Chapter 9 Absorption by Atmospheric Gases KEY POINTS
Chapter 9: Absorption by Atmospheric Gases KEY POINTS • Visible and UV Absorption: due to electronic transitions. Monatomic polyatomic. • IR Absorption: due to vibration and rotation transitions. Polyatomic. • Microwave Absorption: due to rotation transitions. Polyatomic. • Absorption cross sections depend on temperature and pressure. • Population of energy levels depends on temperature (thermal energy, k. T). Transitions between levels therefore depend on temperature. • Temperature (Doppler) broadening of absorption lines in the mesosphere. • Pressure broadening of absorption lines (due to molecular collisions) in the troposphere. Pat Arnott, ATMS 749, UNR, 2008
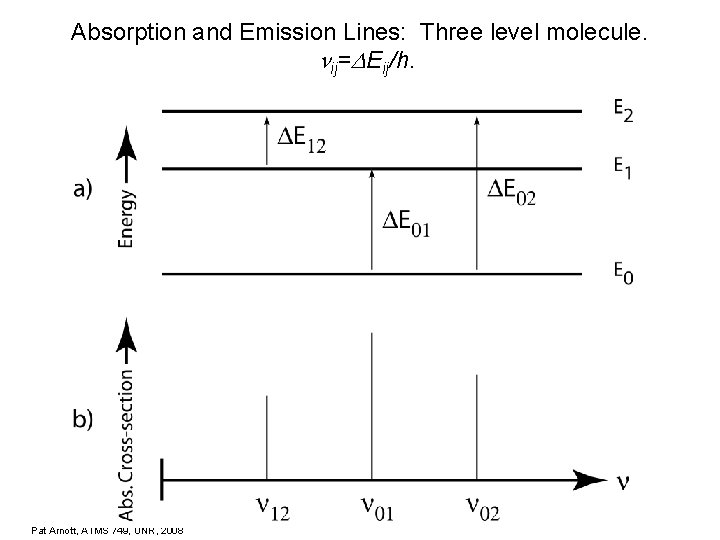
Absorption and Emission Lines: Three level molecule. ij= Eij/h. Pat Arnott, ATMS 749, UNR, 2008
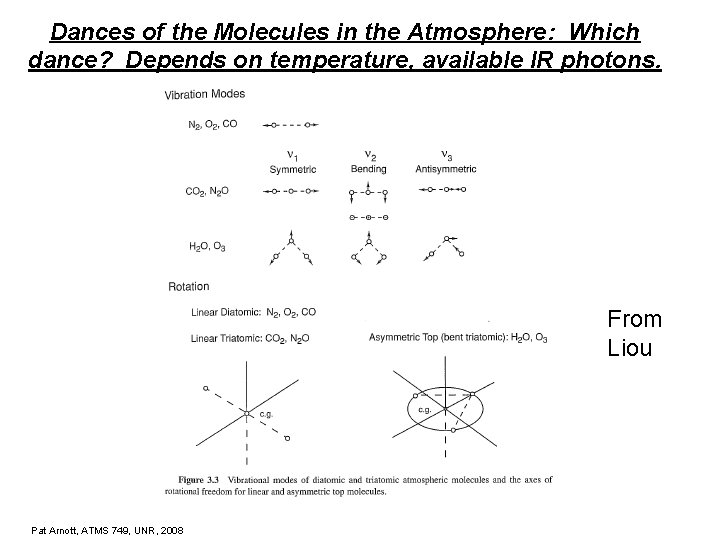
Dances of the Molecules in the Atmosphere: Which dance? Depends on temperature, available IR photons. From Liou Pat Arnott, ATMS 749, UNR, 2008
