Chapter 9 Acids Bases Salts 9 1 9

Chapter 9 Acids, Bases, & Salts 9. 1 & 9. 3 The Arrhenius Theory and Naming Acids Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Acids Arrhenius acids • produce H+ ions in water. H 2 O HCl(g) H+(aq) + Cl- (aq) • • are electrolytes. have a sour taste. turn litmus paper red. neutralize bases. 2
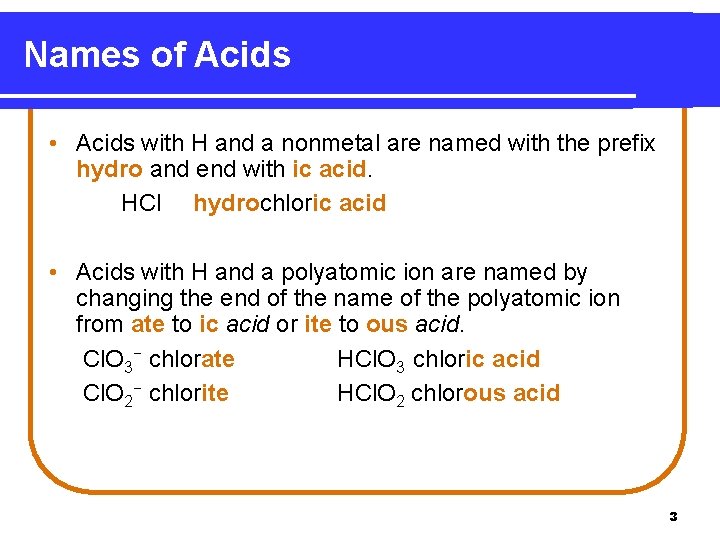
Names of Acids • Acids with H and a nonmetal are named with the prefix hydro and end with ic acid. HCl hydrochloric acid • Acids with H and a polyatomic ion are named by changing the end of the name of the polyatomic ion from ate to ic acid or ite to ous acid. Cl. O 3− chlorate HCl. O 3 chloric acid Cl. O 2− chlorite HCl. O 2 chlorous acid 3

Names of Some Common Acids 4
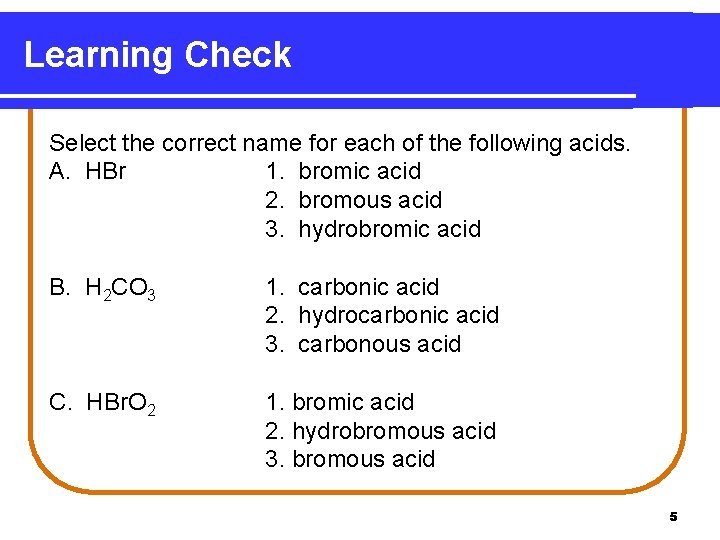
Learning Check Select the correct name for each of the following acids. A. HBr 1. bromic acid 2. bromous acid 3. hydrobromic acid B. H 2 CO 3 1. carbonic acid 2. hydrocarbonic acid 3. carbonous acid C. HBr. O 2 1. bromic acid 2. hydrobromous acid 3. bromous acid 5
