Chapter 9 C Heat Nature of Heat Heat



- Slides: 18

Chapter 9 C - Heat

Nature of Heat • Heat – Flow of thermal energy from one place to another – Sometimes used for thermal energy or temperature – In science, heat means amount of thermal energy that is moved

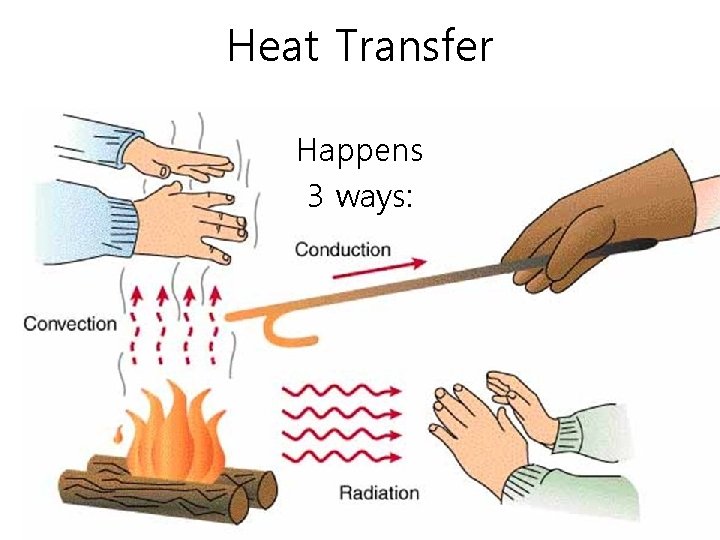
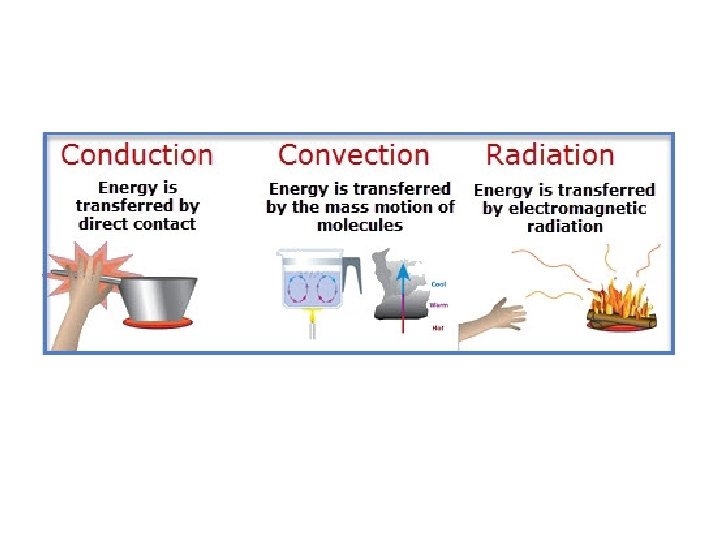
Heat Transfer Happens 3 ways:


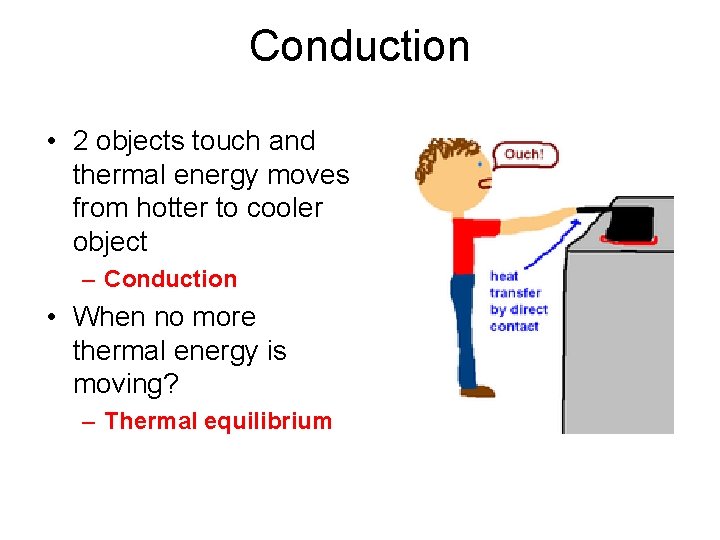
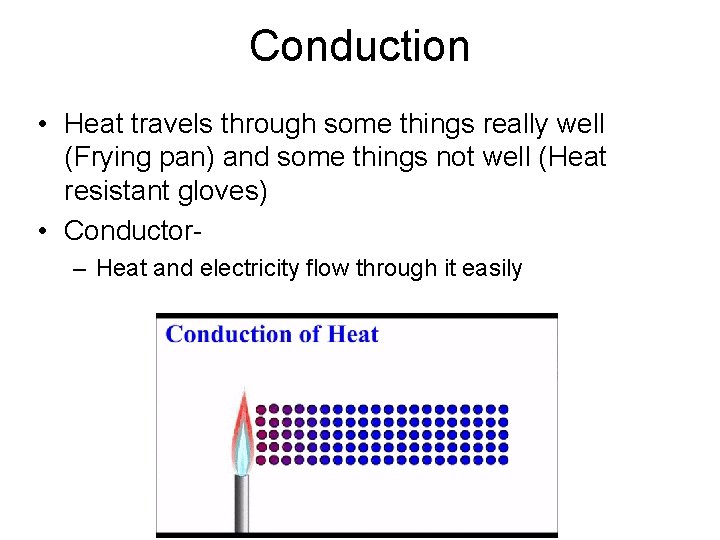
Conduction • 2 objects touch and thermal energy moves from hotter to cooler object – Conduction • When no more thermal energy is moving? – Thermal equilibrium

