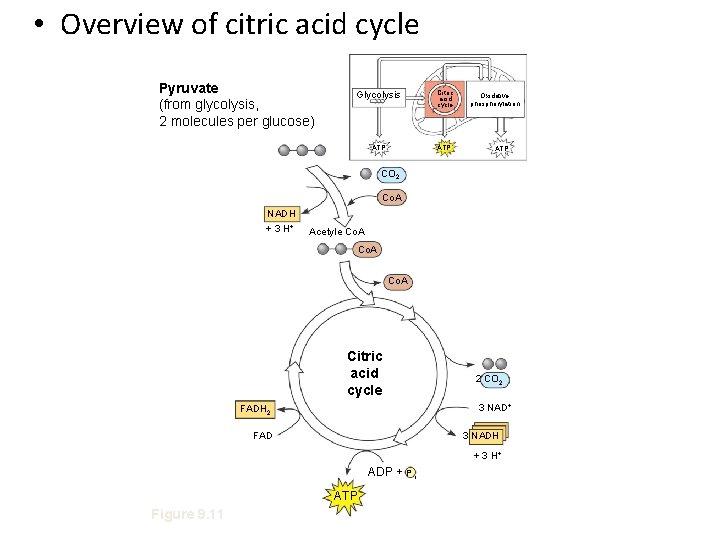
• Overview of citric acid cycle Pyruvate (from glycolysis, 2 molecules per glucose) Glycolysis Citric acid cycle ATP Oxidative phosphorylation ATP CO 2 Co. A NADH + 3 H+ Acetyle Co. A Citric acid cycle 2 CO 2 3 NAD+ FADH 2 FAD 3 NADH + 3 H+ ADP + P i ATP Figure 9. 11

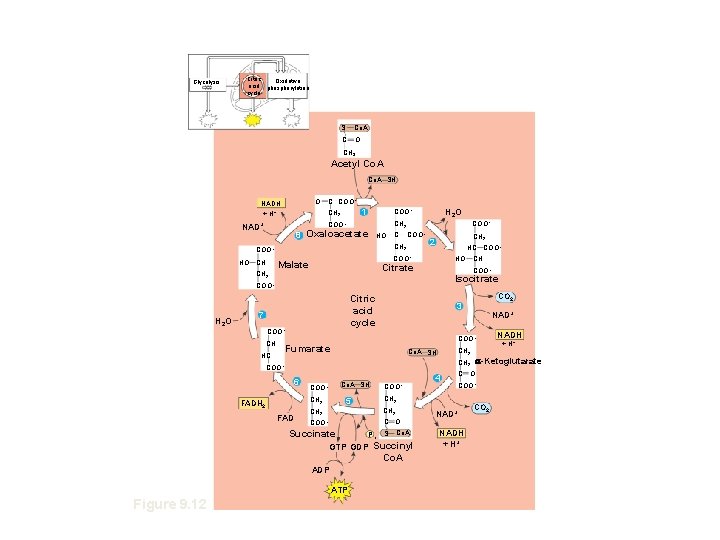
Glycolysis Citric Oxidative acid phosphorylation cycle S Co. A C O CH 3 Acetyl Co. A SH O NADH + H+ C COO– 1 CH 2 8 Oxaloacetate HO C CH 2 COO– HO CH COO– CH 2 COO– NAD+ H 2 O COO– CH 2 2 HC COO– Malate Figure HO Citrate 9. 12 Isocitrate COO– H 2 O Citric acid cycle 7 COO– CH NAD+ COO– CH 2 Co. A SH 6 Co. A SH COO– FAD CH 2 C O COO– Succinate Pi S Co. A GTP GDP Succinyl Co. A ADP ATP 4 C O COO– CH 2 5 CH 2 FADH 2 COO– NAD+ NADH + H+ a-Ketoglutarate CH 2 COO– Figure 9. 12 CO 2 3 Fumarate HC CH COO– CO 2

