Chapter 9 Acids and Bases Dr Ronnie Phillips

Chapter 9 Acids and Bases Dr. Ronnie Phillips CHEM 1151
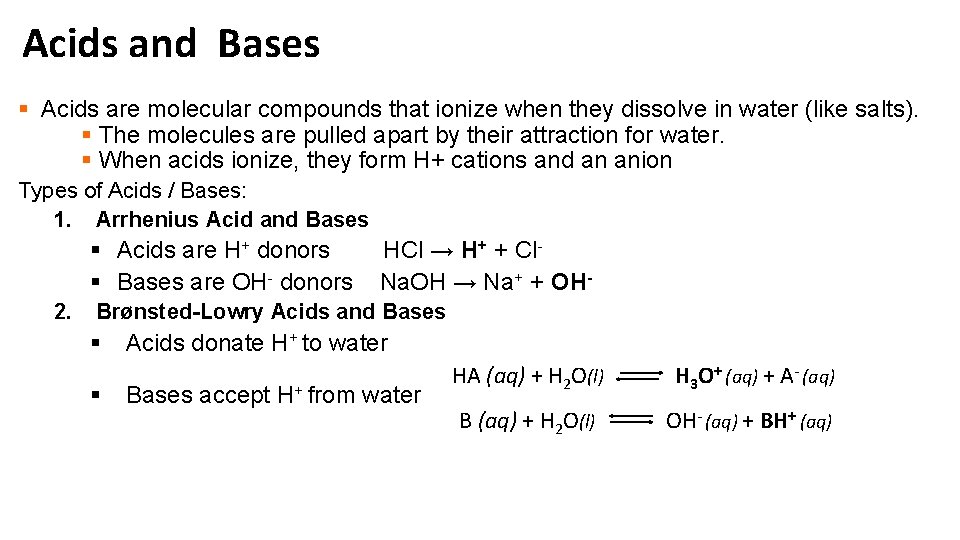
Acids and Bases § Acids are molecular compounds that ionize when they dissolve in water (like salts). § The molecules are pulled apart by their attraction for water. § When acids ionize, they form H+ cations and an anion Types of Acids / Bases: 1. Arrhenius Acid and Bases § Acids are H+ donors § Bases are OH- donors 2. HCl → H+ + Cl. Na. OH → Na+ + OH- Brønsted-Lowry Acids and Bases § § Acids donate H+ to water Bases accept H+ from water HA (aq) + H 2 O(l) H 3 O+ (aq) + A- (aq) B (aq) + H 2 O(l) OH- (aq) + BH+ (aq)
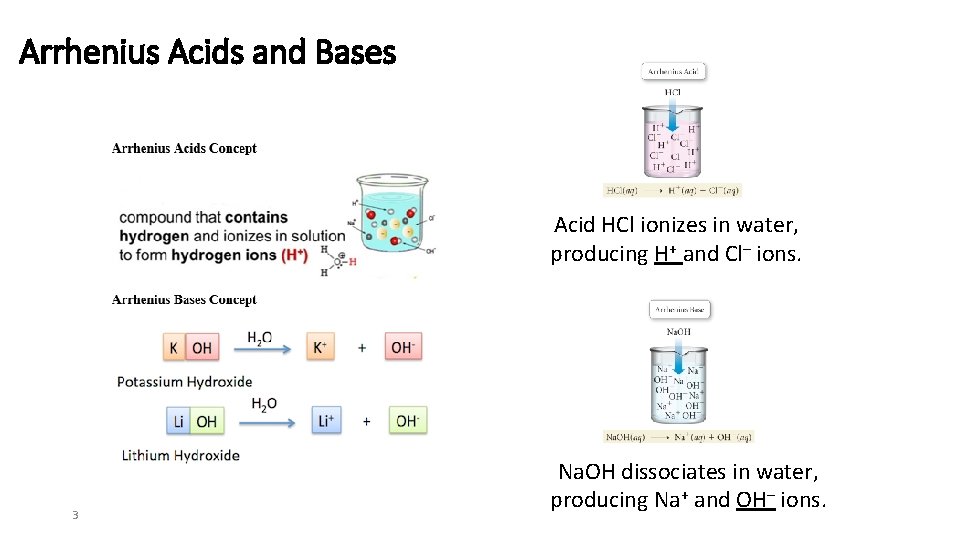
Arrhenius Acids and Bases Acid HCl ionizes in water, producing H+ and Cl– ions. 3 Na. OH dissociates in water, producing Na+ and OH– ions.
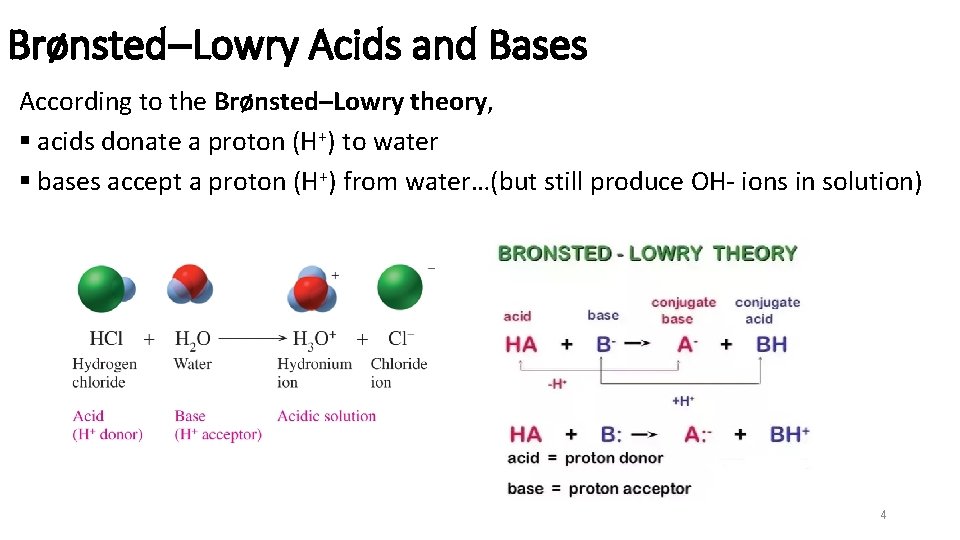
Brønsted–Lowry Acids and Bases According to the Brønsted–Lowry theory, § acids donate a proton (H+) to water § bases accept a proton (H+) from water…(but still produce OH- ions in solution) 4
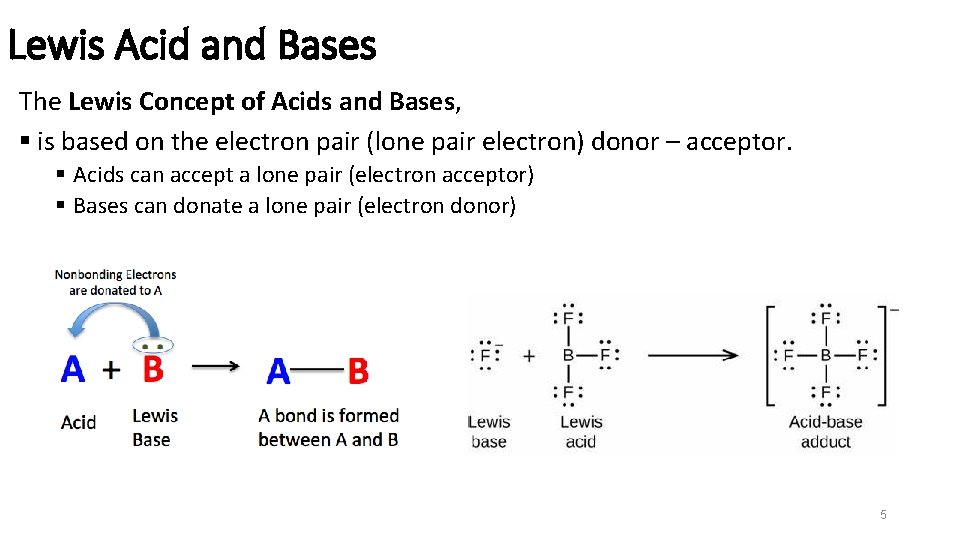
Lewis Acid and Bases The Lewis Concept of Acids and Bases, § is based on the electron pair (lone pair electron) donor – acceptor. § Acids can accept a lone pair (electron acceptor) § Bases can donate a lone pair (electron donor) 5
Naming Acids named as: § Binary acids have H+ cation and nonmetal anion. § Example: HCl § Oxyacids have H+ cation and polyatomic anion. § Example HNO 3 6

Naming Binary Acids § Write a hydro prefix. § Follow with the nonmetal name. § Change ending on nonmetal name to -ic. § Write the word acid at the end of the name § NOTE: Acid means dissolved in water, if not dissolved in water, the compound is named as an ionic compound 7

Example—Naming Binary Acids: HCl (aq) 1. Identify the anion. Cl = Cl−, chloride because Group 7 A 2. Name the anion with an -ic suffix. Cl− = chloride chloric 3. Add a hydro- prefix to the anion name. hydrochloric 4. Add the word acid to the end. hydrochloric acid *If not dissolved in water the name would be: Hydrogen Chloride 8
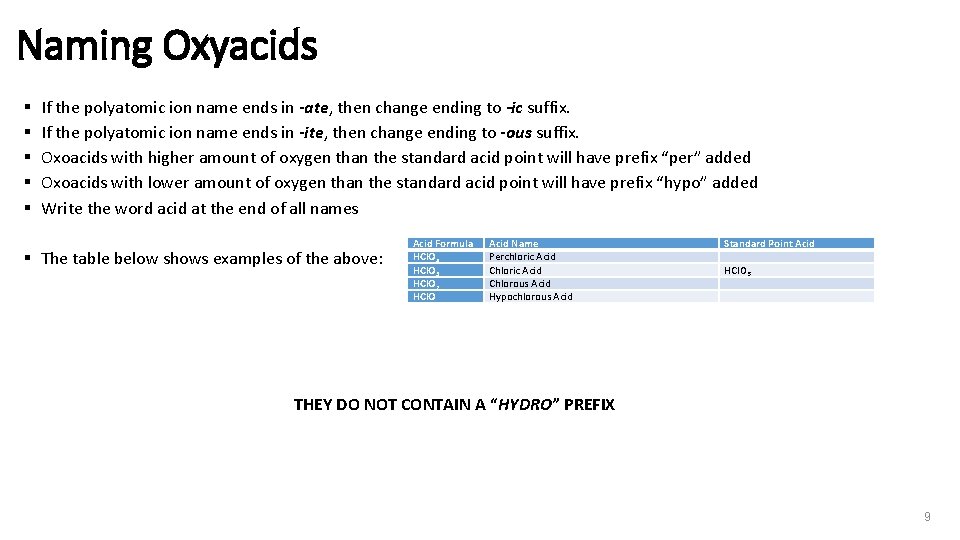
Naming Oxyacids § § § If the polyatomic ion name ends in -ate, then change ending to -ic suffix. If the polyatomic ion name ends in -ite, then change ending to -ous suffix. Oxoacids with higher amount of oxygen than the standard acid point will have prefix “per” added Oxoacids with lower amount of oxygen than the standard acid point will have prefix “hypo” added Write the word acid at the end of all names § The table below shows examples of the above: Acid Formula HCl. O 4 HCl. O 3 HCl. O 2 HCl. O Acid Name Perchloric Acid Chlorous Acid Hypochlorous Acid Standard Point Acid HCl. O 3 THEY DO NOT CONTAIN A “HYDRO” PREFIX 9
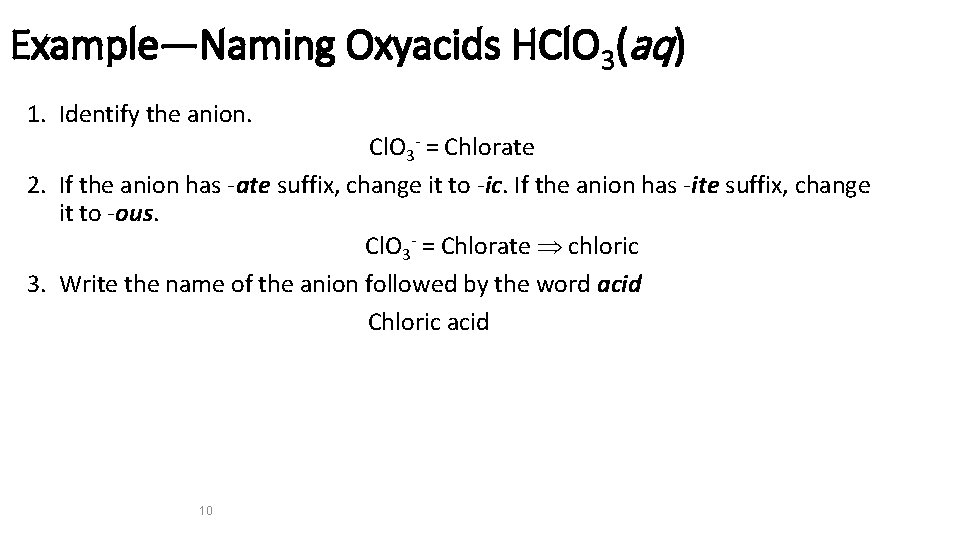
Example—Naming Oxyacids HCl. O 3(aq) 1. Identify the anion. Cl. O 3 - = Chlorate 2. If the anion has -ate suffix, change it to -ic. If the anion has -ite suffix, change it to -ous. Cl. O 3 - = Chlorate chloric 3. Write the name of the anion followed by the word acid Chloric acid 10
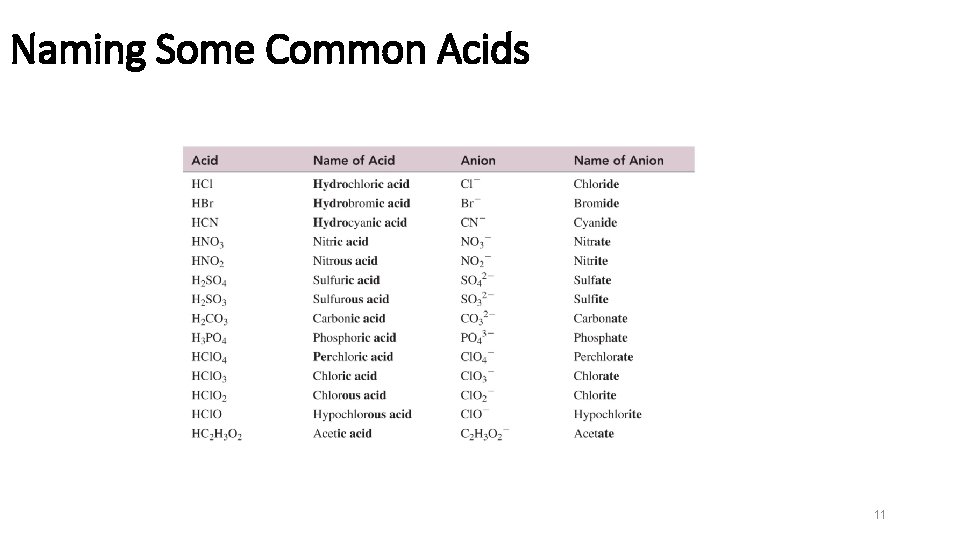
Naming Some Common Acids 11