Conduction • Heat travels through some things really well (Frying pan) and some things not well (Heat resistant gloves) • Conductor– Heat and electricity flow through it easily

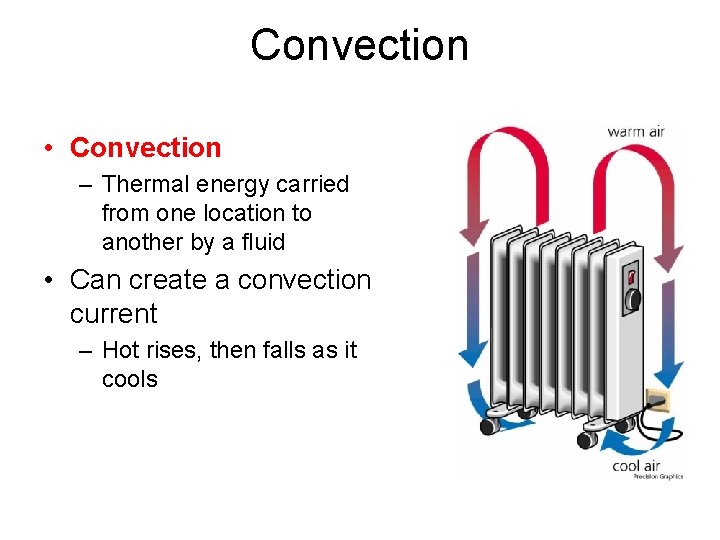
Convection • Convection – Thermal energy carried from one location to another by a fluid • Can create a convection current – Hot rises, then falls as it cools

Air is heated to cook food. Example of convection

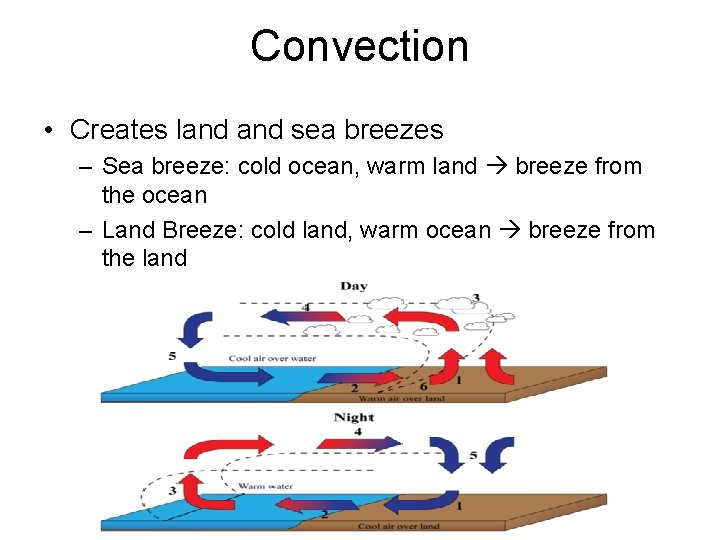
Convection • Creates land sea breezes – Sea breeze: cold ocean, warm land breeze from the ocean – Land Breeze: cold land, warm ocean breeze from the land

Radiation • Heat transfer through radiant (electromagnetic) energy: – Radiation • Sun- radiation