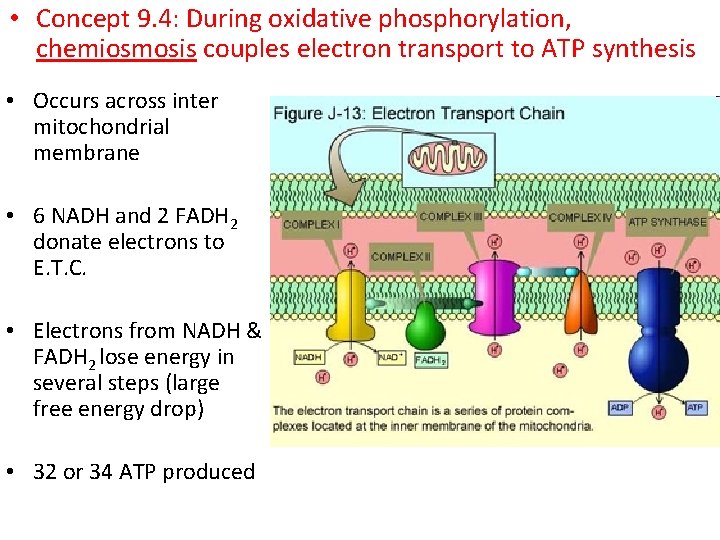
• Concept 9. 4: During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis • Occurs across inter mitochondrial membrane • 6 NADH and 2 FADH 2 donate electrons to E. T. C. • Electrons from NADH & FADH 2 lose energy in several steps (large free energy drop) • 32 or 34 ATP produced

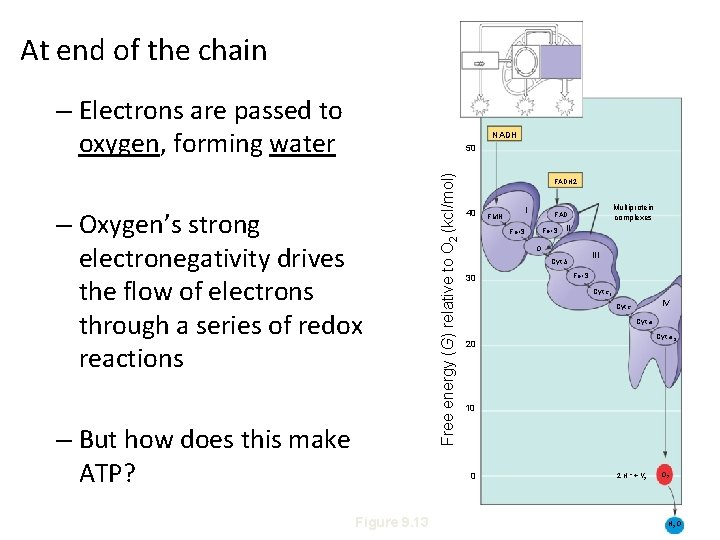
At end of the chain – Electrons are passed to oxygen, forming water NADH – Oxygen’s strong electronegativity drives the flow of electrons through a series of redox reactions – But how does this make ATP? Free energy (G) relative to O 2 (kcl/mol) 50 FADH 2 40 Multiprotein complexes FAD Fe • S II O III Cyt b 30 Fe • S Cyt c 1 IV Cyt c Cyt a 3 20 10 0 Figure 9. 13 I FMN 2 H + + 1 2 O 2 H 2 O