Learning Check Select the correct name for each of the following acids: A. HBr 1) bromic acid 2) bromous acid 3) hydrobromic acid B. H 2 CO 3 1) carbonic acid (Carbonate) 2) hydrocarbonic acid 3) carbonous acid C. HBr. O 2 1) bromic acid (Bromite) 2) hydrobromous acid 3) bromous acid 12

Naming Common Bases § Bases with OH ions are named as ionic compounds § the metal + the hydroxide in the formula. Examples: Na. OH (sodium hydroxide) KOH (potassium hydroxide) Ba(OH)2 (barium hydroxide) Al(OH)3 (aluminum hydroxide) Fe(OH)3 (iron (III) hydroxide) 13
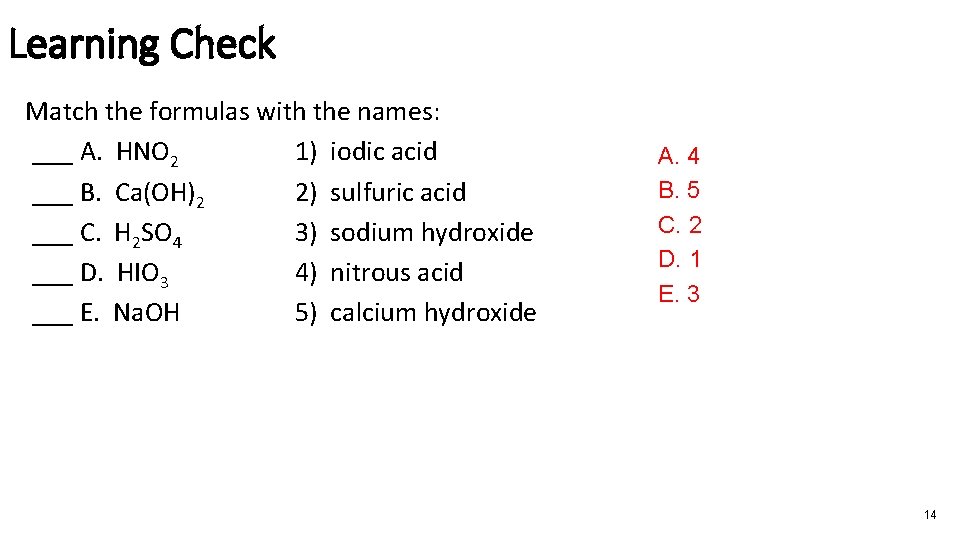
Learning Check Match the formulas with the names: ___ A. HNO 2 1) iodic acid ___ B. Ca(OH)2 2) sulfuric acid ___ C. H 2 SO 4 3) sodium hydroxide ___ D. HIO 3 4) nitrous acid ___ E. Na. OH 5) calcium hydroxide A. 4 B. 5 C. 2 D. 1 E. 3 14
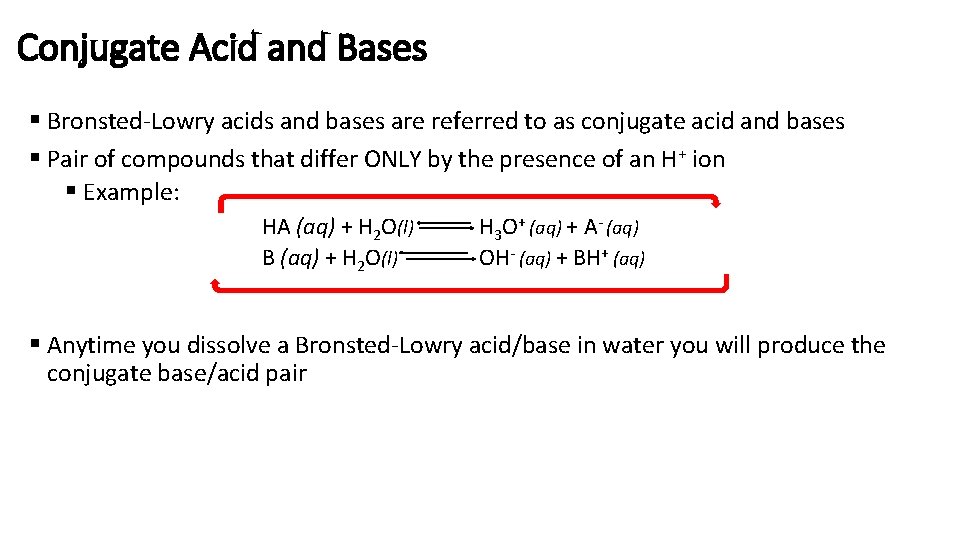
Conjugate Acid and Bases § Bronsted-Lowry acids and bases are referred to as conjugate acid and bases § Pair of compounds that differ ONLY by the presence of an H+ ion § Example: HA (aq) + H 2 O(l) B (aq) + H 2 O(l) H 3 O+ (aq) + A- (aq) OH- (aq) + BH+ (aq) § Anytime you dissolve a Bronsted-Lowry acid/base in water you will produce the conjugate base/acid pair
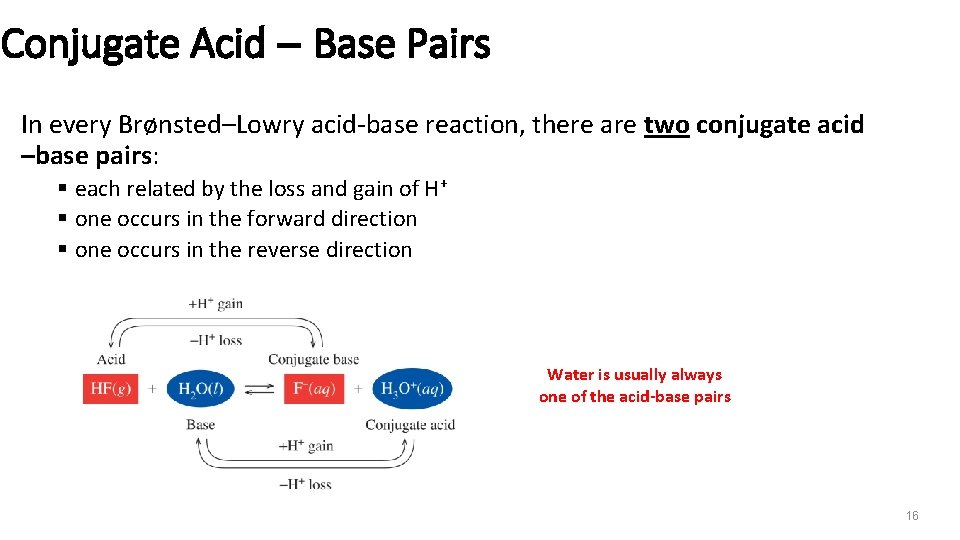
Conjugate Acid – Base Pairs In every Brønsted–Lowry acid-base reaction, there are two conjugate acid –base pairs: § each related by the loss and gain of H+ § one occurs in the forward direction § one occurs in the reverse direction Water is usually always one of the acid-base pairs 16
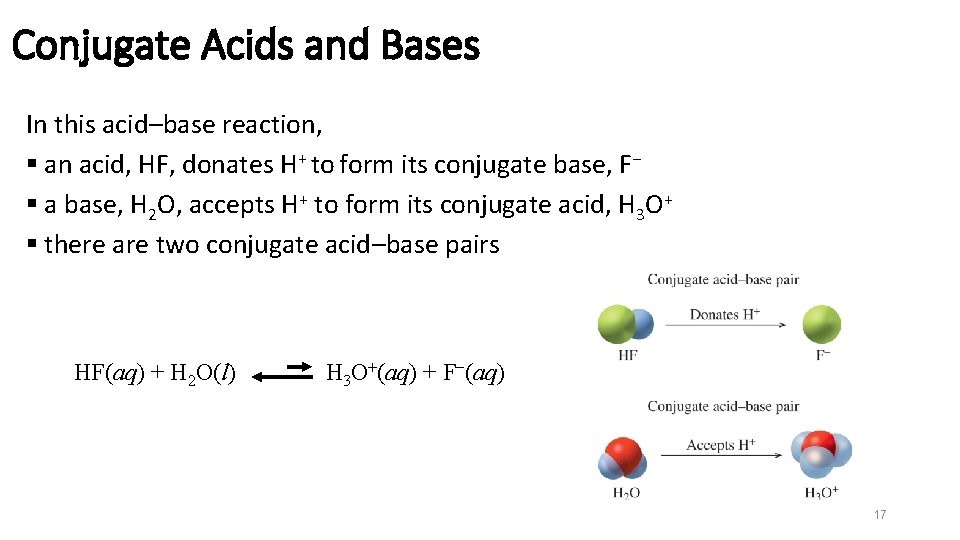
Conjugate Acids and Bases In this acid–base reaction, § an acid, HF, donates H+ to form its conjugate base, F− § a base, H 2 O, accepts H+ to form its conjugate acid, H 3 O+ § there are two conjugate acid–base pairs HF(aq) + H 2 O(l) H 3 O+(aq) + F−(aq) 17

Learning Check A. Write the conjugate base of the following: Br 1) HBr HS 2) H 2 S HCO 3 3) H 2 CO 3 B. Write the conjugate acid of the following: 1) NO 2 HNO 2 2) NH 3 NH 4+ H 2 O 3) OH 18

Learning Check Identify the sets that contain acid–base conjugate pairs. 1) HNO 2, NO 2− 2) H 2 CO 3, CO 32− 3) HCl, Cl. O 4− 4) HS−, H 2 S 5) NH 3, NH 4+ Answer: 1), 4), and 5) 19
Conjugate Acid Base Pairs Amphoteric Compounds: § Amphoteric compounds are compounds can act as an acid or as a base depending on the reaction they are participating in. § Examples of such amphoteric compounds are given amphoteric compounds: § H 2 O is an amphoteric compound: § H 2 O as an acid: § H 2 O as a base: H 2 O(l) + NH 3(aq) NH 4+(aq) + OH-(aq) H 2 O(l) + HCl H 3 O+(aq) + Cl-(aq)
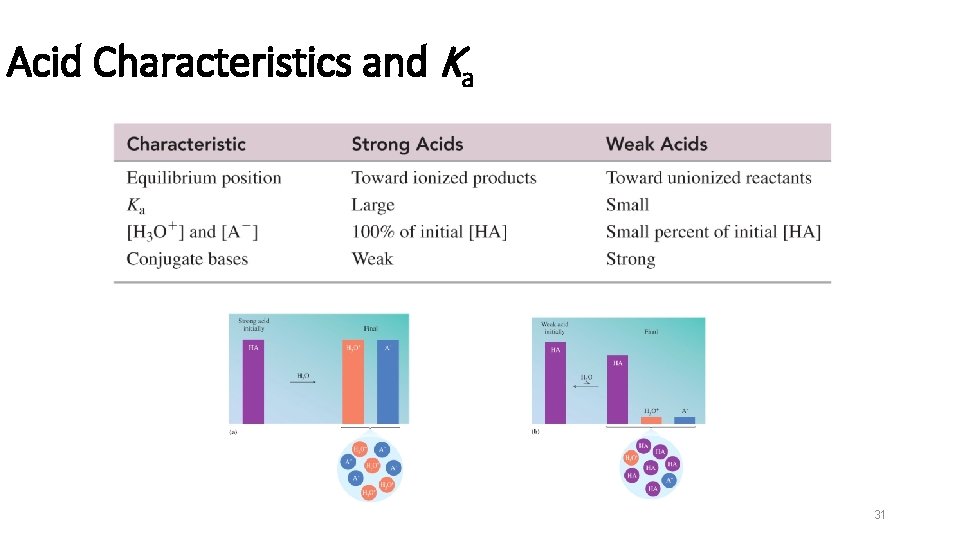
Acids § Acids are molecular compounds that ionize when they dissolve in water. § The percentage of molecules that ionize varies from one acid to another. § Acids that ionize virtually 100% are called strong acids. (same as strong electrolyte) HCl(g) + H 2 O(l) H+(aq) + Cl−(aq) § Acids that only ionize a small percentage are called weak acids. HF(aq) H+(aq) + F−(aq) + HF(aq) 21
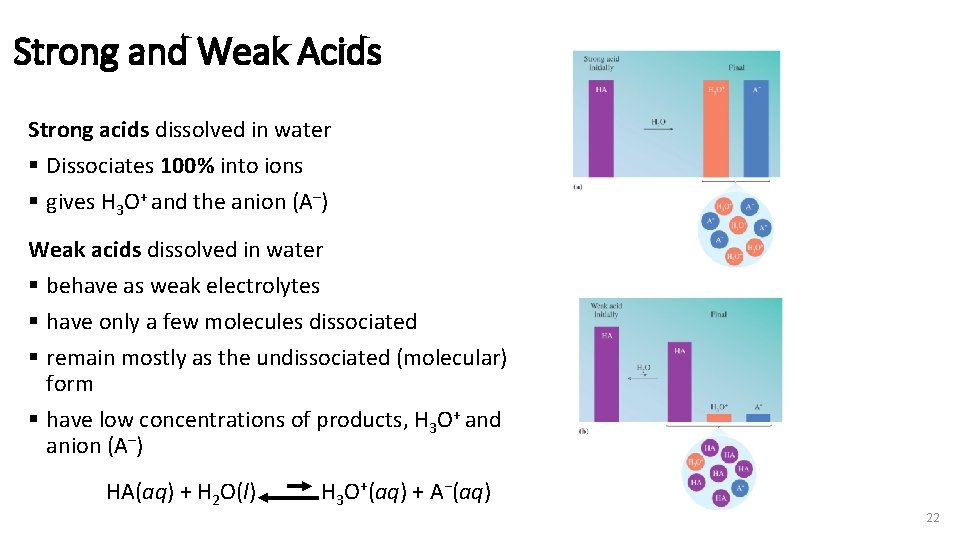
Strong and Weak Acids Strong acids dissolved in water § Dissociates 100% into ions § gives H 3 O+ and the anion (A–) Weak acids dissolved in water § behave as weak electrolytes § have only a few molecules dissociated § remain mostly as the undissociated (molecular) form § have low concentrations of products, H 3 O+ and anion (A–) HA(aq) + H 2 O(l) H 3 O+(aq) + A−(aq) 22
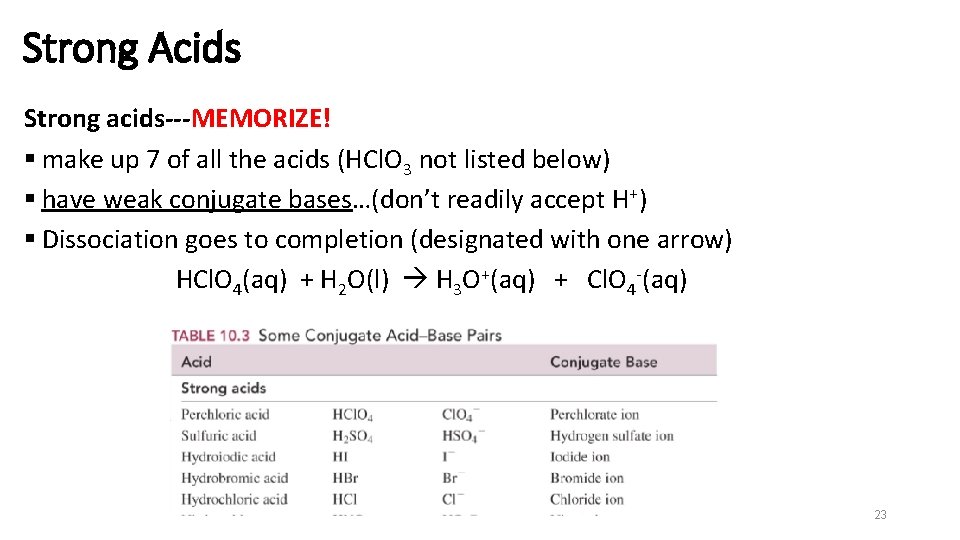
Strong Acids Strong acids---MEMORIZE! § make up 7 of all the acids (HCl. O 3 not listed below) § have weak conjugate bases…(don’t readily accept H+) § Dissociation goes to completion (designated with one arrow) HCl. O 4(aq) + H 2 O(l) H 3 O+(aq) + Cl. O 4 -(aq) 23
Weak Acids § make up most of the acids § have ‘stronger’ conjugate bases…(readily accept H+) § Dissociate only partially (designated with double arrow) 24