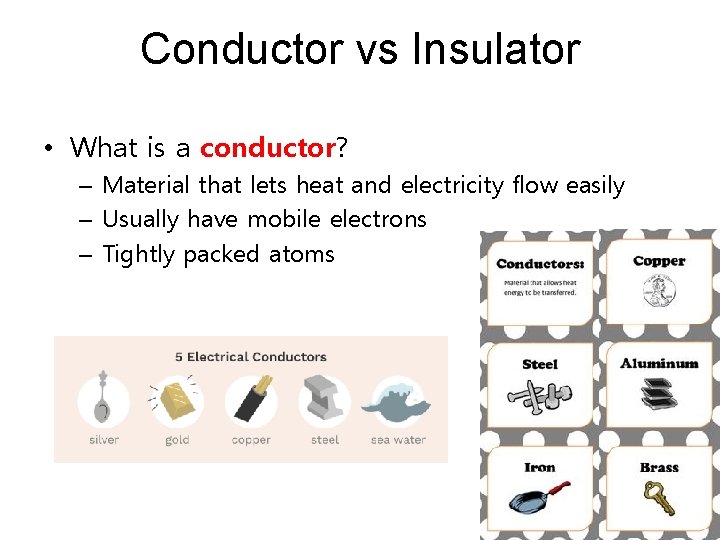
Conductor vs Insulator • What is a conductor? – Material that lets heat and electricity flow easily – Usually have mobile electrons – Tightly packed atoms

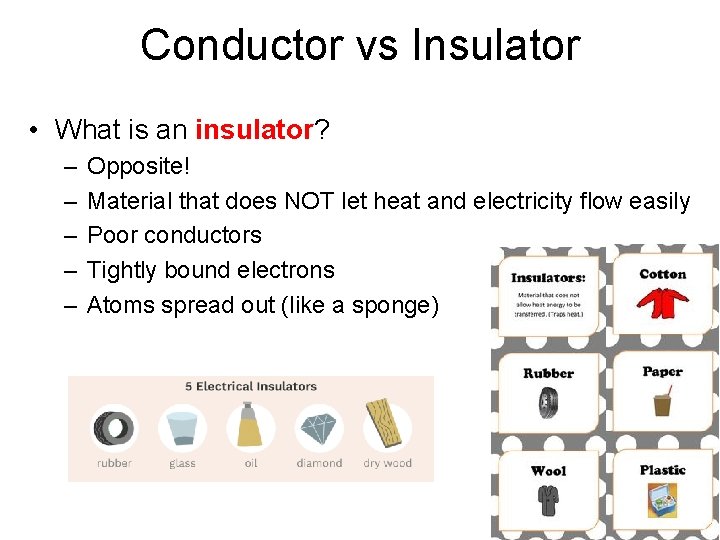
Conductor vs Insulator • What is an insulator? – – – Opposite! Material that does NOT let heat and electricity flow easily Poor conductors Tightly bound electrons Atoms spread out (like a sponge)


Measuring Heat • Every object can gain or loose heat – Heat capacity (C) - amount of thermal energy (J) it must gain or lose to change temp by 1° C • Change in thermal energy in something is called heat – Symbol is Q