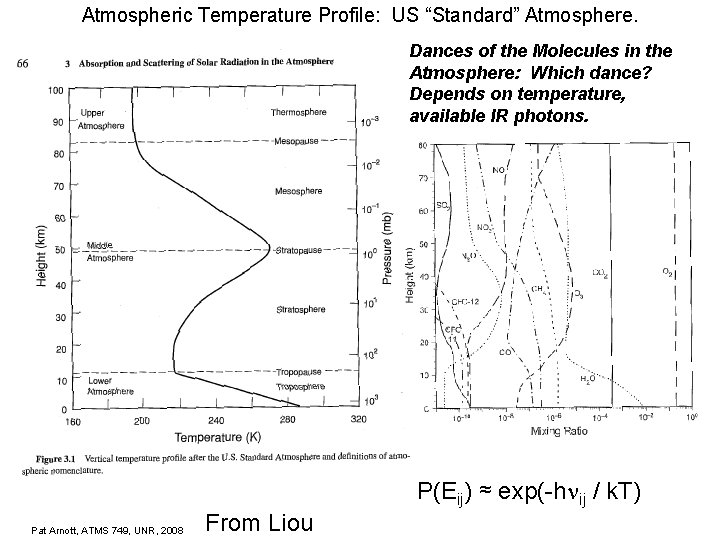
Atmospheric Temperature Profile: US “Standard” Atmosphere. Dances of the Molecules in the Atmosphere: Which dance? Depends on temperature, available IR photons. P(Eij) ≈ exp(-h ij / k. T) Pat Arnott, ATMS 749, UNR, 2008 From Liou
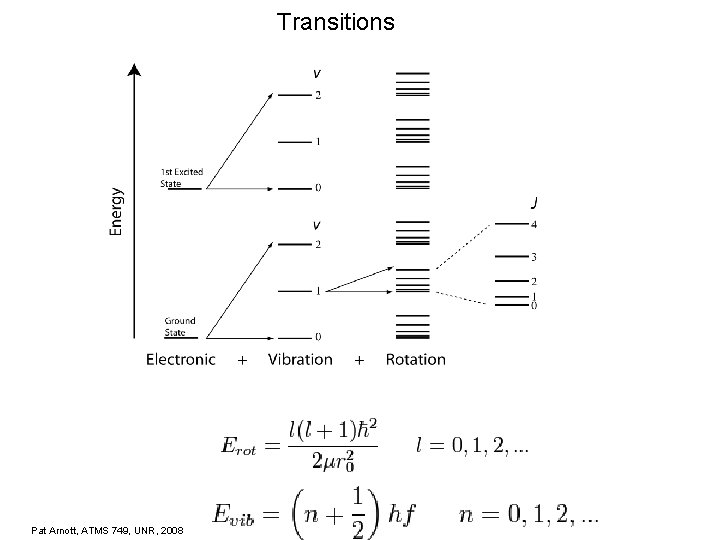
Transitions Pat Arnott, ATMS 749, UNR, 2008
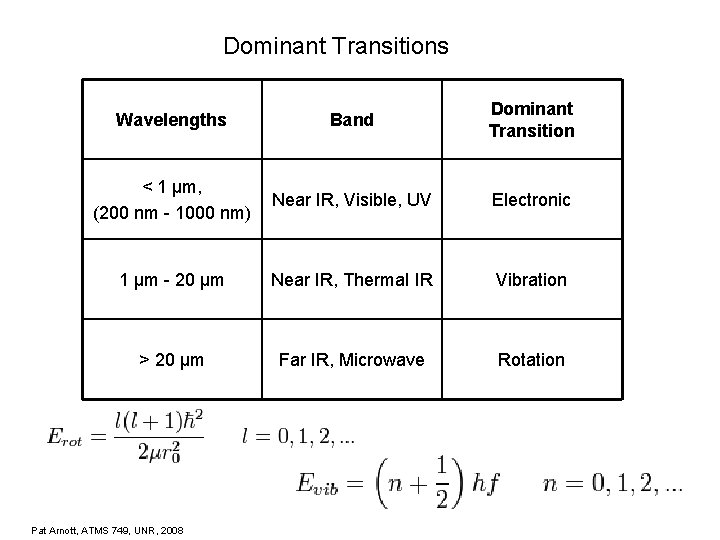
Dominant Transitions Wavelengths Band Dominant Transition < 1 µm, (200 nm - 1000 nm) Near IR, Visible, UV Electronic 1 µm - 20 µm Near IR, Thermal IR Vibration > 20 µm Far IR, Microwave Rotation Pat Arnott, ATMS 749, UNR, 2008
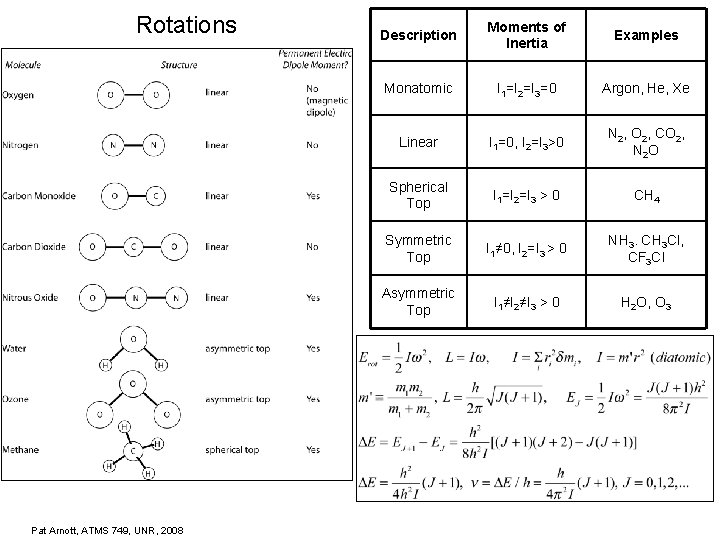
Rotations Pat Arnott, ATMS 749, UNR, 2008 Description Moments of Inertia Examples Monatomic I 1=I 2=I 3=0 Argon, He, Xe Linear I 1=0, I 2=I 3>0 N 2, O 2, CO 2, N 2 O Spherical Top I 1=I 2=I 3 > 0 CH 4 Symmetric Top I 1≠ 0, I 2=I 3 > 0 NH 3. CH 3 Cl, CF 3 Cl Asymmetric Top I 1≠I 2≠I 3 > 0 H 2 O, O 3