Strong (Arrhenius) Bases Strong Bases – MEMORIZE! § Are ONLY Arrhenius bases § are formed from metals of Group 1 A and 2 A § Group 1 A: Li. OH, Na. OH, KOH…etc § Group 2 A: Mg(OH)2, Ca(OH)2, Ba(OH)2. . etc. § dissociate completely in water (designated with one arrow) KOH(s) K+(aq) + OH−(aq) 25

Weak Bases § are most other bases § Arrhenius bases using Grp 2, Al, or transition metals § All Bronsted-Lowry Bases § dissociate only slightly in water (designated with double arrow) § form only a few ions in water NH 3(g) + H 2 O(l) NH 4+(aq) + OH−(aq) 26
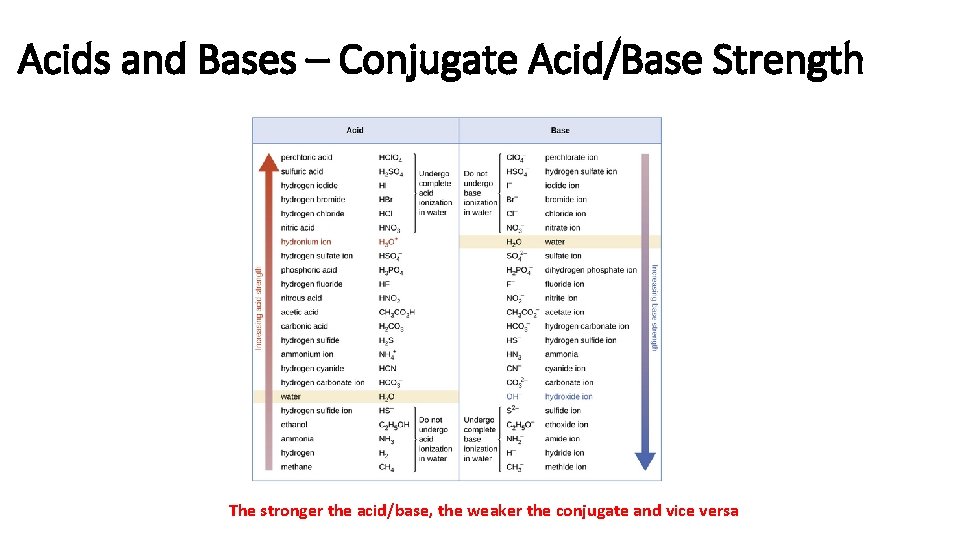
Acids and Bases – Conjugate Acid/Base Strength The stronger the acid/base, the weaker the conjugate and vice versa
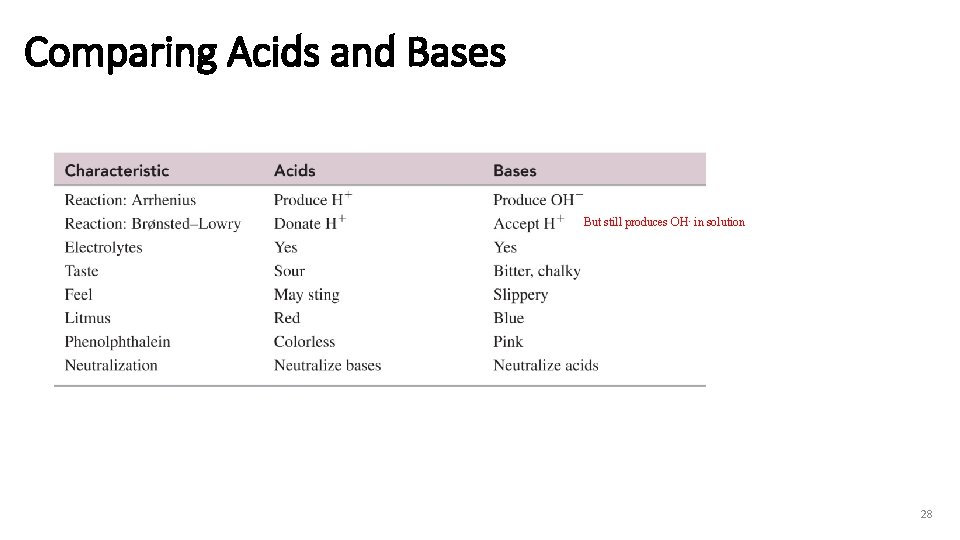
Comparing Acids and Bases But still produces OH- in solution 28

Learning Check Identify each of the following as a strong or weak acid or base. A. HBr strong acid B. HNO 2 weak acid strong base C. Na. OH strong acid D. H 2 SO 4 weak base E. Cu(OH)2 29
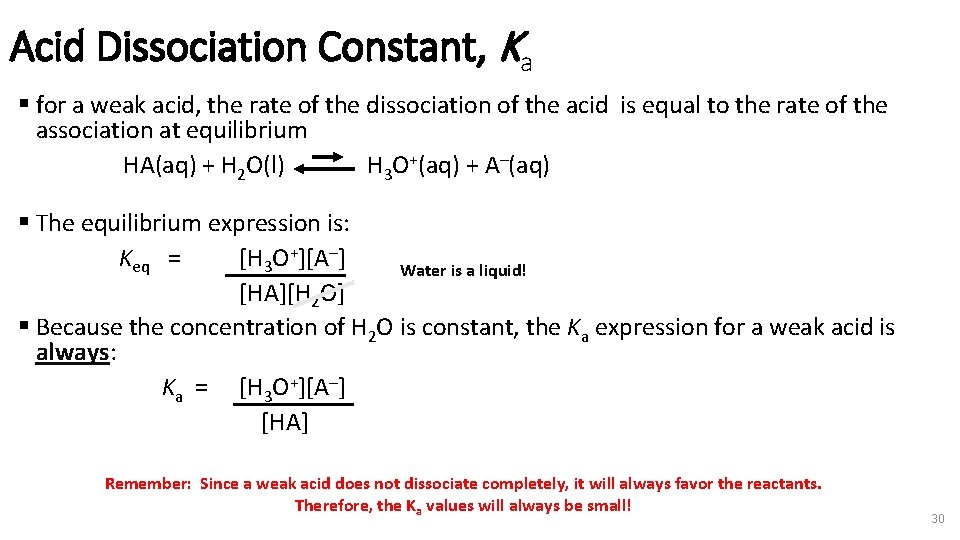
Acid Dissociation Constant, Ka § for a weak acid, the rate of the dissociation of the acid is equal to the rate of the association at equilibrium HA(aq) + H 2 O(l) H 3 O+(aq) + A–(aq) § The equilibrium expression is: Keq = [H 3 O+][A–] Water is a liquid! [HA][H 2 O] § Because the concentration of H 2 O is constant, the Ka expression for a weak acid is always: Ka = [H 3 O+][A–] [HA] Remember: Since a weak acid does not dissociate completely, it will always favor the reactants. Therefore, the Ka values will always be small! 30
Acid Characteristics and Ka 31
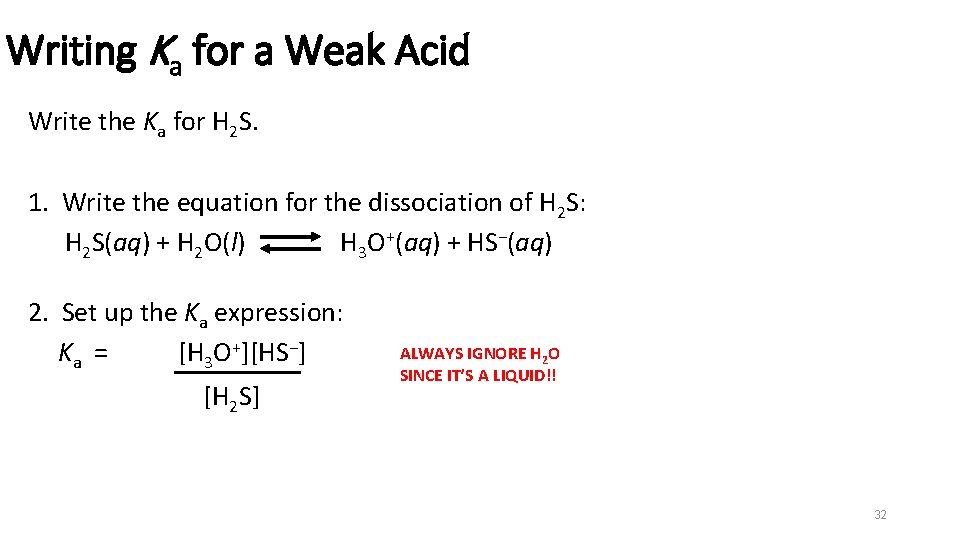
Writing Ka for a Weak Acid Write the Ka for H 2 S. 1. Write the equation for the dissociation of H 2 S: H 2 S(aq) + H 2 O(l) H 3 O+(aq) + HS−(aq) 2. Set up the Ka expression: Ka = [H 3 O+][HS−] [H 2 S] ALWAYS IGNORE H 2 O SINCE IT’S A LIQUID!! 32