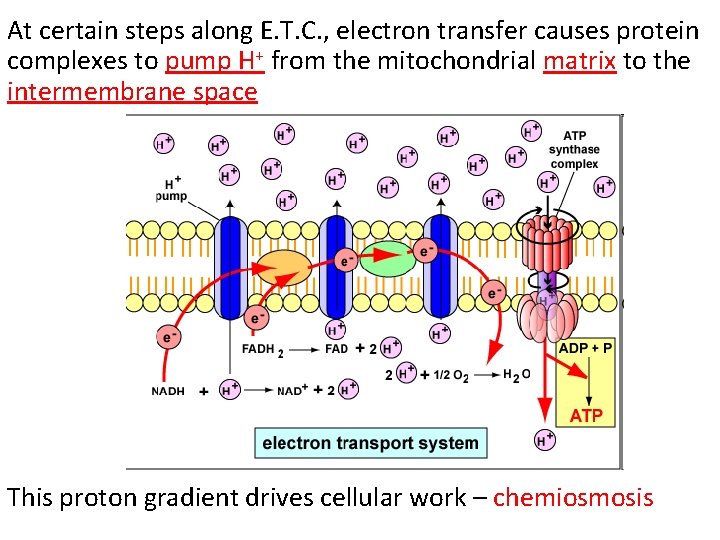
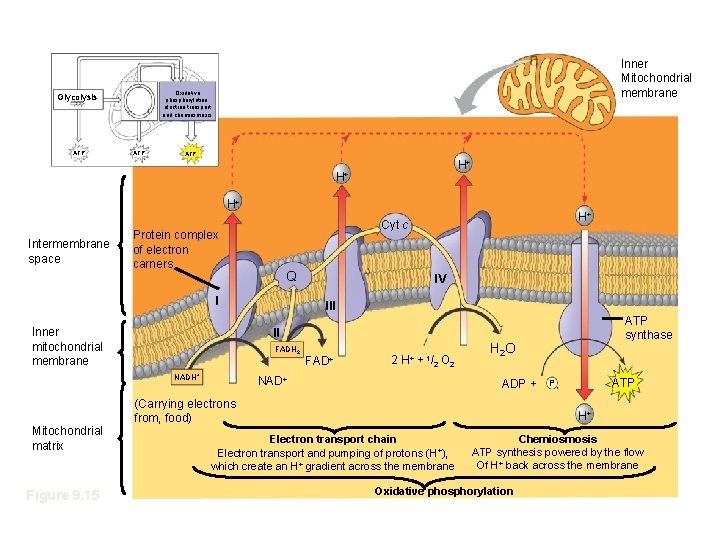
At certain steps along E. T. C. , electron transfer causes protein complexes to pump H+ from the mitochondrial matrix to the intermembrane space This proton gradient drives cellular work – chemiosmosis

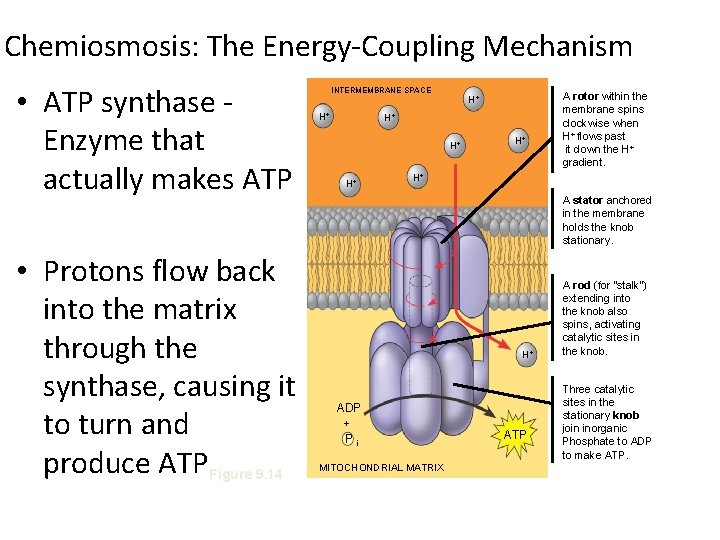
Chemiosmosis: The Energy-Coupling Mechanism • ATP synthase Enzyme that actually makes ATP • Protons flow back into the matrix through the synthase, causing it to turn and produce ATPFigure 9. 14 INTERMEMBRANE SPACE H+ H+ H+ A rotor within the membrane spins clockwise when H+ flows past it down the H+ gradient. H+ A stator anchored in the membrane holds the knob stationary. H+ ADP + Pi MITOCHONDRIAL MATRIX ATP A rod (for “stalk”) extending into the knob also spins, activating catalytic sites in the knob. Three catalytic sites in the stationary knob join inorganic Phosphate to ADP to make ATP.

This is finally starting to all make sense! • Resulting H+ gradient – Stores energy – Drives chemiosmosis in ATP synthase – Referred to as proton-motive force • Chemiosmosis – Uses energy in form of H+ gradient across a membrane to drive cellular work

Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP Inner Mitochondrial membrane ATP • Chemiosmosis and E. T. C. H+ H+ H+ Intermembrane space Protein complex of electron carners Q I Inner mitochondrial membrane Figure 9. 15 IV III ATP synthase II FADH 2 NADH+ Mitochondrial matrix H+ Cyt c FAD+ 2 H+ + 1/2 O 2 NAD+ H 2 O ADP + (Carrying electrons from, food) ATP Pi H+ Electron transport chain Electron transport and pumping of protons (H+), which create an H+ gradient across the membrane Chemiosmosis ATP synthesis powered by the flow Of H+ back across the membrane Oxidative phosphorylation

• When electrons flow along the electron transport chains of mitochondria, which of the following changes occur? a) The p. H of the matrix increases. b) ATP synthase pumps protons by active transport. c) The electrons gain free energy. d) The cytochromes of the chain phosphorylate ADP to form ATP. e) NAD+ is oxidized.