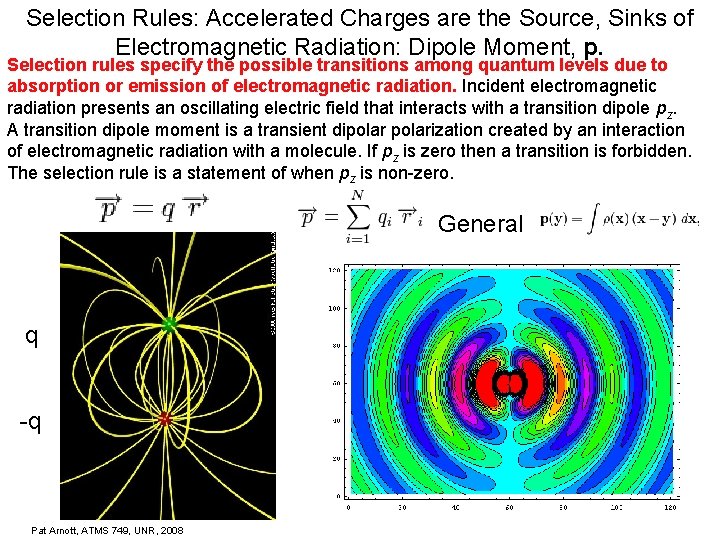
Selection Rules: Accelerated Charges are the Source, Sinks of Electromagnetic Radiation: Dipole Moment, p. Selection rules specify the possible transitions among quantum levels due to absorption or emission of electromagnetic radiation. Incident electromagnetic radiation presents an oscillating electric field that interacts with a transition dipole pz. A transition dipole moment is a transient dipolarization created by an interaction of electromagnetic radiation with a molecule. If pz is zero then a transition is forbidden. The selection rule is a statement of when pz is non-zero. General q -q Pat Arnott, ATMS 749, UNR, 2008
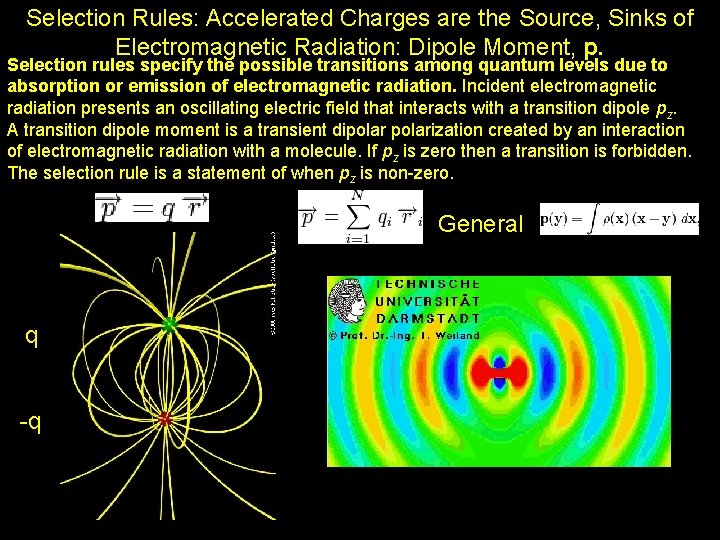
Selection Rules: Accelerated Charges are the Source, Sinks of Electromagnetic Radiation: Dipole Moment, p. Selection rules specify the possible transitions among quantum levels due to absorption or emission of electromagnetic radiation. Incident electromagnetic radiation presents an oscillating electric