Learning Check Write the Ka for HCN. (HINT: Must write balanced equation first) 1. Write the equation for the dissociation of HCN: HCN(aq) + H 2 O(l) H 3 O+(aq) + CN (aq) 2. Set up the Ka expression: Ka = [H 3 O+][CN ] [HCN] 33
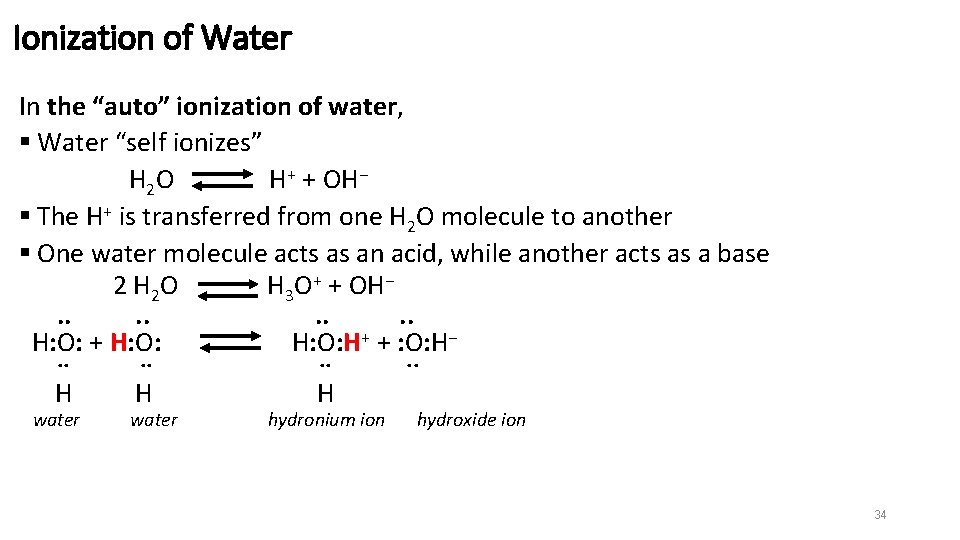
Ionization of Water In the “auto” ionization of water, § Water “self ionizes” H 2 O H+ + OH− § The H+ is transferred from one H 2 O molecule to another § One water molecule acts as an acid, while another acts as a base 2 H 2 O H 3 O+ + OH−. . . . H: O: + H: O: H+ + : O: H−. . . H H H water hydronium ion . . hydroxide ion 34
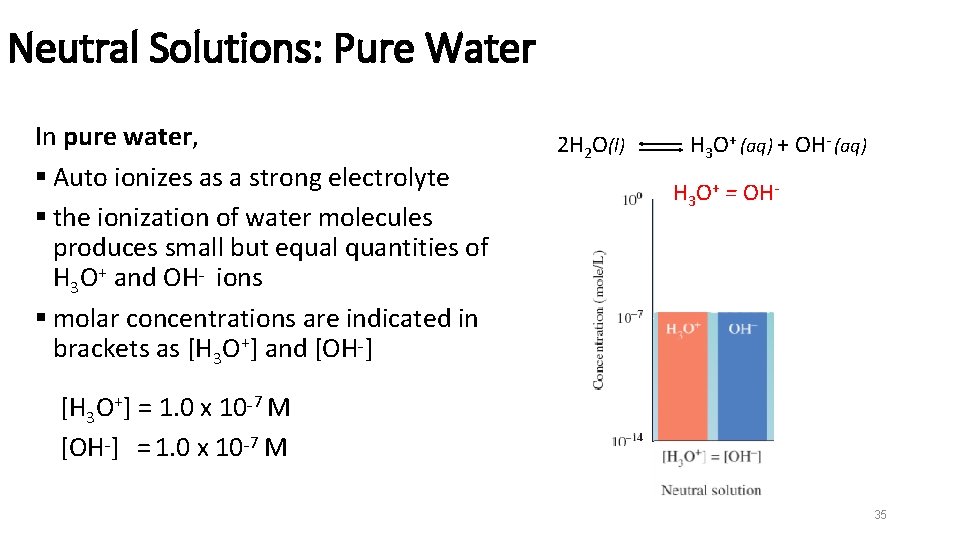
Neutral Solutions: Pure Water In pure water, § Auto ionizes as a strong electrolyte § the ionization of water molecules produces small but equal quantities of H 3 O+ and OH− ions § molar concentrations are indicated in brackets as [H 3 O+] and [OH−] 2 H 2 O(l) H 3 O+ (aq) + OH- (aq) H 3 O+ = OH- [H 3 O+] = 1. 0 x 10− 7 M [OH−] = 1. 0 x 10− 7 M 35
![Acidic Solutions Adding an acid to pure water § increases the [H 3 O+] Acidic Solutions Adding an acid to pure water § increases the [H 3 O+]](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-36.jpg)
Acidic Solutions Adding an acid to pure water § increases the [H 3 O+] § causes the [H 3 O+] to exceed 1. 0 x 10− 7 M § decreases the [OH−] HA (aq) + H 2 O(l) H 3 O+ (aq) + A- (aq) H 3 O+ > OH 36
![Basic Solutions Adding a base to pure water, § increases the [OH−] § causes Basic Solutions Adding a base to pure water, § increases the [OH−] § causes](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-37.jpg)
Basic Solutions Adding a base to pure water, § increases the [OH−] § causes the [OH−] to exceed 1. 0 x 10− 7 M § decreases the [H 3 O+] B (aq) + H 2 O(l) OH- (aq) + BH+ (aq) OH- > H 3 O+ 37
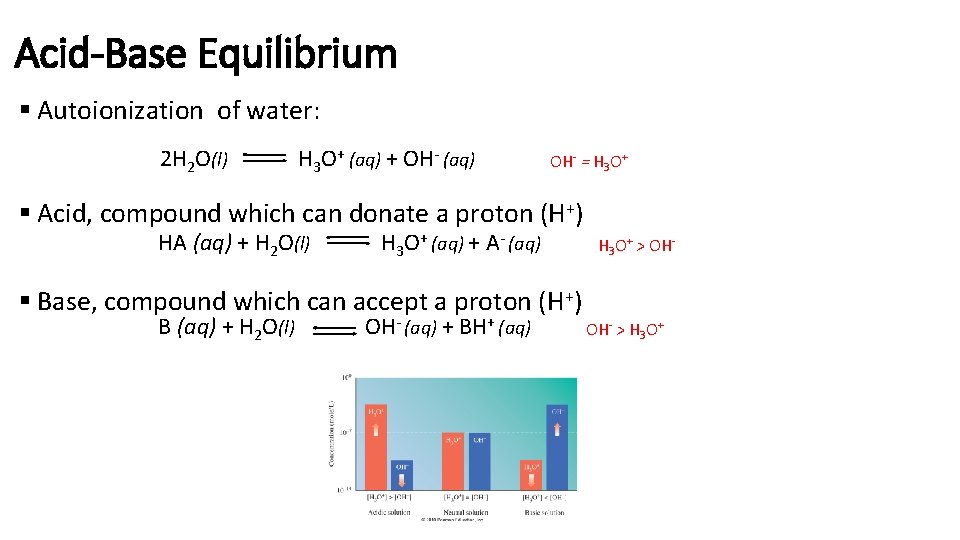
Acid-Base Equilibrium § Autoionization of water: 2 H 2 O(l) H 3 O+ (aq) + OH- (aq) OH- = H 3 O+ § Acid, compound which can donate a proton (H+) HA (aq) + H 2 O(l) H 3 O+ (aq) + A- (aq) § Base, compound which can accept a proton (H+) B (aq) + H 2 O(l) OH- (aq) + BH+ (aq) H 3 O+ > OH- > H 3 O+
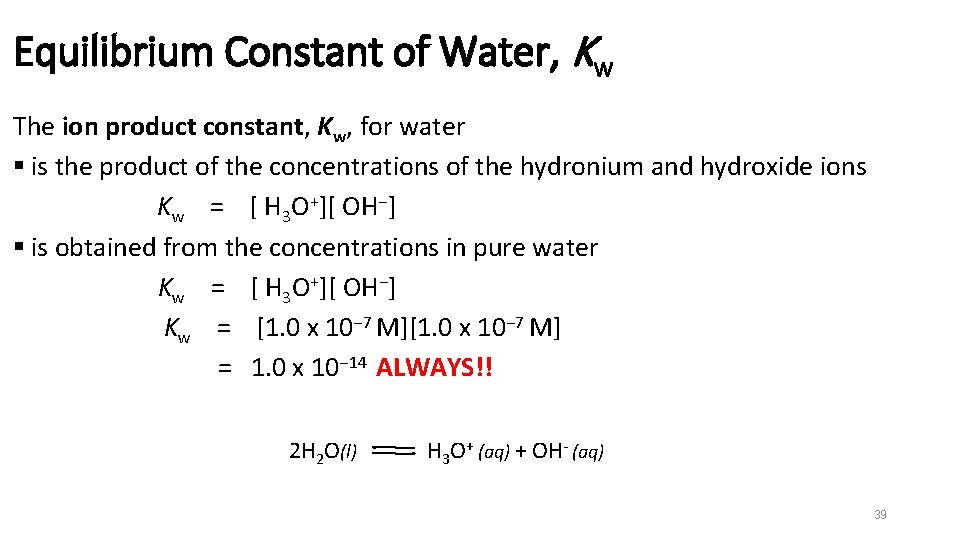
Equilibrium Constant of Water, Kw The ion product constant, Kw, for water § is the product of the concentrations of the hydronium and hydroxide ions Kw = [ H 3 O+][ OH−] § is obtained from the concentrations in pure water Kw = [ H 3 O+][ OH−] Kw = [1. 0 x 10− 7 M] = 1. 0 x 10− 14 ALWAYS!! 2 H 2 O(l) H 3 O+ (aq) + OH- (aq) 39
![[H 3 O+] and [OH−] in Solutions In neutral, acidic, or basic solutions, the [H 3 O+] and [OH−] in Solutions In neutral, acidic, or basic solutions, the](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-40.jpg)
[H 3 O+] and [OH−] in Solutions In neutral, acidic, or basic solutions, the Kw at 25 °C is always 1. 0 x 10− 14. Neutral solutions always have 1. 0 x 10− 7 M [H 3 O+] and [OH−] Acidic solutions always have > 1. 0 x 10− 7 M [H 3 O+] and < 1. 0 x 10− 7 M [OH−] Basic solutions always have < 1. 0 x 10− 7 M [H 3 O+] and > 1. 0 x 10− 7 M [OH−] 40
![Guide to Calculating [H 3 + O] Anytime you know [OH−], can find the Guide to Calculating [H 3 + O] Anytime you know [OH−], can find the](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-41.jpg)
Guide to Calculating [H 3 + O] Anytime you know [OH−], can find the [H 3 O+] using Kw (and vice versa) 41
![Example of Calculating [H 3 + O] What is the [H 3 O+] of Example of Calculating [H 3 + O] What is the [H 3 O+] of](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-42.jpg)
Example of Calculating [H 3 + O] What is the [H 3 O+] of a solution if [OH−] is 5. 0 x 10− 8 M? Write the Kw for water: Kw = [H 3 O+ ][OH− ] = 1. 0 x 10− 14 Rearrange the Kw expression for [H 3 O+]: [H 3 O+] = 1. 0 x 10− 14 [OH−] Substitute the known [OH−] and calculate: [H 3 O+] = 1. 0 x 10− 14 = 2. 0 x 10− 7 M [5. 0 x 10− 8] 42
![Learning Check If lemon juice has [H 3 O+] of 2 x 10− 3 Learning Check If lemon juice has [H 3 O+] of 2 x 10− 3](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-43.jpg)
Learning Check If lemon juice has [H 3 O+] of 2 x 10− 3 M, what is the [OH−] of the solution? Write the Kw for water: Kw = [H 3 O+][OH−] = 1. 0 x 10− 14 Rearrange the Kw expression for unknown [OH−]: [OH−] = 1. 0 x 10− 14 [H 3 O+ ] Substitute the known [H 3 O+] and calculate: [OH−] = 1. 0 x 10− 14 = 5 x 10− 12 M [2 x 10− 3 ] 43
![Learning Check The [OH−] of an ammonia solution is 4. 0 x 10− 2 Learning Check The [OH−] of an ammonia solution is 4. 0 x 10− 2](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-44.jpg)
Learning Check The [OH−] of an ammonia solution is 4. 0 x 10− 2 M. What is the [H 3 O+ ] of the solution? Write the Kw for water: 1) 2. 5 x 10− 11 M + ][OH− ] = 1. 0 x 10− 14 K = [H O w 3 2) 2. 5 x 10− 12 M +]: Rearrange the K expression for [H O − 13 w 3 3) 2. 5 x 10 M [H 3 O+] = 1. 0 x 10− 14 [OH−] Substitute the known [OH−] and calculate: [H 3 O+] = 1. 0 x 10− 14 = 2. 5 x 10− 13 M 4. 0 x 10− 2 44
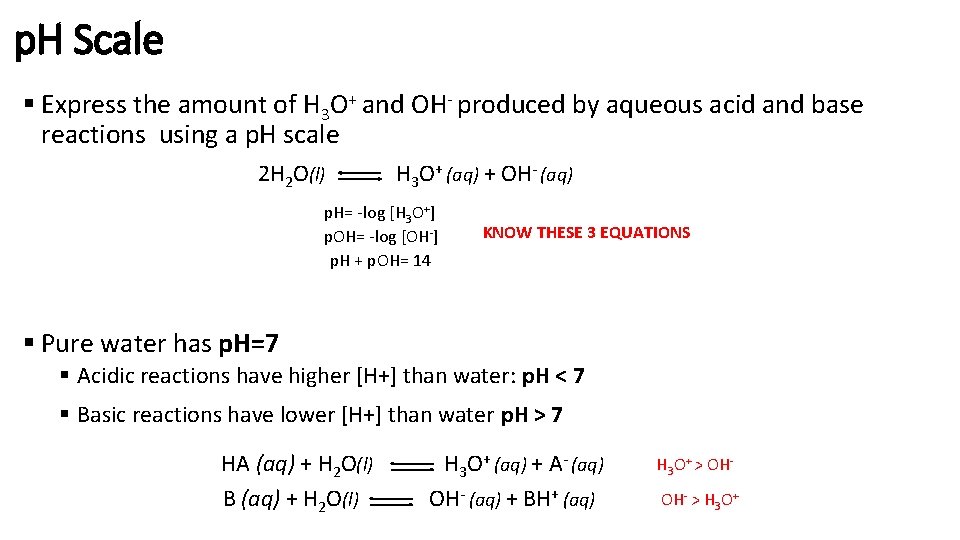
p. H Scale § Express the amount of H 3 O+ and OH- produced by aqueous acid and base reactions using a p. H scale 2 H 2 O(l) H 3 O+ (aq) + OH- (aq) p. H= -log [H 3 O+] p. OH= -log [OH-] p. H + p. OH= 14 KNOW THESE 3 EQUATIONS § Pure water has p. H=7 § Acidic reactions have higher [H+] than water: p. H < 7 § Basic reactions have lower [H+] than water p. H > 7 HA (aq) + H 2 O(l) B (aq) + H 2 O(l) H 3 O+ (aq) + A- (aq) OH- (aq) + BH+ (aq) H 3 O+ > OHOH- > H 3 O+
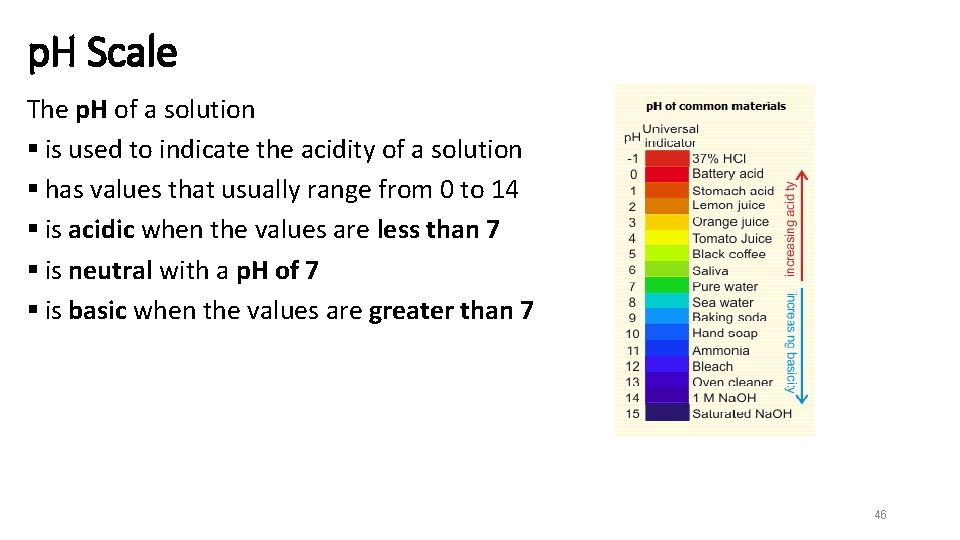
p. H Scale The p. H of a solution § is used to indicate the acidity of a solution § has values that usually range from 0 to 14 § is acidic when the values are less than 7 § is neutral with a p. H of 7 § is basic when the values are greater than 7 46
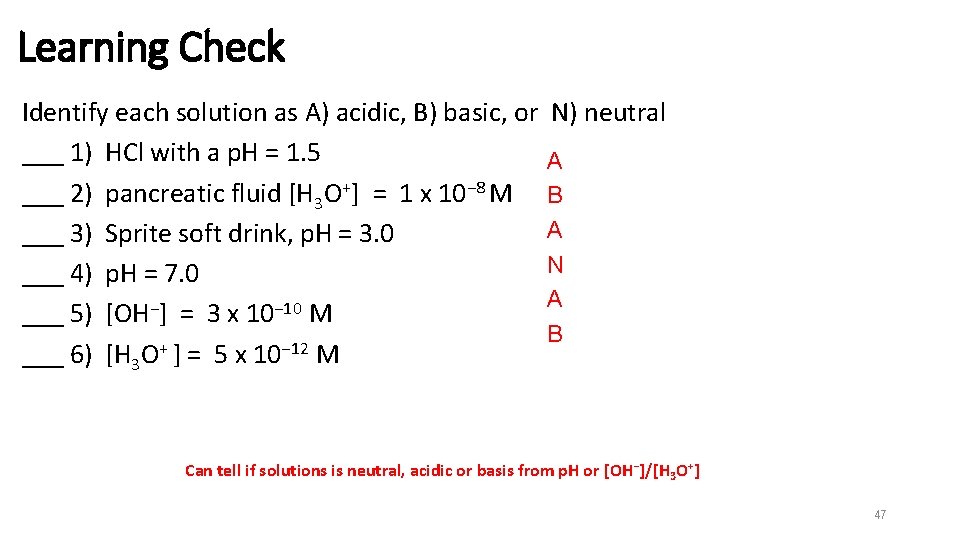
Learning Check Identify each solution as A) acidic, B) basic, or ___ 1) HCl with a p. H = 1. 5 ___ 2) pancreatic fluid [H 3 O+] = 1 x 10− 8 M ___ 3) Sprite soft drink, p. H = 3. 0 ___ 4) p. H = 7. 0 ___ 5) [OH−] = 3 x 10− 10 M ___ 6) [H 3 O+ ] = 5 x 10− 12 M N) neutral A B A N A B Can tell if solutions is neutral, acidic or basis from p. H or [OH−]/[H 3 O+] 47