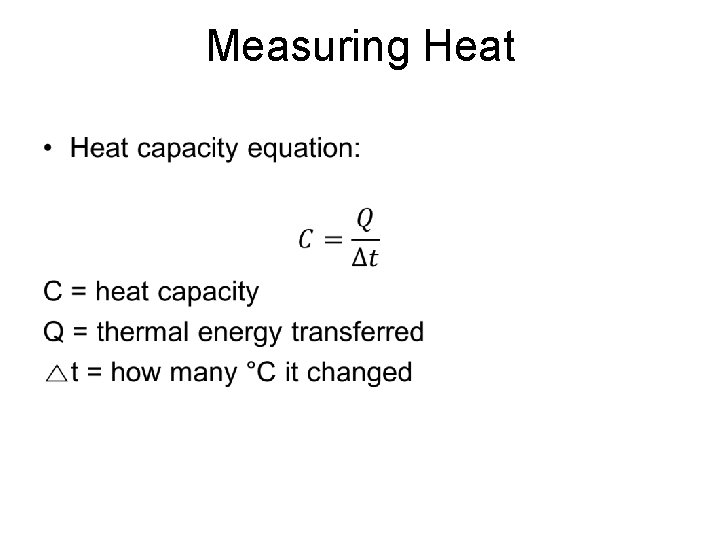
Measuring Heat •

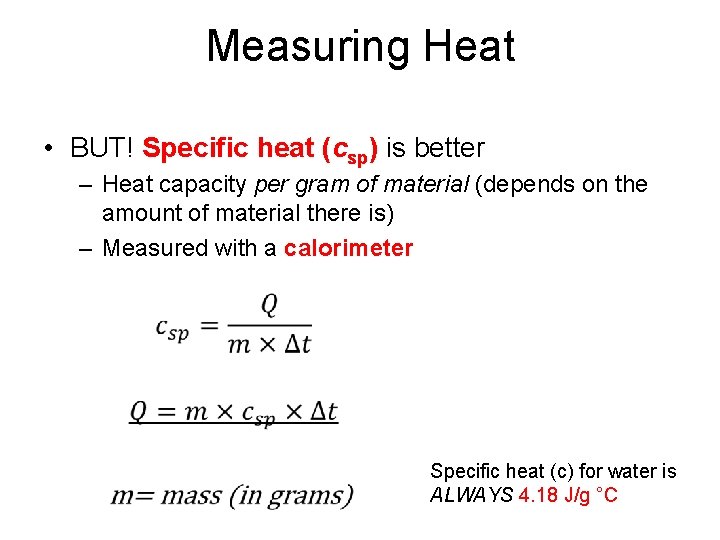
Measuring Heat • BUT! Specific heat (csp) is better – Heat capacity per gram of material (depends on the amount of material there is) – Measured with a calorimeter • Specific heat (c) for water is ALWAYS 4. 18 J/g °C

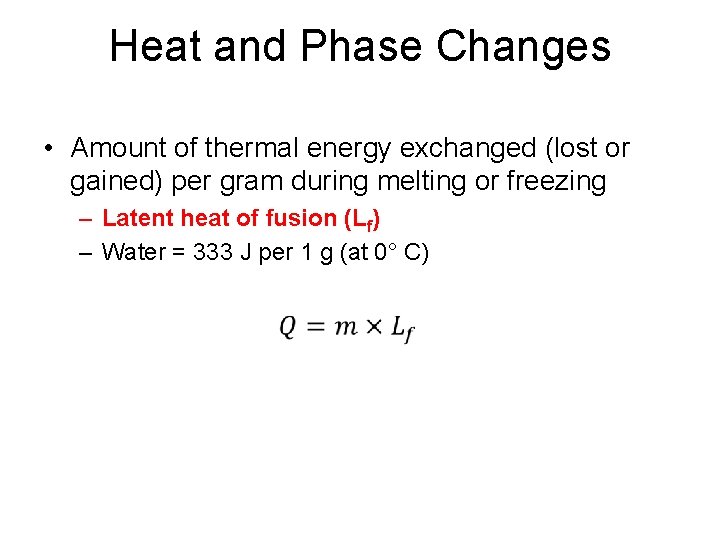
Heat and Phase Changes • Amount of thermal energy exchanged (lost or gained) per gram during melting or freezing – Latent heat of fusion (Lf) – Water = 333 J per 1 g (at 0° C) •

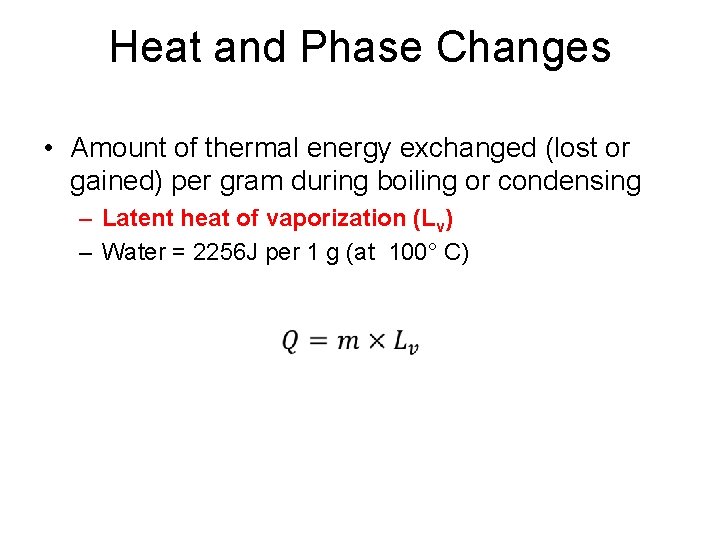
Heat and Phase Changes • Amount of thermal energy exchanged (lost or gained) per gram during boiling or condensing – Latent heat of vaporization (Lv) – Water = 2256 J per 1 g (at 100° C) •