field that interacts with a transition dipole pz. A transition dipole moment is a transient dipolarization created by an interaction of electromagnetic radiation with a molecule. If pz is zero then a transition is forbidden. The selection rule is a statement of when pz is non-zero. General q -q Pat Arnott, ATMS 749, UNR, 2008
Pat Arnott, ATMS 749, UNR, 2008
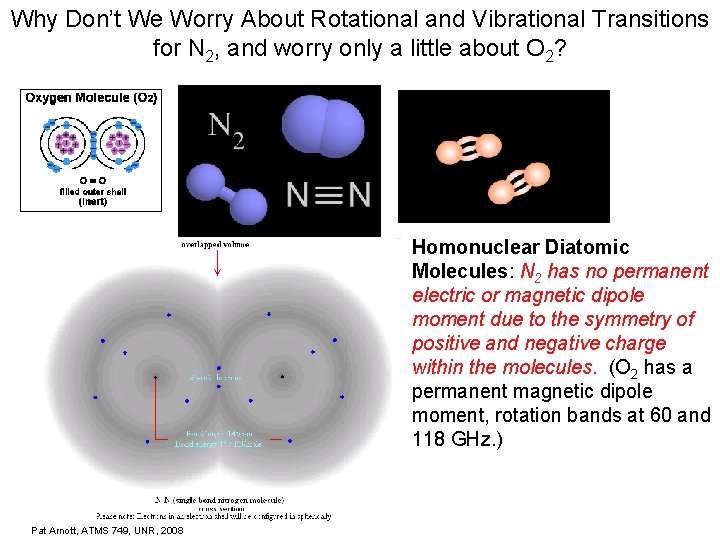
Why Don’t We Worry About Rotational and Vibrational Transitions for N 2, and worry only a little about O 2? Homonuclear Diatomic Molecules: N 2 has no permanent electric or magnetic dipole moment due to the symmetry of positive and negative charge within the molecules. (O 2 has a permanent magnetic dipole moment, rotation bands at 60 and 118 GHz. ) Pat Arnott, ATMS 749, UNR, 2008
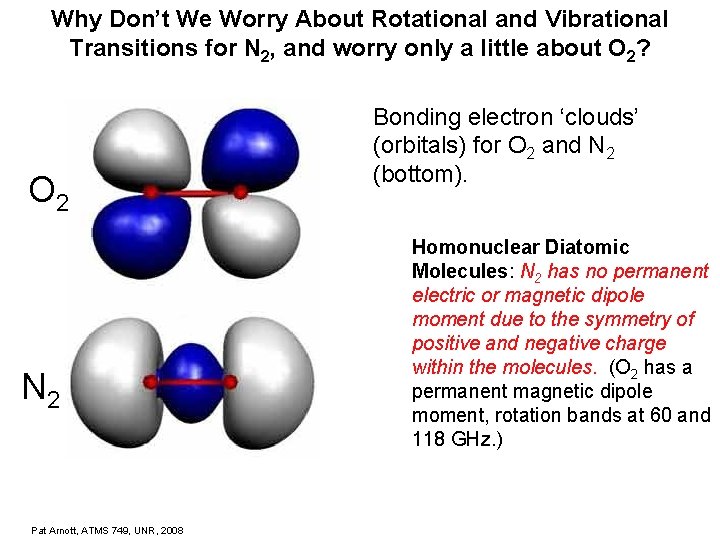
Why Don’t We Worry About Rotational and Vibrational Transitions for N 2, and worry only a little about O 2? O 2 N 2 Pat Arnott, ATMS 749, UNR, 2008 Bonding electron ‘clouds’ (orbitals) for O 2 and N 2 (bottom). Homonuclear Diatomic Molecules: N 2 has no permanent electric or magnetic dipole moment due to the symmetry of positive and negative charge within the molecules. (O 2 has a permanent magnetic dipole moment, rotation bands at 60 and 118 GHz. )
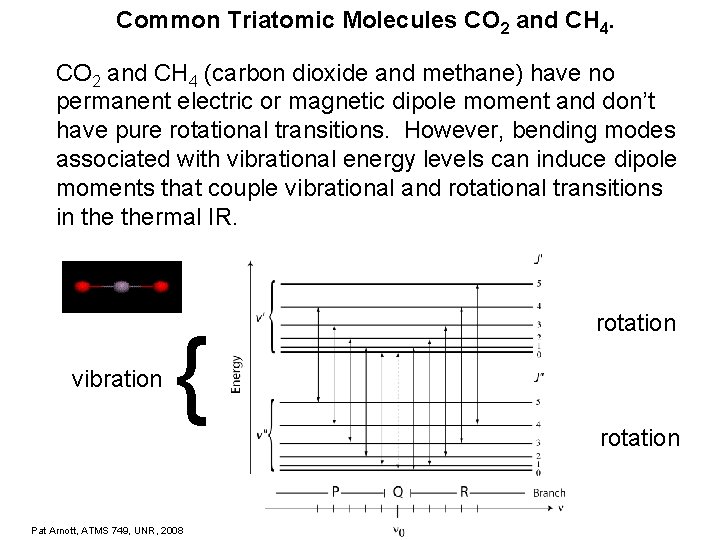
Common Triatomic Molecules CO 2 and CH 4 (carbon dioxide and methane) have no permanent electric or magnetic dipole moment and don’t have pure rotational transitions. However, bending modes associated with vibrational energy levels can induce dipole moments that couple vibrational and rotational transitions in thermal IR. vibration { Pat Arnott, ATMS 749, UNR, 2008 rotation
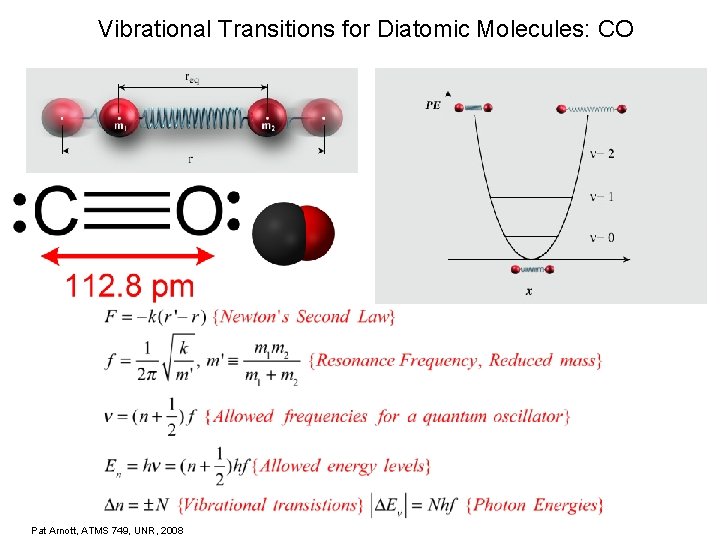
Vibrational Transitions for Diatomic Molecules: CO Pat Arnott, ATMS 749, UNR, 2008
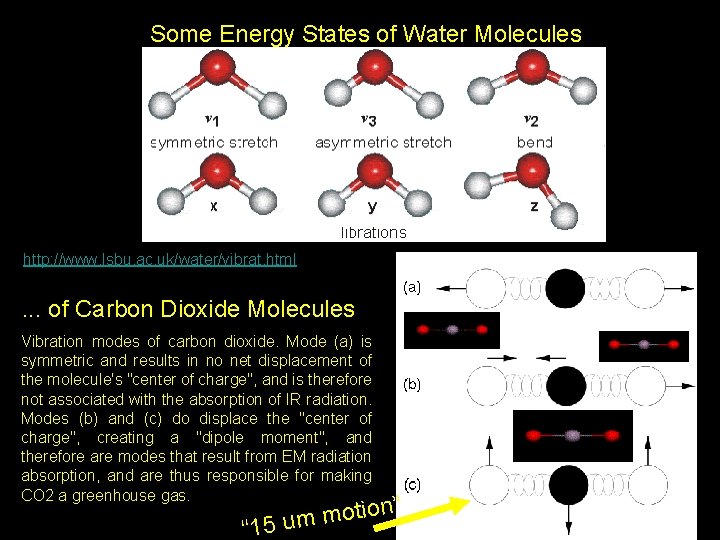
Some Energy States of Water Molecules http: //www. lsbu. ac. uk/water/vibrat. html . . . of Carbon Dioxide Molecules Vibration modes of carbon dioxide. Mode (a) is symmetric and results in no net displacement of the molecule's "center of charge", and is therefore not associated with the absorption of IR radiation. Modes (b) and (c) do displace the "center of charge", creating a "dipole moment", and therefore are modes that result from EM radiation absorption, and are thus responsible for making CO 2 a greenhouse gas. Pat Arnott, ATMS 749, UNR, 2008 “ n” o i t o m 15 um
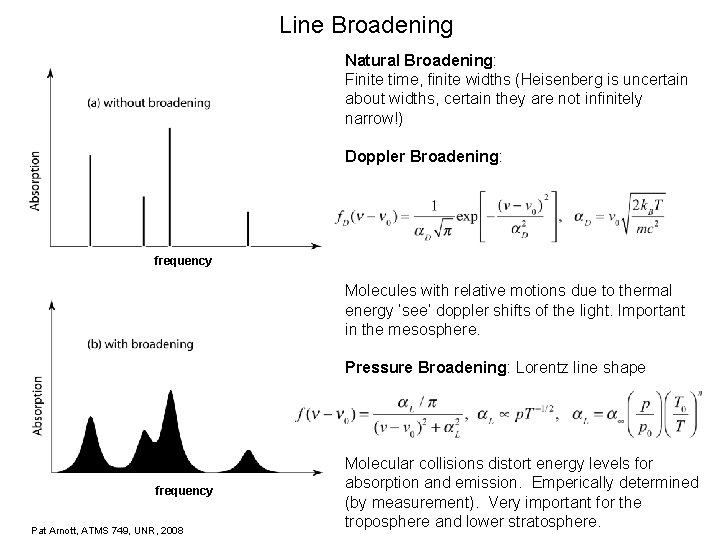
Line Broadening Natural Broadening: Finite time, finite widths (Heisenberg is uncertain about widths, certain they are not infinitely narrow!) Doppler Broadening: frequency Molecules with relative motions due to thermal energy ‘see’ doppler shifts of the light. Important in the mesosphere. Pressure Broadening: Lorentz line shape frequency Pat Arnott, ATMS 749, UNR, 2008 Molecular collisions distort energy levels for absorption and emission. Emperically determined (by measurement). Very important for the troposphere and lower stratosphere.