Testing the p. H of Solutions § The p. H of solutions can be determined using § a p. H meter § p. H paper § Acid—turns blue litmus red § Base—turns red litmus blue § indicators that have specific colors at different p. H values 48
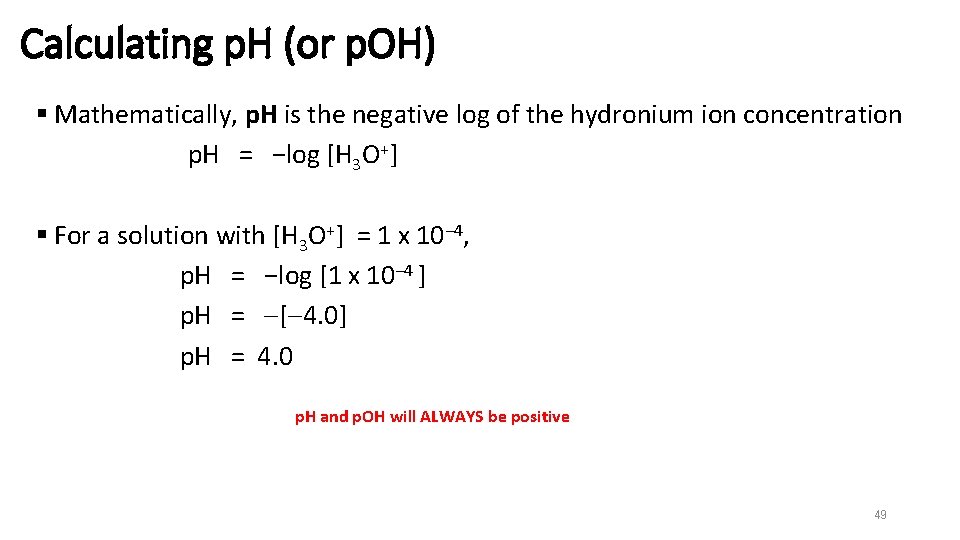
Calculating p. H (or p. OH) § Mathematically, p. H is the negative log of the hydronium ion concentration p. H = −log [H 3 O+] § For a solution with [H 3 O+] = 1 x 10− 4, p. H = −log [1 x 10− 4 ] p. H = [ 4. 0] p. H = 4. 0 p. H and p. OH will ALWAYS be positive 49
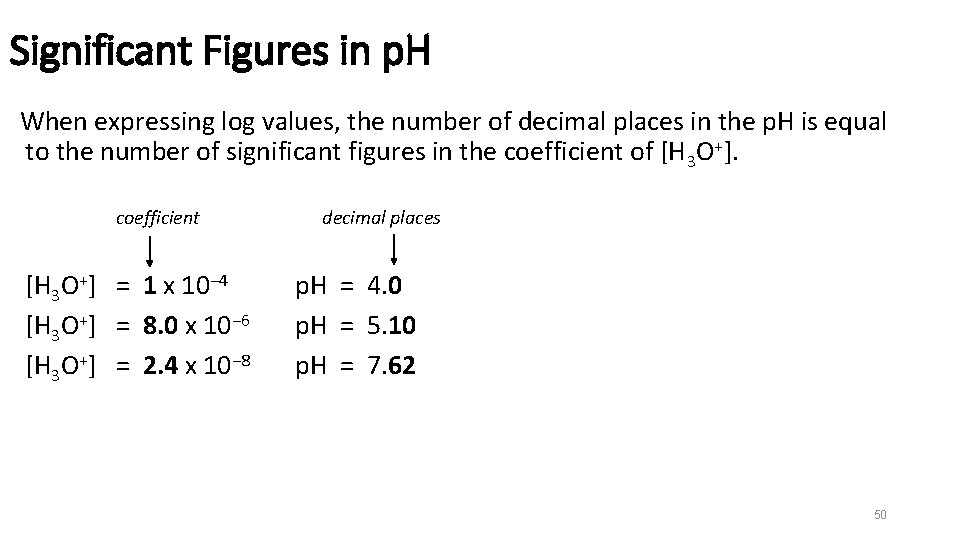
Significant Figures in p. H When expressing log values, the number of decimal places in the p. H is equal to the number of significant figures in the coefficient of [H 3 O+]. coefficient [H 3 O+] = 1 x 10− 4 [H 3 O+] = 8. 0 x 10− 6 [H 3 O+] = 2. 4 x 10− 8 decimal places p. H = 4. 0 p. H = 5. 10 p. H = 7. 62 50
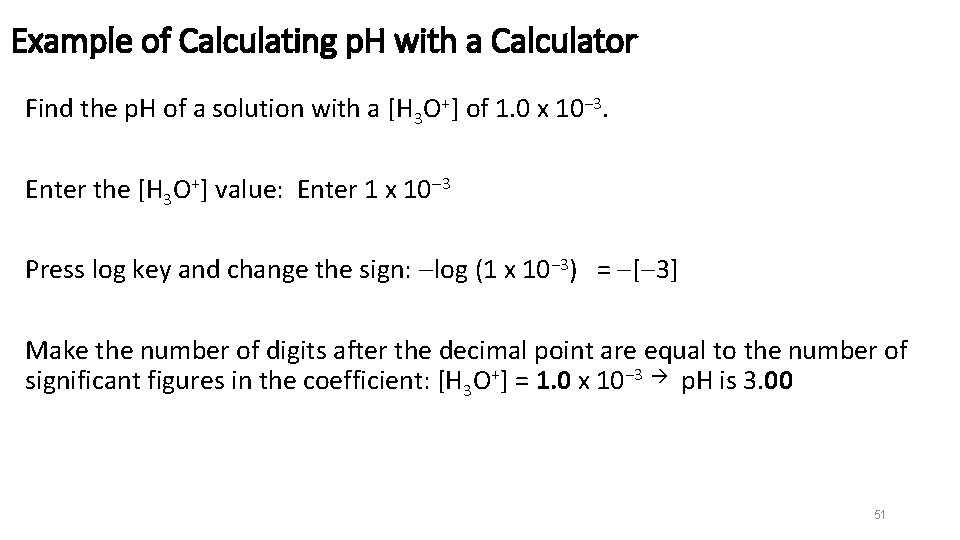
Example of Calculating p. H with a Calculator Find the p. H of a solution with a [H 3 O+] of 1. 0 x 10− 3. Enter the [H 3 O+] value: Enter 1 x 10 3 Press log key and change the sign: log (1 x 10− 3) = [ 3] Make the number of digits after the decimal point are equal to the number of significant figures in the coefficient: [H 3 O+] = 1. 0 x 10− 3 p. H is 3. 00 51
![Learning Check What is the p. H of coffee if the [H 3 O+] Learning Check What is the p. H of coffee if the [H 3 O+]](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-52.jpg)
Learning Check What is the p. H of coffee if the [H 3 O+] is 1 x 10− 5 M? p. H= -log [H 3 O+] log (1 x 10− 5) = [ 5] [H 3 O+]= 1 x 10− 5, p. H is 5. 0 52
![Learning Check A. 1) 2) 3) The [H 3 O+] of tomato juice is Learning Check A. 1) 2) 3) The [H 3 O+] of tomato juice is](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-53.jpg)
Learning Check A. 1) 2) 3) The [H 3 O+] of tomato juice is 2 x 10− 4 M. What is the p. H of the solution? 4. 0 log (2 x 10− 4) = [ 3. 7] 3. 7 [H 3 O+] = 2 x 10− 4, p. H is = 3. 7 10. 3 B. 1) 2) 3) The [OH−] of a solution is 1. 0 x 10− 3 M. What is the p. H? 3. 00 − 11) = [ 11] log (1. 0 x 10 11. 00 +] =1. 0 x 10− 11, p. H is = 11. 00 [H O – 11. 00 3 53
![Calculating [H 3 O+] from p. H using Calculator Calculate the [H 3 O+] Calculating [H 3 O+] from p. H using Calculator Calculate the [H 3 O+]](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-54.jpg)
Calculating [H 3 O+] from p. H using Calculator Calculate the [H 3 O+] for a p. H value of 8. 0. p. H = -log [H 3 O+] = 1 x 10−p. H For p. H = 8. 0, the [H 3 O+] = 1 x 10− 8 § Enter the p. H value, change sign: – 8. 0 § Convert p. H to concentration: § Adjust the significant figures in the coefficient (1 digit following decimal point = 1 digit in the coefficient): 54
![Learning Check What is the [H 3 O+] of a solution with a p. Learning Check What is the [H 3 O+] of a solution with a p.](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-55.jpg)
Learning Check What is the [H 3 O+] of a solution with a p. H of 10. 0? 1) 1 x 10− 4 M − 10 M 3) 1 x 10 2) 1 x 1010 M 3) 1 x 10− 10 M What is the [H 3 O+] of a solution with a p. H of 2. 85? 1) 1. 0 x 10− 2. 85 M 2) 1. 4 x 10− 3 M 3) 8. 5 x 10− 2 M 55
![[H 3 O+], [OH-], p. H, & p. OH Values You should be able [H 3 O+], [OH-], p. H, & p. OH Values You should be able](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-56.jpg)
[H 3 O+], [OH-], p. H, & p. OH Values You should be able to calculate [H 3 O+], [OH-], p. H, & p. OH Using: Kw = [ H 3 O+][ OH−] = 1. 0 x 10− 14 p. H= -log [H 3 O+] p. OH= -log [OH-] p. H + p. OH= 14 56
![Calculating p. OH § p. OH calculated just like p. H, but with [OH-] Calculating p. OH § p. OH calculated just like p. H, but with [OH-]](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-57.jpg)
Calculating p. OH § p. OH calculated just like p. H, but with [OH-] instead of [H 3 O+] p. OH = −log [OH-] § Can also calculate p. OH from p. H using the following relation p. H + p. OH = 14 Calculating [OH-] and [H 3 O+] § Can be calculated two ways § From p. H: p. H= -log [H 3 O+] p. OH= -log [OH-] § From Kw: Kw = [ H 3 O+][ OH−] = 1. 0 x 10− 14 57
![Learning Check A. What is the [OH-] of a solution with a p. H Learning Check A. What is the [OH-] of a solution with a p. H](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-58.jpg)
Learning Check A. What is the [OH-] of a solution with a p. H of 10. 0? p. H + p. OH = 14 So p. OH = 4. 00 [OH-] p. OH= -log [OH-] = 1 x 10−p. OH [OH-] = 1. 0 x 10− 4 M OR p. H= -log [H 3 O+] = 1 x 10−p. H So [H 3 O+] = 1. 0 x 10− 10 M Kw = [ H 3 O+][ OH−] = 1. 0 x 10− 14 = [1. 0 x 10− 10 M ][ OH−] = 1. 0 x 10− 14 [OH-] = 1. 0 x 10− 4 M 58