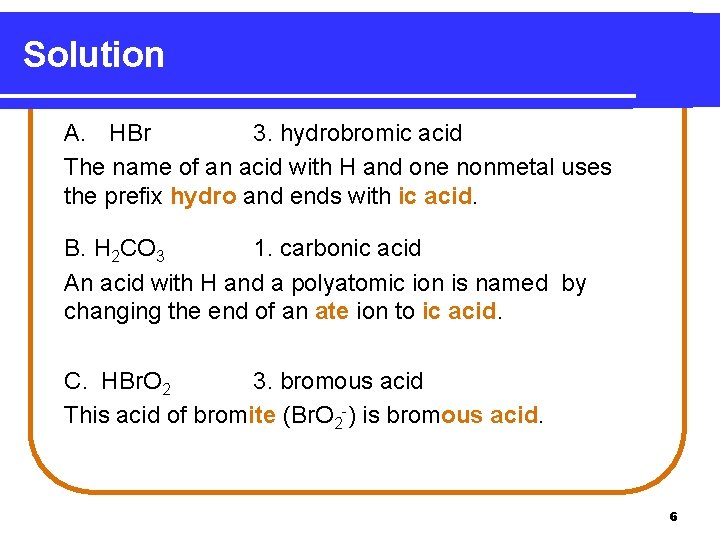
Solution A. HBr 3. hydrobromic acid The name of an acid with H and one nonmetal uses the prefix hydro and ends with ic acid. B. H 2 CO 3 1. carbonic acid An acid with H and a polyatomic ion is named by changing the end of an ate ion to ic acid. C. HBr. O 2 3. bromous acid This acid of bromite (Br. O 2 -) is bromous acid. 6
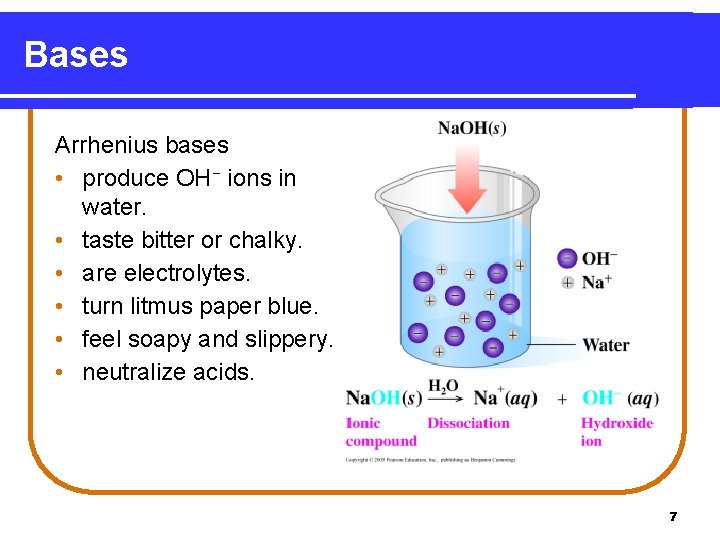
Bases Arrhenius bases • produce OH− ions in water. • taste bitter or chalky. • are electrolytes. • turn litmus paper blue. • feel soapy and slippery. • neutralize acids. 7
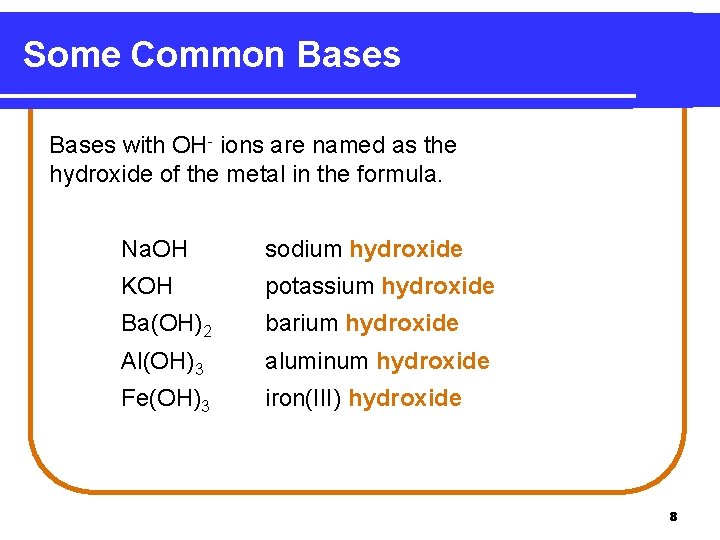
Some Common Bases with OH- ions are named as the hydroxide of the metal in the formula. Na. OH sodium hydroxide KOH potassium hydroxide Ba(OH)2 barium hydroxide Al(OH)3 aluminum hydroxide Fe(OH)3 iron(III) hydroxide 8

