Chapter 9 Aqueous Solutions Predicting Solubilities Solubility Chemists

Chapter 9 Aqueous Solutions Predicting Solubilities

Solubility Chemists express solubility (S) as: mass of solute dissolved per 100 m. L of water or moles of solute per litre of solution

Solubility Generally, it is agreed that: if solute has S > 0. 1 mol/L, it is classified as SOLUBLE. if solute has S < 0. 01 mol/L, it is classified as INSOLUBLE. if solute has S between 0. 1 mol/L and 0. 01 mol/L, it is classified as PARTLY or SLIGHTLY SOLUBLE. for our purposes, if S < 0. 1 mol/L, it is classified as INSOLUBLE.

Solubility Rules …are determined experimentally. …can be used as a guideline to predict solubility. Use the solubility table on periodic table handout from class
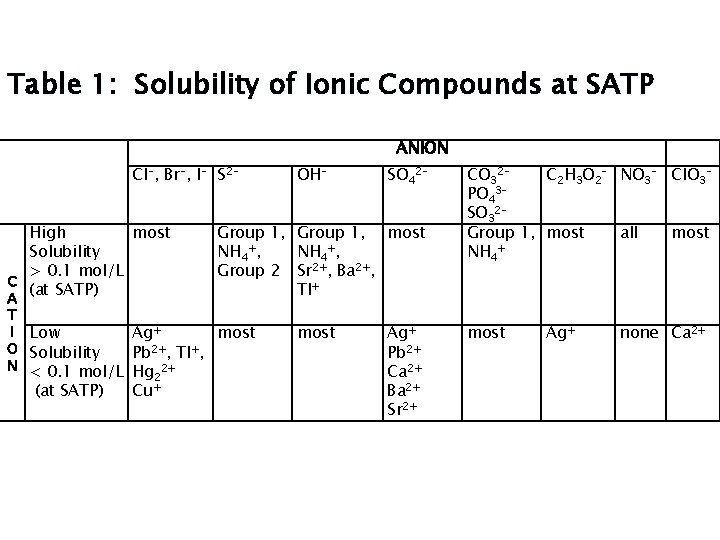
Table 1: Solubility of Ionic Compounds at SATP ANION Cl-, Br-, I- S 2 High most Solubility > 0. 1 mol/L C (at SATP) A 2 Cu+ SO 42 - Group 1, most NH 4+, Group 2 Sr 2+, Ba 2+, Tl+ T I Low Ag+ most O Solubility Pb 2+, Tl+, N < 0. 1 mol/L Hg 2+ (at SATP) OH- most Ag+ Pb 2+ Ca 2+ Ba 2+ Sr 2+ CO 32 C 2 H 3 O 2 - NO 3 - Cl. O 3 PO 43 SO 32 Group 1, most all most + NH 4 most Ag+ none Ca 2+

Solubility Rules- Others? See p 334 Table 9. 1 (5 rules, higher number takes priority) See p 336 Language Link !
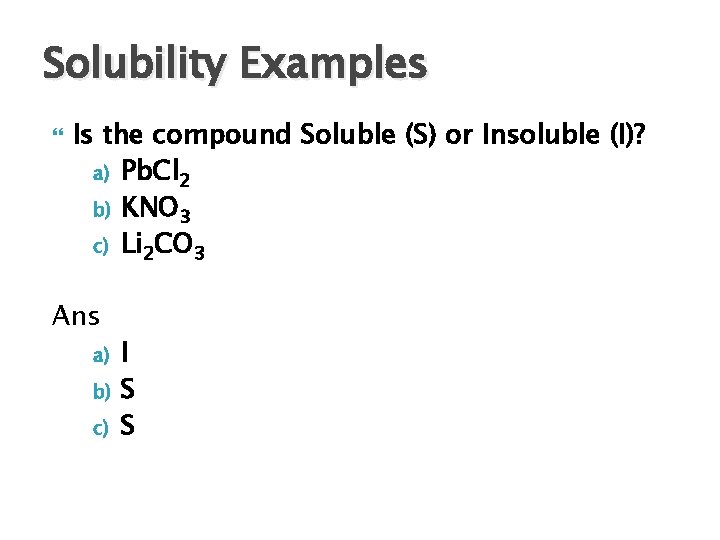
Solubility Examples Is the compound Soluble (S) or Insoluble (I)? a) Pb. Cl 2 b) KNO 3 c) Li 2 CO 3 Ans a) b) c) I S S

Solubility Factors Many, but not all ionic compounds, are soluble in water. Why not all? There are several factors: 1. Ion Charge Compounds with higher ion charge tend to be insoluble because when the charge is inc. , the force that holds ions together is inc. e. g PO 43 - has large ion charge and is insoluble
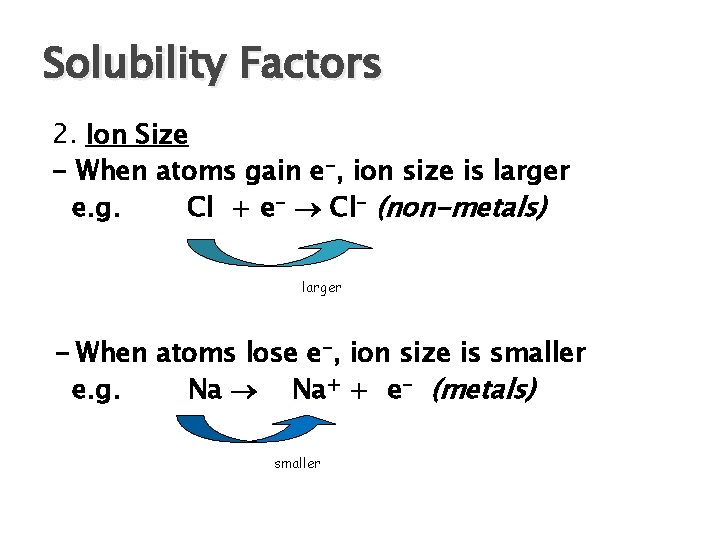
Solubility Factors 2. Ion Size - When atoms gain e-, ion size is larger e. g. Cl + e- Cl- (non-metals) larger - When atoms lose e-, ion size is smaller e. g. Na Na+ + e- (metals) smaller

Solubility Factors 2. Ion Size - continued Small ions bond more closely than large ions ∴cmpds with small ions are less soluble If you compare ions in the same family in the periodic table, as you go down the family, ion size inc ∴ solubility inc.

Homework Textbook ü Read p 330 -331, 334 ü Do p 335 #1 -3 p 336 #1 -6 ü (See answers on p 367) BLM 9 -1 and 9 -4 ü (See answers on website)
- Slides: 11