![Learning Check What is the [OH-] of a solution with a p. H of Learning Check What is the [OH-] of a solution with a p. H of](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-59.jpg)
Learning Check What is the [OH-] of a solution with a p. H of 2. 85? p. H + p. OH = 14 So p. OH = 11. 15 p. OH = - log [OH-] = 7. 1 x 10− 12 M 59
Acids and Metals Acids react with metals § such as K, Na, Ca, Mg, Al, Zn, Fe, and Sn § to produce hydrogen gas and the salt of the metal § Single replacement reactions Equations: 2 K(s) + 2 HCl(aq) Zn(s) + 2 HCl(aq) 2 KCl(aq) + H 2(g) Zn. Cl 2(aq) + H 2(g) Net ionic equations: 2 K(s) + 2 H+(aq) Zn(s) + 2 H+(aq) 2 K+(aq) + H 2(g) Zn 2+ (aq) + H 2(g) 60
Acids and Carbonates Acids react with carbonates and hydrogen carbonates to produce carbon dioxide gas, a salt, and water. 2 HCl(aq) + Ca. CO 3(s) HCl(aq) + Na. HCO 3(s) CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) CO 2(g) + Na. Cl (aq) + H 2 O(l) 61
Learning Check Write the products of the following reactions of acids as the complete equation and net ionic equation: A. Zn(s) + 2 HCl (aq) Zn(s) + 2 H+(aq) B. Mg. CO 3 (s) + 2 HCl(aq) Mg. CO 3(s) + 2 H+(aq) ? Zn. Cl 2(aq) + H 2(g) Zn+2(aq) + H 2(g) ? Mg. Cl 2(aq) + CO 2(g) + H 2 O(l) Mg+2(aq) + CO 2(g) + H 2 O(l) 62
Neutralization Reactions § Called neutralization reactions because the acid and base “neutralize” each other’s properties Neutralization is the reaction of § an acid such as HCl and a base such as Na. OH HCl(aq) H+ (aq) + Cl−(aq) Na. OH(aq) Na+ (aq) + OH−(aq) HCl(aq) + Na. OH(aq) Na. Cl + H 2 O(l) § Net ionic equations: the H 3 O+ from the acid and the OH− from the base to form water H+(aq) + OH−(aq) → H 2 O(l) 63
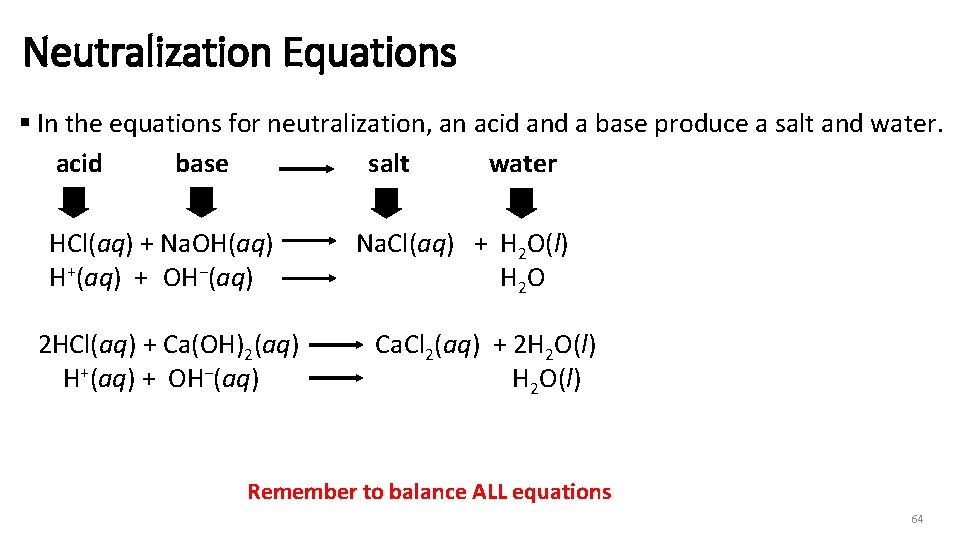
Neutralization Equations § In the equations for neutralization, an acid and a base produce a salt and water. acid base salt water v HCl(aq) + Na. OH(aq) H+(aq) + OH−(aq) 2 HCl(aq) + Ca(OH)2(aq) H+(aq) + OH−(aq) Na. Cl(aq) + H 2 O(l) H 2 O Ca. Cl 2(aq) + 2 H 2 O(l) Remember to balance ALL equations 64
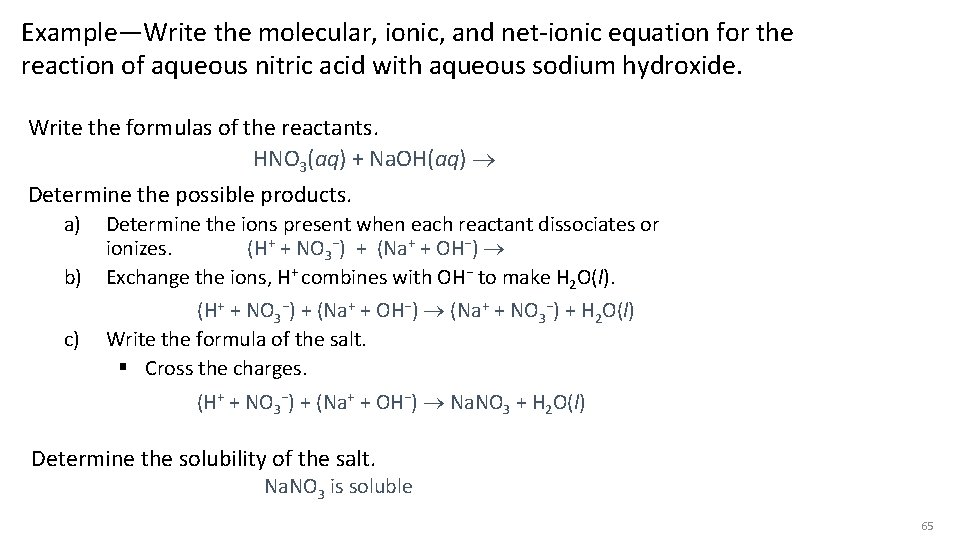
Example—Write the molecular, ionic, and net-ionic equation for the reaction of aqueous nitric acid with aqueous sodium hydroxide. Write the formulas of the reactants. HNO 3(aq) + Na. OH(aq) Determine the possible products. a) b) c) Determine the ions present when each reactant dissociates or ionizes. (H+ + NO 3−) + (Na+ + OH−) Exchange the ions, H+ combines with OH− to make H 2 O(l). (H+ + NO 3−) + (Na+ + OH−) (Na+ + NO 3−) + H 2 O(l) Write the formula of the salt. § Cross the charges. (H+ + NO 3−) + (Na+ + OH−) Na. NO 3 + H 2 O(l) Determine the solubility of the salt. Na. NO 3 is soluble 65
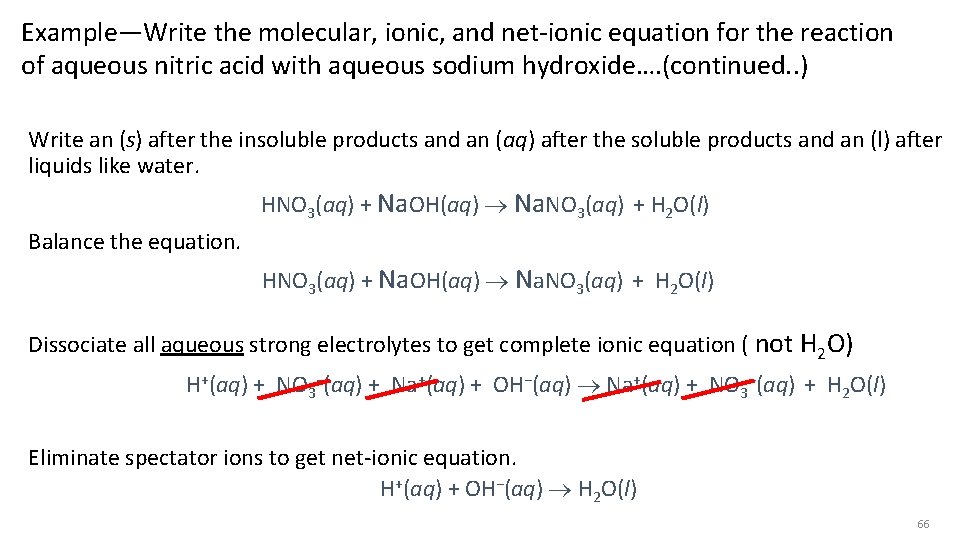
Example—Write the molecular, ionic, and net-ionic equation for the reaction of aqueous nitric acid with aqueous sodium hydroxide…. (continued. . ) Write an (s) after the insoluble products and an (aq) after the soluble products and an (l) after liquids like water. HNO 3(aq) + Na. OH(aq) Na. NO 3(aq) + H 2 O(l) Balance the equation. HNO 3(aq) + Na. OH(aq) Na. NO 3(aq) + H 2 O(l) Dissociate all aqueous strong electrolytes to get complete ionic equation ( not H 2 O) H+(aq) + NO 3−(aq) + Na+(aq) + OH−(aq) Na+(aq) + NO 3−(aq) + H 2 O(l) Eliminate spectator ions to get net-ionic equation. H+(aq) + OH−(aq) H 2 O(l) 66
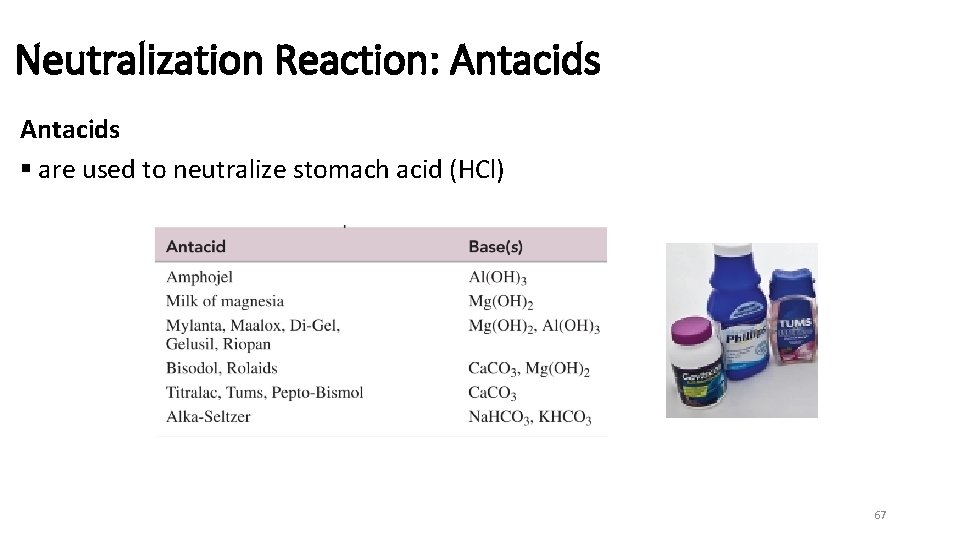
Neutralization Reaction: Antacids § are used to neutralize stomach acid (HCl) 67
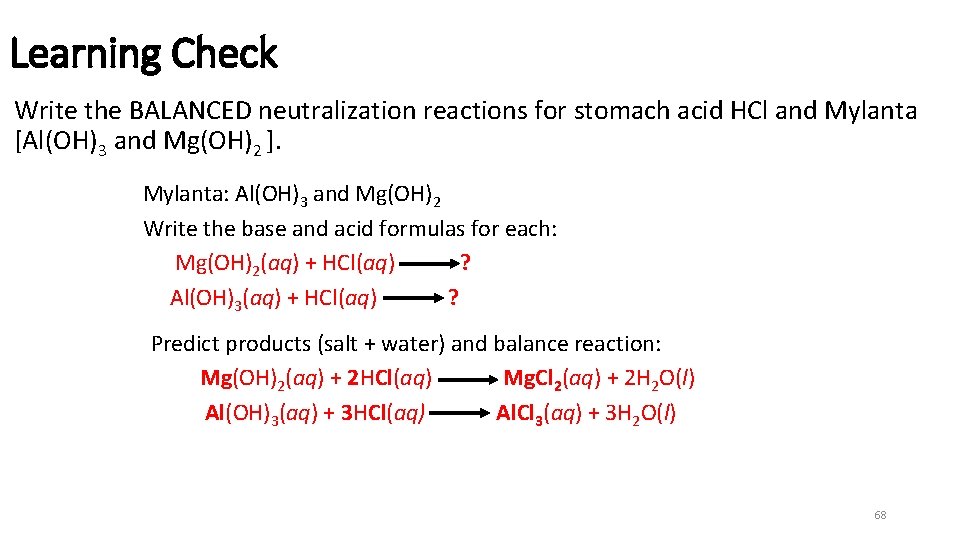
Learning Check Write the BALANCED neutralization reactions for stomach acid HCl and Mylanta [Al(OH)3 and Mg(OH)2 ]. Mylanta: Al(OH)3 and Mg(OH)2 Write the base and acid formulas for each: Mg(OH)2(aq) + HCl(aq) ? Al(OH)3(aq) + HCl(aq) ? Predict products (salt + water) and balance reaction: Mg(OH)2(aq) + 2 HCl(aq) Mg. Cl 2(aq) + 2 H 2 O(l) Al(OH)3(aq) + 3 HCl(aq) Al. Cl 3(aq) + 3 H 2 O(l) 68
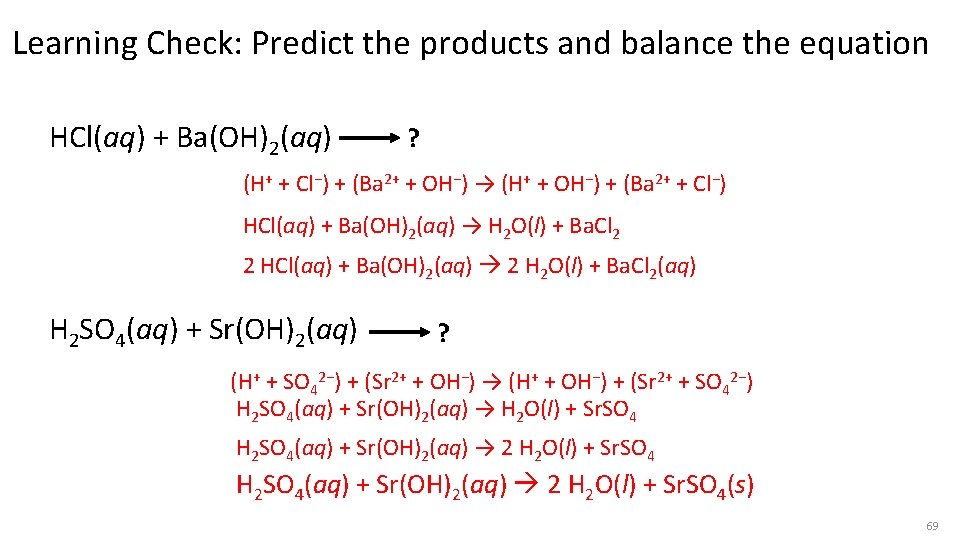
Learning Check: Predict the products and balance the equation HCl(aq) + Ba(OH)2(aq) ? (H+ + Cl−) + (Ba 2+ + OH−) → (H+ + OH−) + (Ba 2+ + Cl−) HCl(aq) + Ba(OH)2(aq) → H 2 O(l) + Ba. Cl 2 2 HCl(aq) + Ba(OH)2(aq) 2 H 2 O(l) + Ba. Cl 2(aq) H 2 SO 4(aq) + Sr(OH)2(aq) ? (H+ + SO 42−) + (Sr 2+ + OH−) → (H+ + OH−) + (Sr 2+ + SO 42−) H 2 SO 4(aq) + Sr(OH)2(aq) → H 2 O(l) + Sr. SO 4 H 2 SO 4(aq) + Sr(OH)2(aq) → 2 H 2 O(l) + Sr. SO 4 H 2 SO 4(aq) + Sr(OH)2(aq) 2 H 2 O(l) + Sr. SO 4(s) 69
Acid–Base Titration § is a laboratory procedure used to determine the molarity of an acid § uses a base such as Na. OH to neutralize a measured volume of an acid § Ultimately undergoing a neutralization reaction HCl(aq) + Na. OH(aq) Base (Na. OH) Acid solution Na. Cl(aq) + H 2 O(l) Click on Link to Simulate a Titration: Acid-Base Titration 70