5. Cyanide is a poison that blocks the passage of electrons along the electron transport chain. Which of the following is a metabolic effect of this poison? a) The lower p. H of the intermembrane space is much lower than normal. b) Electrons are passed directly to oxygen, causing cells to explode. c) Alcohol would build up in the cells. d) NADH supplies would be exhausted, and ATP synthesis would cease. e) No proton gradient would be produced, and ATP synthesis would cease.

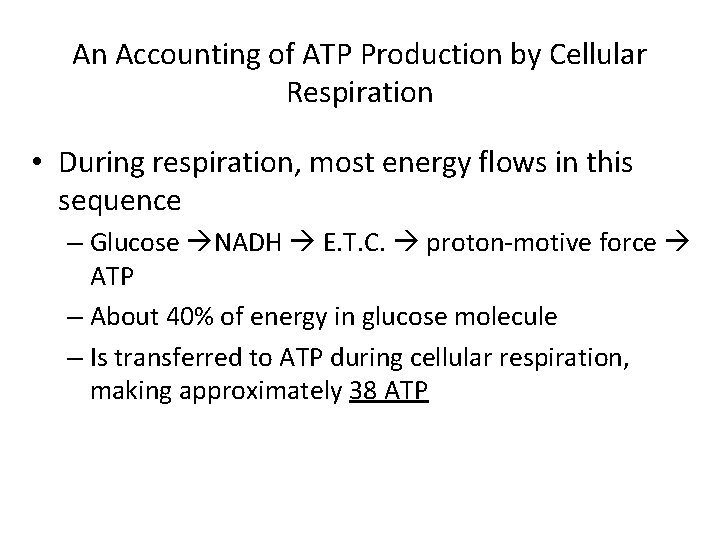
An Accounting of ATP Production by Cellular Respiration • During respiration, most energy flows in this sequence – Glucose NADH E. T. C. proton-motive force ATP – About 40% of energy in glucose molecule – Is transferred to ATP during cellular respiration, making approximately 38 ATP

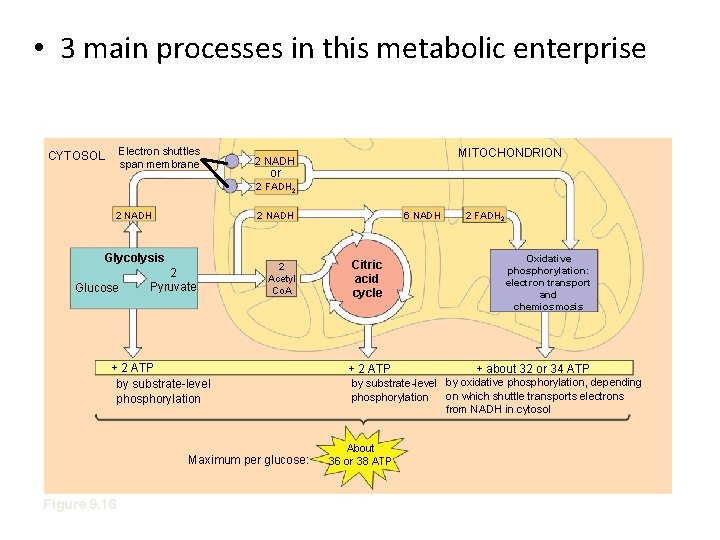
• 3 main processes in this metabolic enterprise Electron shuttles span membrane CYTOSOL MITOCHONDRION 2 NADH or 2 FADH 2 2 NADH Glycolysis Glucose 2 Pyruvate 2 Acetyl Co. A + 2 ATP by substrate-level phosphorylation Maximum per glucose: Figure 9. 16 6 NADH Citric acid cycle + 2 ATP 2 FADH 2 Oxidative phosphorylation: electron transport and chemiosmosis + about 32 or 34 ATP by substrate-level by oxidative phosphorylation, depending on which shuttle transports electrons phosphorylation from NADH in cytosol About 36 or 38 ATP