Learning Check Match the formulas with the names. A. ___HNO 2 1) iodic acid B. ___Ca(OH)2 2) sulfuric acid C. ___H 2 SO 4 3) sodium hydroxide D. ___HIO 3 4) nitrous acid E. ___Na. OH 5) calcium hydroxide 9
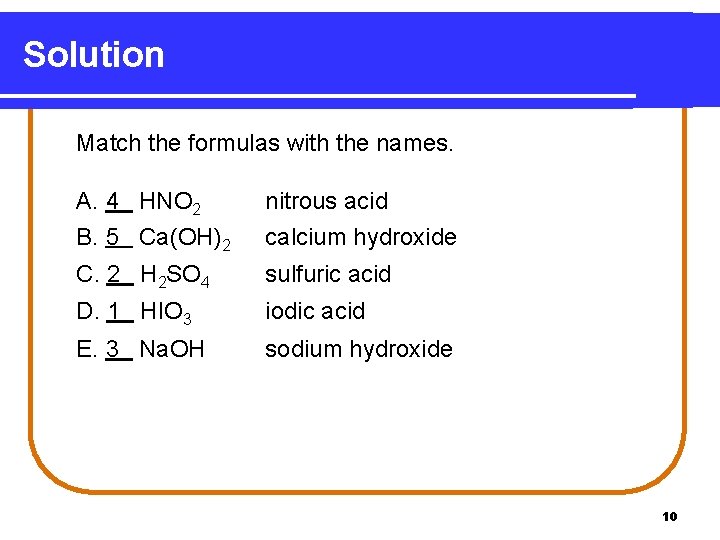
Solution Match the formulas with the names. A. 4 HNO 2 B. 5 Ca(OH)2 nitrous acid calcium hydroxide C. 2 H 2 SO 4 sulfuric acid D. 1 HIO 3 iodic acid E. 3 Na. OH sodium hydroxide 10
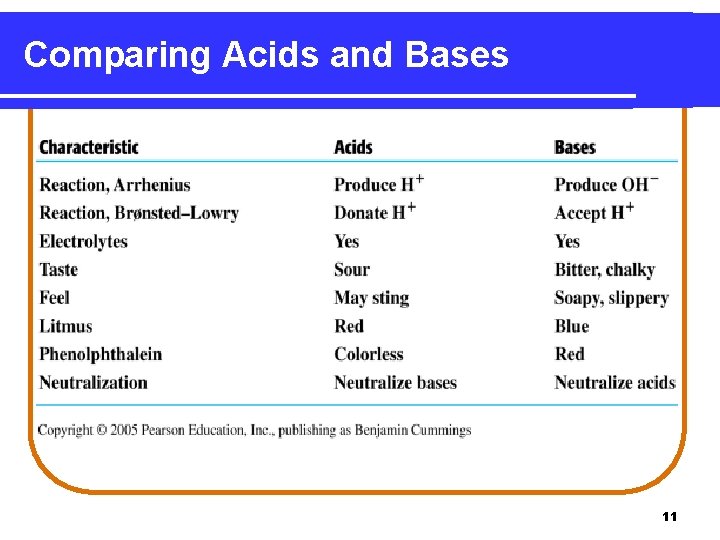
Comparing Acids and Bases 11
Learning Check Identify each as a characteristic of an A) acid or B) base. ____1. ____2. ____3. ____4. ____5. has a sour taste produces OH- in aqueous solutions has a chalky taste is an electrolyte produces H+ in aqueous solutions 12
Solution Identify each as a characteristic of an A) acid or B) base. A B B A, B A 1. 2. 3. 4. 5. has a sour taste produces OH- in aqueous solutions has a chalky taste is an electrolyte produces H+ in aqueous solutions 13
- Slides: 13