p. H Indicators An indicator § is added to the acid in the flask § causes the solution to change color when the acid is neutralized HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) 71

Acid–Base Titrations: § Titration – delivery of a measured volume of a solution of known concentration (the titrant/base) into a solution containing the substance of unknown concentration being analyzed (the analyte/acid). § Equivalence point – when enough titrant has been added to react exactly with the analyte. § Endpoint – the indicator changes color so you can tell the equivalence point has been reached. 72
End Point of Titration At the end point, § The equivalence point has been reached § the indicator has a permanent color § the volume of the base used to reach the end point is measured § the molarity of the acid is calculated using the neutralization equation for the reaction (stoichiometry) HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) 73
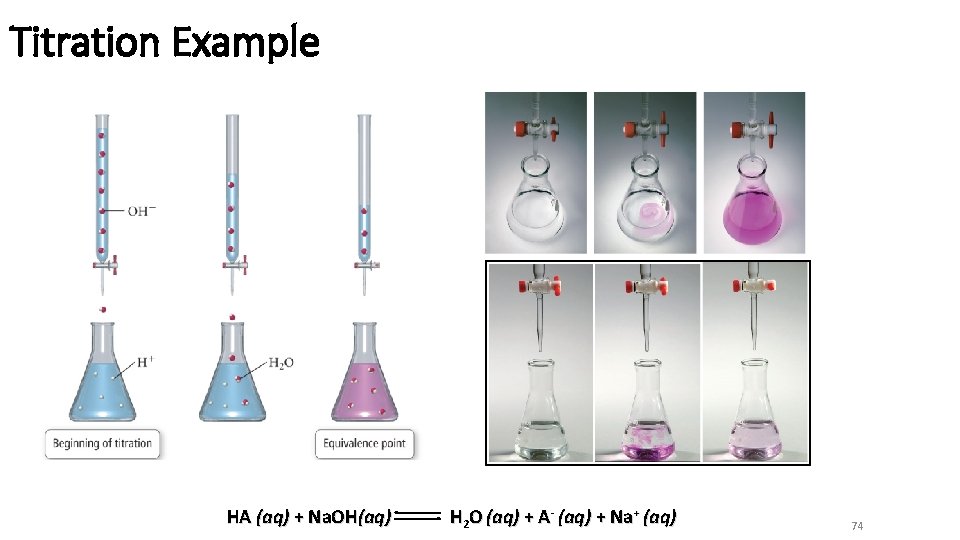
Titration Example HA (aq) + Na. OH(aq) H 2 O (aq) + A- (aq) + Na+ (aq) 74
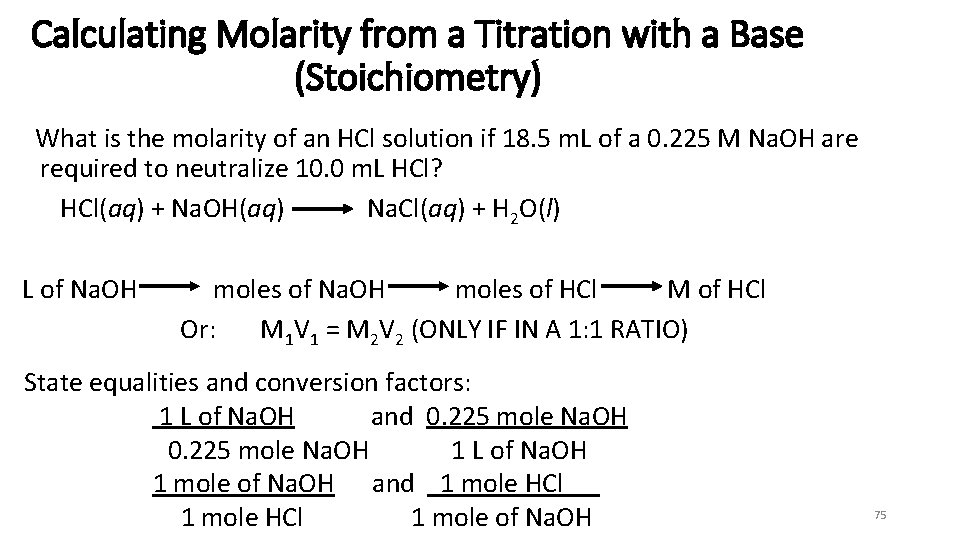
Calculating Molarity from a Titration with a Base (Stoichiometry) What is the molarity of an HCl solution if 18. 5 m. L of a 0. 225 M Na. OH are required to neutralize 10. 0 m. L HCl? HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) L of Na. OH moles of HCl M of HCl Or: M 1 V 1 = M 2 V 2 (ONLY IF IN A 1: 1 RATIO) State equalities and conversion factors: 1 L of Na. OH and 0. 225 mole Na. OH 1 L of Na. OH 1 mole of Na. OH and 1 mole HCl 1 mole of Na. OH 75
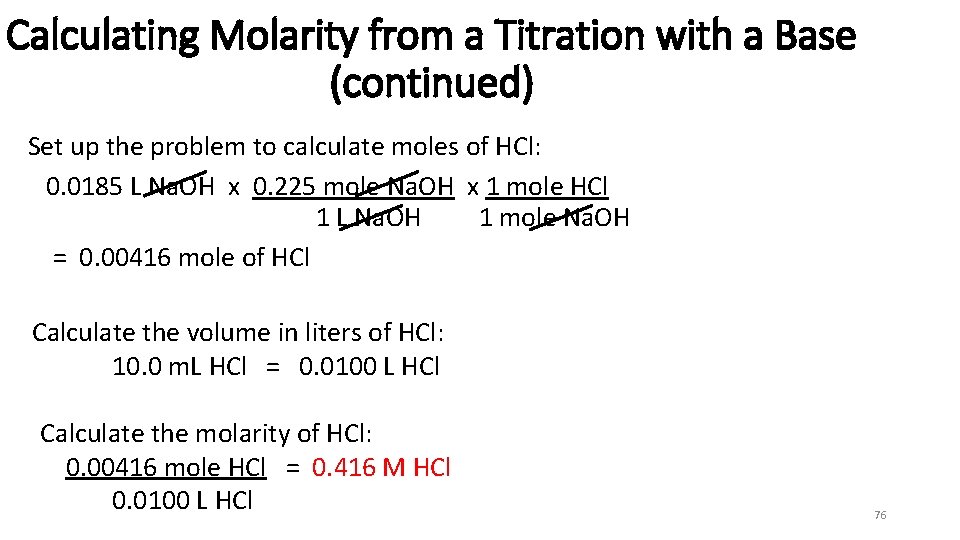
Calculating Molarity from a Titration with a Base (continued) Set up the problem to calculate moles of HCl: 0. 0185 L Na. OH x 0. 225 mole Na. OH x 1 mole HCl 1 L Na. OH 1 mole Na. OH = 0. 00416 mole of HCl Calculate the volume in liters of HCl: 10. 0 m. L HCl = 0. 0100 L HCl Calculate the molarity of HCl: 0. 00416 mole HCl = 0. 416 M HCl 0. 0100 L HCl 76
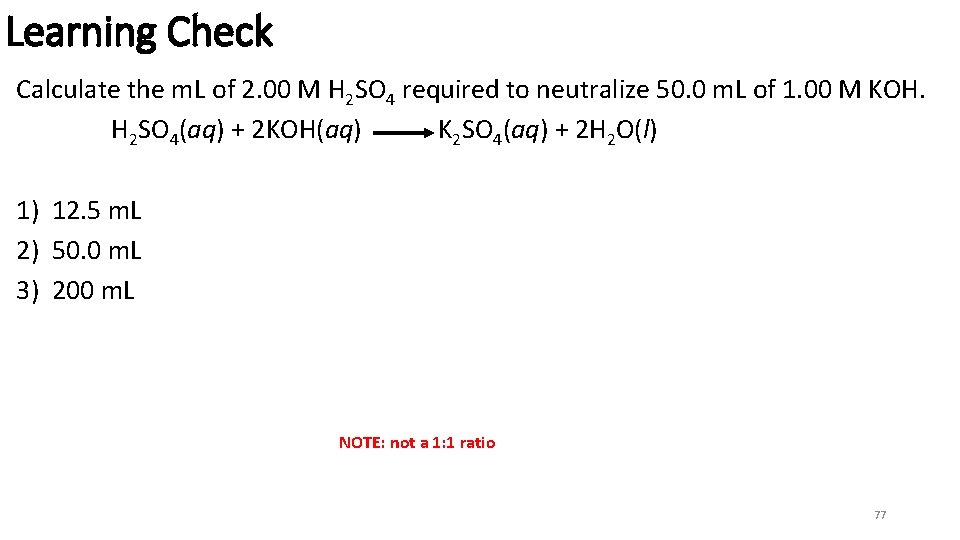
Learning Check Calculate the m. L of 2. 00 M H 2 SO 4 required to neutralize 50. 0 m. L of 1. 00 M KOH. H 2 SO 4(aq) + 2 KOH(aq) K 2 SO 4(aq) + 2 H 2 O(l) 1) 12. 5 m. L 2) 50. 0 m. L 3) 200 m. L NOTE: not a 1: 1 ratio 77
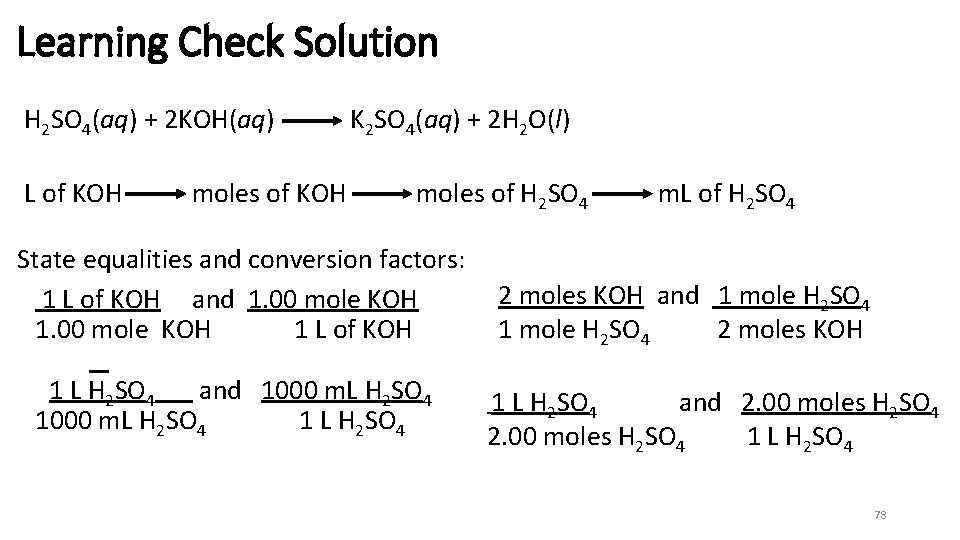
Learning Check Solution H 2 SO 4(aq) + 2 KOH(aq) L of KOH moles of KOH K 2 SO 4(aq) + 2 H 2 O(l) moles of H 2 SO 4 State equalities and conversion factors: 1 L of KOH and 1. 00 mole KOH 1 L of KOH 1 L H 2 SO 4 and 1000 m. L H 2 SO 4 1 L H 2 SO 4 m. L of H 2 SO 4 2 moles KOH and 1 mole H 2 SO 4 2 moles KOH 1 L H 2 SO 4 and 2. 00 moles H 2 SO 4 1 L H 2 SO 4 78
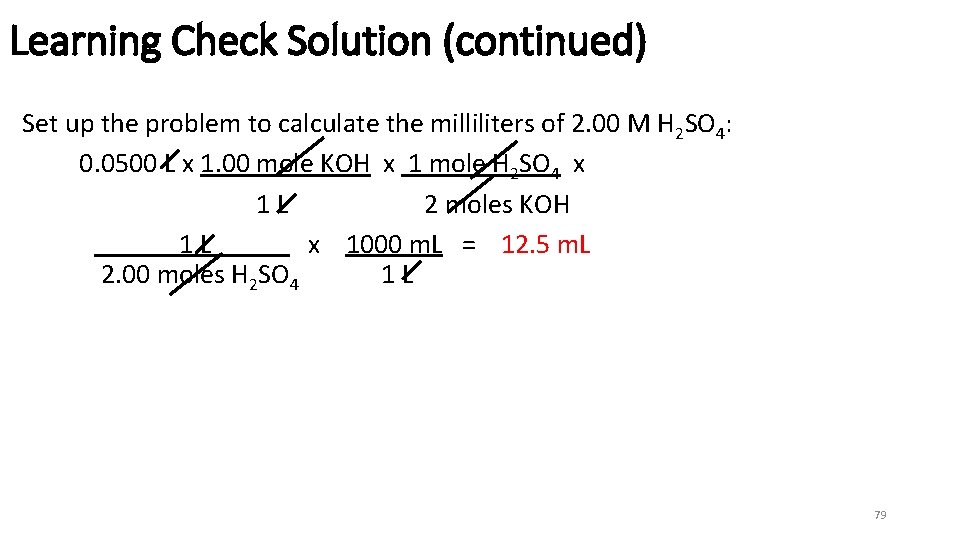
Learning Check Solution (continued) Set up the problem to calculate the milliliters of 2. 00 M H 2 SO 4: 0. 0500 L x 1. 00 mole KOH x 1 mole H 2 SO 4 x 1 L 2 moles KOH 1 L x 1000 m. L = 12. 5 m. L 2. 00 moles H 2 SO 4 1 L 79
Buffer Solutions § Buffers are solutions that resist changes in p. H when an acid or base is added. § They act by neutralizing the added acid or base, similar to a neutralization reaction § Many buffers are made by mixing relatively large amounts of conjugate acids and bases § An acid, HA and its conjugate base A§ A base, B, and its conjugate acid (BH+) 80
Making an Acid Buffer Make buffer by adding relative large and equal concentrations of Conjugate Base and Conjugate Acid 81
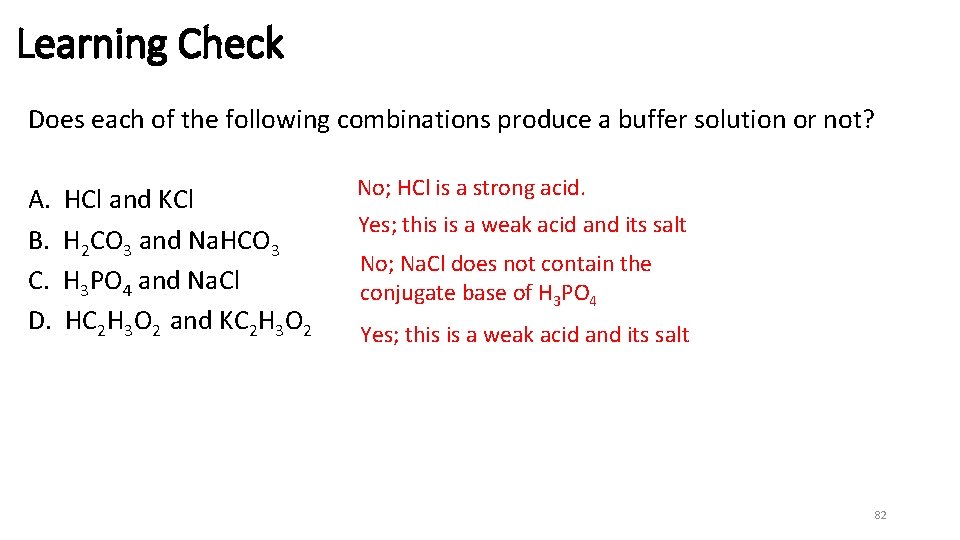
Learning Check Does each of the following combinations produce a buffer solution or not? A. B. C. D. HCl and KCl H 2 CO 3 and Na. HCO 3 H 3 PO 4 and Na. Cl HC 2 H 3 O 2 and KC 2 H 3 O 2 No; HCl is a strong acid. Yes; this is a weak acid and its salt No; Na. Cl does not contain the conjugate base of H 3 PO 4 Yes; this is a weak acid and its salt 82