Concept 9. 5: Fermentation enables some cells to produce ATP w/o use of oxygen • Cellular respiration – Relies on oxygen to produce ATP • In absence of oxygen – Cells can still produce ATP through fermentation

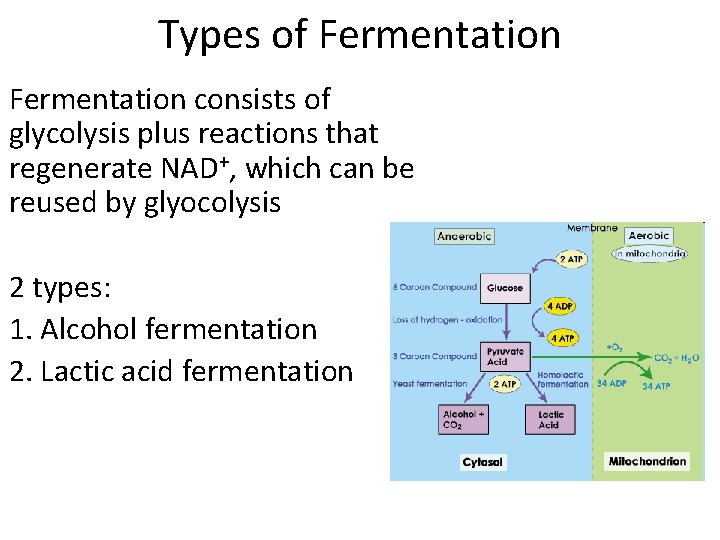
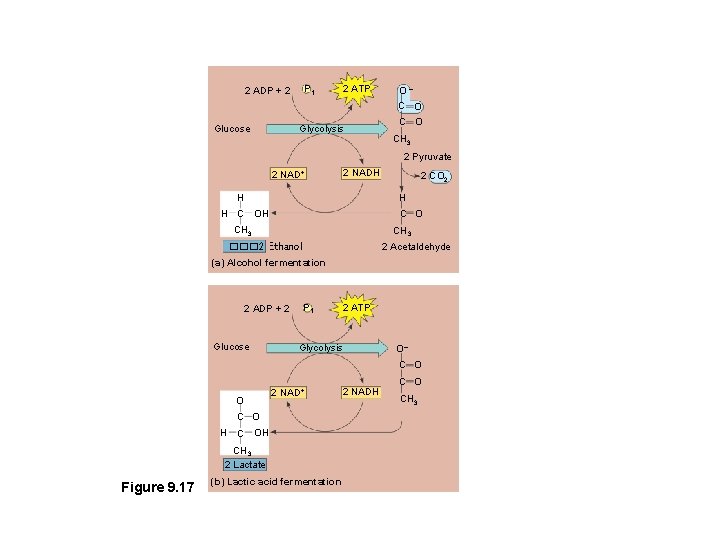
Types of Fermentation consists of glycolysis plus reactions that regenerate NAD+, which can be reused by glyocolysis 2 types: 1. Alcohol fermentation 2. Lactic acid fermentation

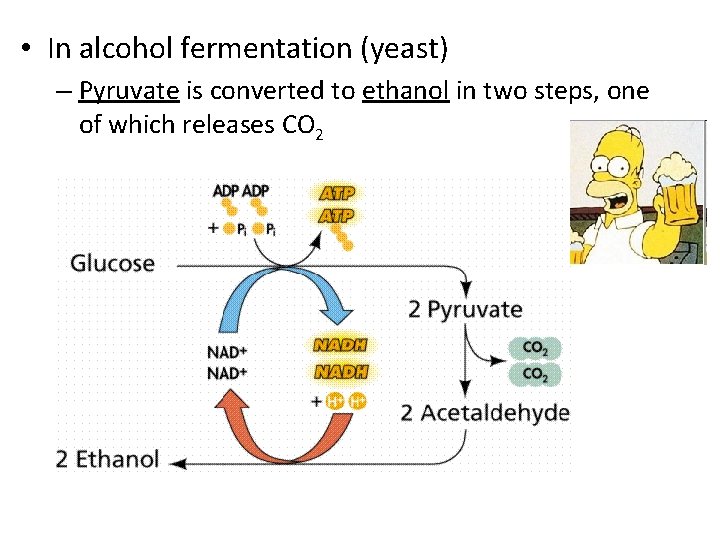
• In alcohol fermentation (yeast) – Pyruvate is converted to ethanol in two steps, one of which releases CO 2