Importance of Buffers § resist changes in p. H from the addition of acid or base…. using a neutralization reaction § are important in the proper functioning of reactions…including those that occur in cells and blood § Blood is a buffer § in blood must maintain a p. H close to 7. 4; a change in the p. H of the blood affects the uptake of oxygen and cellular processes causing death! § in the body you absorb H 3 O+ or OH from foods and cellular processes …. these would initially change the p. H of you body if you did not have a buffer (blood) 83
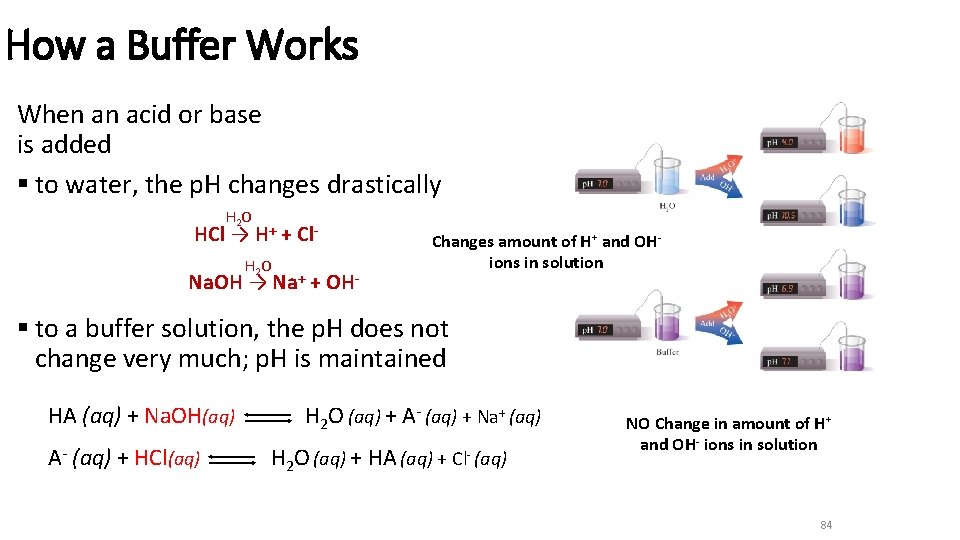
How a Buffer Works When an acid or base is added § to water, the p. H changes drastically H 2 O HCl → H+ + Cl. H 2 O Na. OH → Na+ + OH- Changes amount of H+ and OHions in solution § to a buffer solution, the p. H does not change very much; p. H is maintained HA (aq) + Na. OH(aq) A- (aq) + HCl(aq) H 2 O (aq) + A- (aq) + Na+ (aq) H 2 O (aq) + HA (aq) + Cl- (aq) NO Change in amount of H+ and OH- ions in solution 84
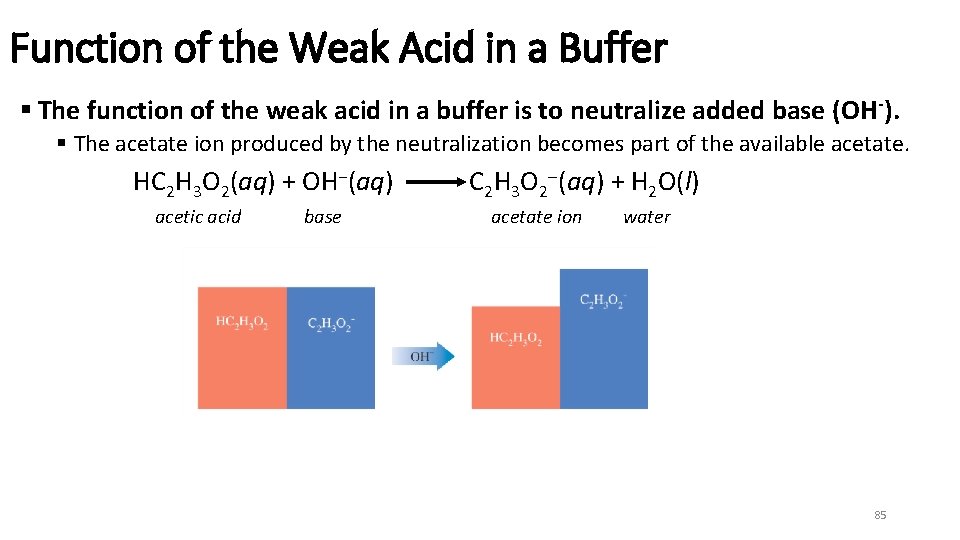
Function of the Weak Acid in a Buffer § The function of the weak acid in a buffer is to neutralize added base (OH -). § The acetate ion produced by the neutralization becomes part of the available acetate. HC 2 H 3 O 2(aq) + OH−(aq) acetic acid base C 2 H 3 O 2 (aq) + H 2 O(l) acetate ion water 85
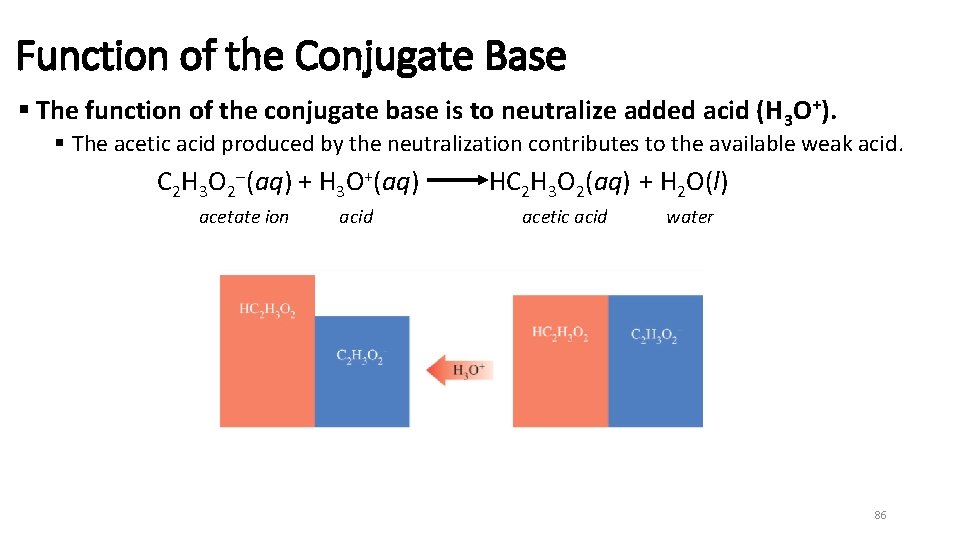
Function of the Conjugate Base § The function of the conjugate base is to neutralize added acid (H 3 O+). § The acetic acid produced by the neutralization contributes to the available weak acid. C 2 H 3 O 2 (aq) + H 3 O+(aq) acetate ion acid HC 2 H 3 O 2(aq) + H 2 O(l) acetic acid water 86
![p. H of a Buffer The [H 3 O+] in the Ka expression is p. H of a Buffer The [H 3 O+] in the Ka expression is](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-87.jpg)
p. H of a Buffer The [H 3 O+] in the Ka expression is used to determine the p. H of a buffer. Weak acid + H 2 O H 3 O+ + conjugate base Ka = [H 3 O+][conjugate base] [weak acid] [H 3 O+] = Ka x [weak acid] [conjugate base] p. H = log [H 3 O+] 87
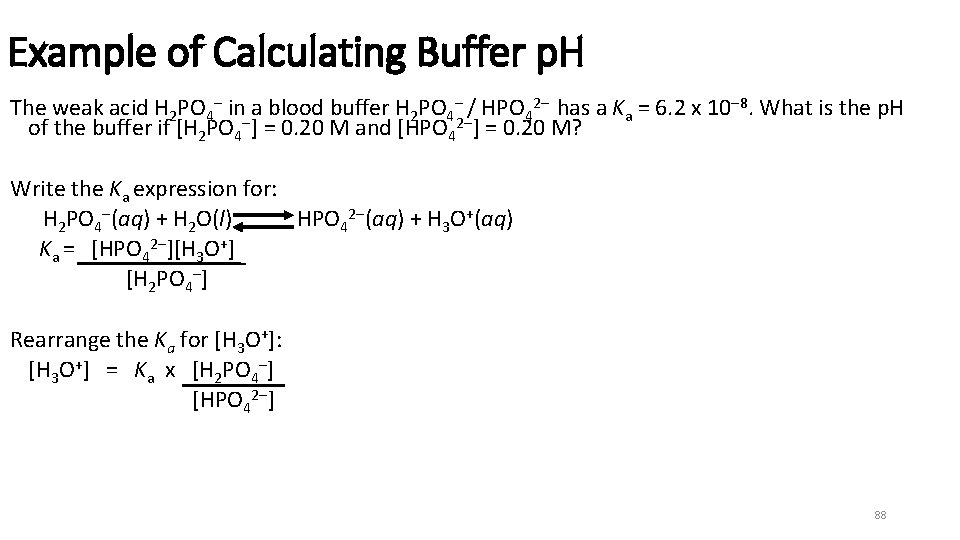
Example of Calculating Buffer p. H The weak acid H 2 PO 4 in a blood buffer H 2 PO 4 / HPO 42 has a Ka = 6. 2 x 10 8. What is the p. H of the buffer if [H 2 PO 4 ] = 0. 20 M and [HPO 42 ] = 0. 20 M? Write the Ka expression for: H 2 PO 4 (aq) + H 2 O(l) HPO 42 (aq) + H 3 O+(aq) Ka = [HPO 42 ][H 3 O+] [H 2 PO 4 ] Rearrange the Ka for [H 3 O+]: [H 3 O+] = Ka x [H 2 PO 4 ] [HPO 42 ] 88
![Example of Calculating Buffer p. H (continued) Substitute [HA] and [A ]: [H 3 Example of Calculating Buffer p. H (continued) Substitute [HA] and [A ]: [H 3](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-89.jpg)
Example of Calculating Buffer p. H (continued) Substitute [HA] and [A ]: [H 3 O+] = 6. 2 x 10 8 x [0. 20 M] = 6. 2 x 10 8 [0. 20 M] Use [H 3 O+] to calculate p. H: p. H = log [6. 2 x 10 8] = 7. 21 89
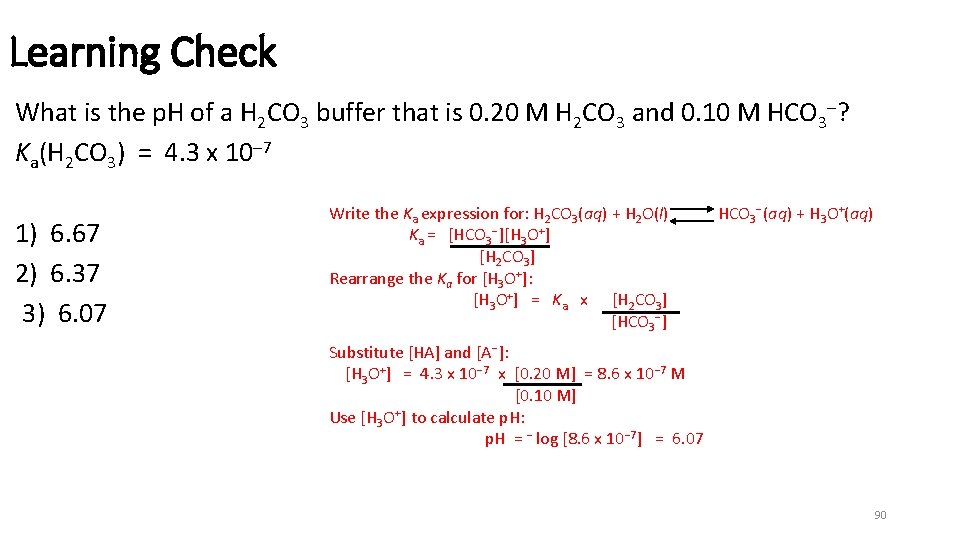
Learning Check What is the p. H of a H 2 CO 3 buffer that is 0. 20 M H 2 CO 3 and 0. 10 M HCO 3 ? Ka(H 2 CO 3) = 4. 3 x 10 7 1) 6. 67 2) 6. 37 3) 6. 07 Write the Ka expression for: H 2 CO 3(aq) + H 2 O(l) Ka = [HCO 3 ][H 3 O+] [H 2 CO 3] Rearrange the Ka for [H 3 O+]: [H 3 O+] = Ka x [H 2 CO 3] [HCO 3 ] HCO 3 (aq) + H 3 O+(aq) Substitute [HA] and [A ]: [H 3 O+] = 4. 3 x 10 7 x [0. 20 M] = 8. 6 x 10 7 M [0. 10 M] Use [H 3 O+] to calculate p. H: p. H = log [8. 6 x 10 7] = 6. 07 90
![Summary of Equations § Kw = [ H 3 O+][ OH−] = 1. 0 Summary of Equations § Kw = [ H 3 O+][ OH−] = 1. 0](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-91.jpg)
Summary of Equations § Kw = [ H 3 O+][ OH−] = 1. 0 x 10− 14 § p. H= -log [H 3 O+] or [H 3 O+] = 10 -p. H § p. OH= -log [OH-] or [OH-] = 10 -p. OH § p. H + p. OH= 14 § M 1 V 1 = M 2 V 2 (for 1: 1 ratios only) or Stoichiometry § Ka = [H 3 O+][conjugate base] [H 3 O+] = Ka x [weak acid] or [conjugate base] [weak acid] 91
![Summary of Equations § Kw = [H 3 O+][OH−] § = 1. 0 x Summary of Equations § Kw = [H 3 O+][OH−] § = 1. 0 x](http://slidetodoc.com/presentation_image_h2/414616e2c2477da13515847e74686961/image-92.jpg)
Summary of Equations § Kw = [H 3 O+][OH−] § = 1. 0 x 10− 14 § p. H + p. OH = 14 § Ex. Given: p. H § Needed: p. OH § Ex. Given: [H 3 O+] § Needed: [OH−] Or vice versa § Given p. H find [OH−] Or vice versa § p. H→ [H 3 O+] →[OH−] § p. H→ p. OH →[OH−] § p. H = -log [H 3 O+] § Ex. Given: [H 3 O+] § Needed: p. H Or vice versa § Given p. OH find [H 3 O+] Or vice versa § p. OH = -log § p. OH→ [OH−] → [H 3 O+] § p. OH→ p. H → [H 3 O+] [OH−] § Ex. Given: [OH−] § Needed: p. OH Or vice versa § Given Ka find p. H Or vice versa § Ka→ [H 3 O+] → p. H Or vice versa 92
- Slides: 92