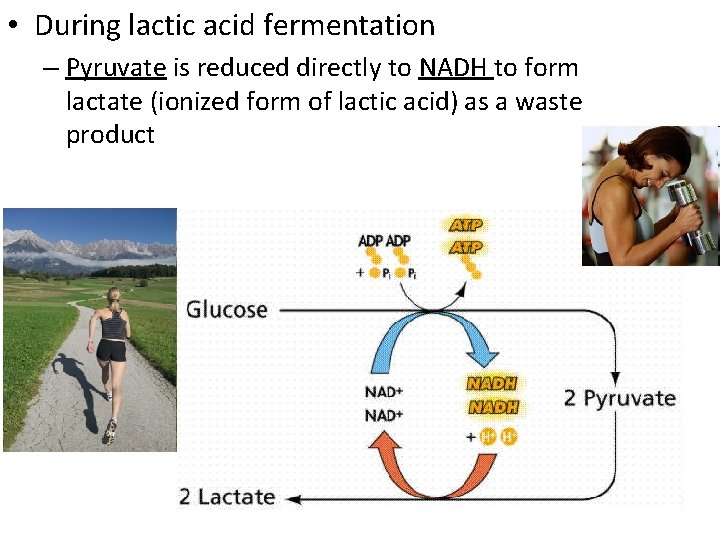
• During lactic acid fermentation – Pyruvate is reduced directly to NADH to form lactate (ionized form of lactic acid) as a waste product
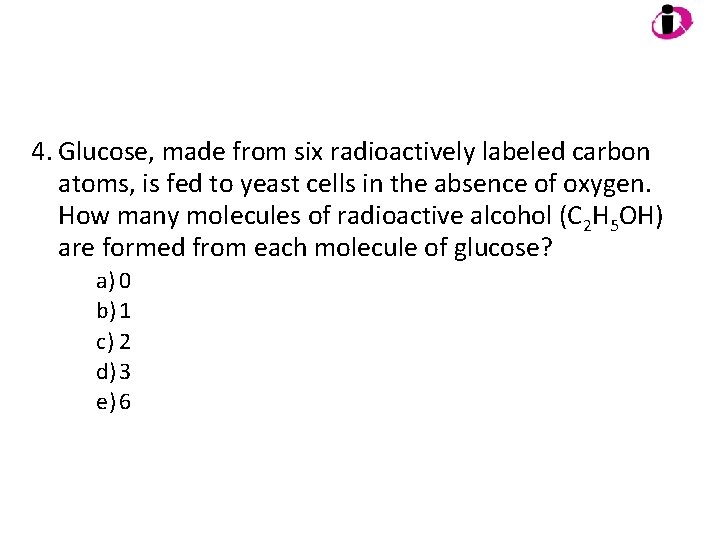
4. Glucose, made from six radioactively labeled carbon atoms, is fed to yeast cells in the absence of oxygen. How many molecules of radioactive alcohol (C 2 H 5 OH) are formed from each molecule of glucose? a) 0 b) 1 c) 2 d) 3 e) 6

P 1 2 ADP + 2 2 ATP O– C O Glucose Glycolysis C O CH 3 2 Pyruvate 2 NAD+ 2 NADH H 2 CO 2 H H C O CH 3 2 Acetaldehyde ��� 2 Ethanol (a) Alcohol fermentation 2 ADP + 2 Glucose P 1 2 ATP Glycolysis O– C O O 2 NAD+ C O H C OH CH 3 2 Lactate Figure 9. 17 (b) Lactic acid fermentation 2 NADH C O CH 3

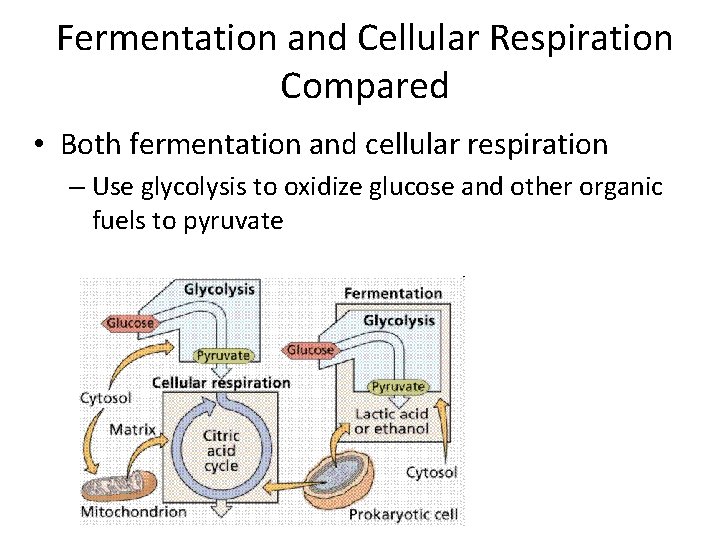
Fermentation and Cellular Respiration Compared • Both fermentation and cellular respiration – Use glycolysis to oxidize glucose and other organic fuels to pyruvate

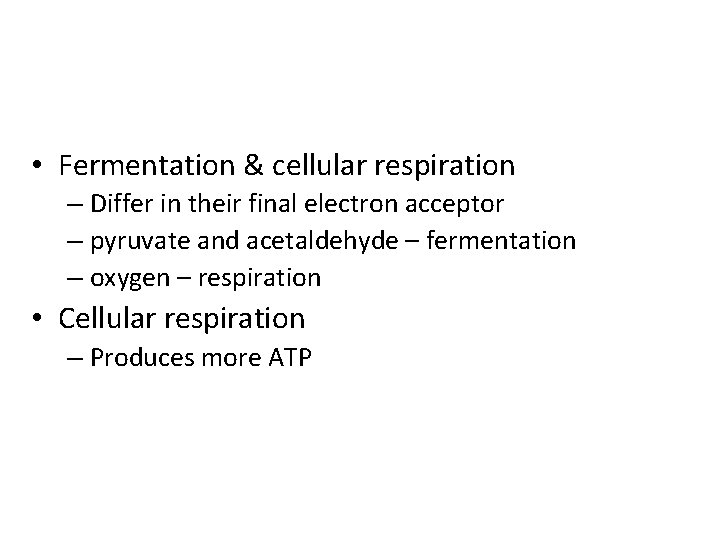
• Fermentation & cellular respiration – Differ in their final electron acceptor – pyruvate and acetaldehyde – fermentation – oxygen – respiration • Cellular respiration – Produces more ATP

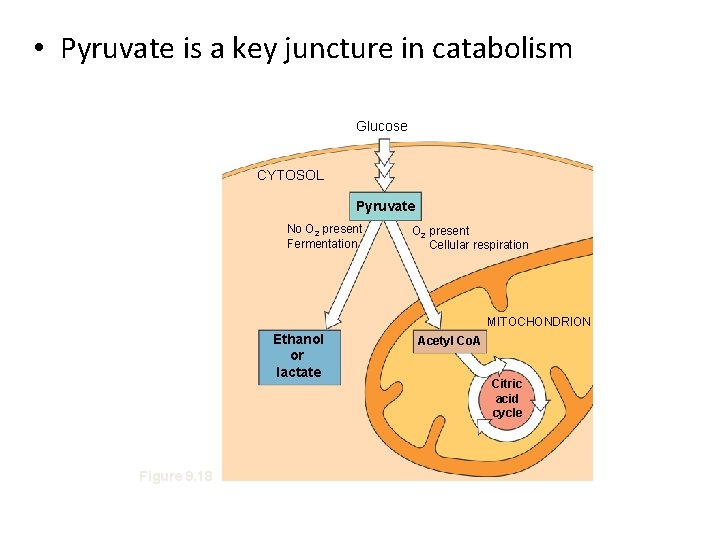
• Pyruvate is a key juncture in catabolism Glucose CYTOSOL Pyruvate No O 2 present Fermentation O 2 present Cellular respiration MITOCHONDRION Ethanol or lactate Figure 9. 18 Acetyl Co. A Citric acid cycle

The Evolutionary Significance of Glycolysis • Glycolysis – Occurs in nearly all organisms – Probably evolved in ancient prokaryotes before there was oxygen in the atmosphere