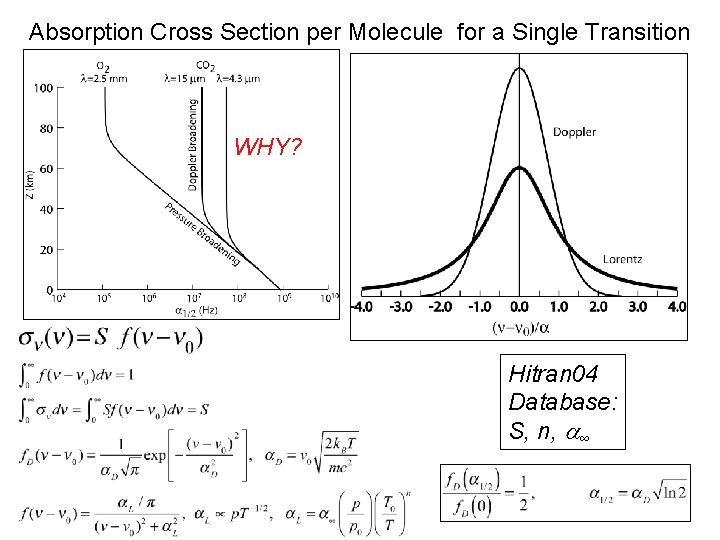
Absorption Cross Section per Molecule for a Single Transition WHY? Hitran 04 Database: S, n, ∞ Pat Arnott, ATMS 749, UNR, 2008
Line Strength Temperature Dependence Summary *** Energy levels are determined from quantum mechanics, electronic, vibration, rotation etc, as related to molecular mass, charge distribution, orientation, number of atoms, etc. *** # of molecules in each state is determined from statistical mechanics, partition function, thermal energy. Is there sufficient thermal energy to populate the energy levels above the ground state? What is the probability molecules are in a given energy state? Pat Arnott, ATMS 749, UNR, 2008

Is it likely that a molecule can be in energy state El? Water Vapor must be in state El before it can absorb photon with energy h 0 c. Molecules are in lower energy states at lower temperature. Pat Arnott, ATMS 749, UNR, 2008
Additional Interactions Continuum Absorption (e. g. water vapor in window region) Broad, weak absorption. From poor model of spectral lines? From water vapor clusters of 2 or more molecules? Both? VERY IMPORTANT!!! ‘Dirties’ the window region in thermal IR. Photoionization (Gamma, X-ray) Continuum absorption, electrons ejected with kinetic energy. Photodissociation (UV, Vis? ) e. g. NO 2 and < 400 nm. Molecules are broken and leave with kinetic energy. NO 2 + h --> NO + O VERY IMPORTANT FOR ATMOSPHERIC CHEMISTRY!!! Pat Arnott, ATMS 749, UNR, 2008
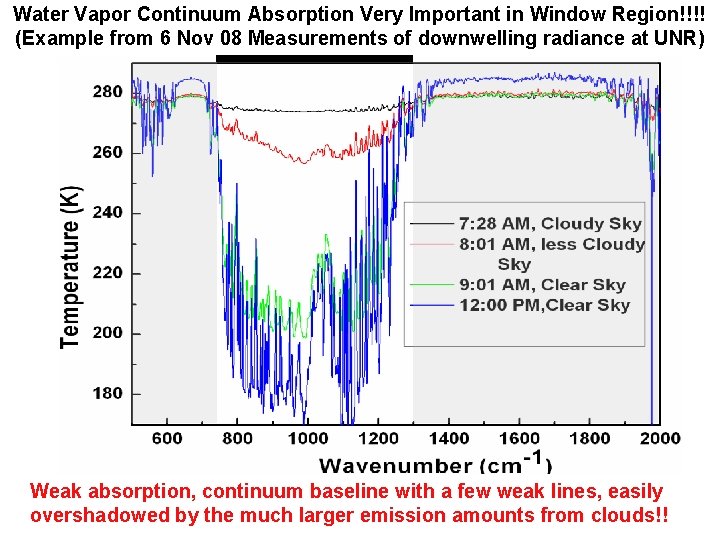
Water Vapor Continuum Absorption Very Important in Window Region!!!! (Example from 6 Nov 08 Measurements of downwelling radiance at UNR) Weak absorption, continuum baseline with a few weak lines, easily overshadowed by the much larger emission amounts from clouds!! Pat Arnott, ATMS 749, UNR, 2008
Number of Lower Energy States for Water Molecules in Wavenumber bins for the Wavenumber Range 500 -750 cm-1. Pat Arnott, ATMS 749, UNR, 2008

Line Strength Temperature Dependence Water Vapor: Weak Line Pat Arnott, ATMS 749, UNR, 2008

Line Strength Temperature Dependence Water Vapor: Strong Line Pat Arnott, ATMS 749, UNR, 2008

Line Strength Temperature Dependence Water Vapor Pat Arnott, ATMS 749, UNR, 2008

Line Strength and Lower Energy States and Temperature Pat Arnott, ATMS 749, UNR, 2008
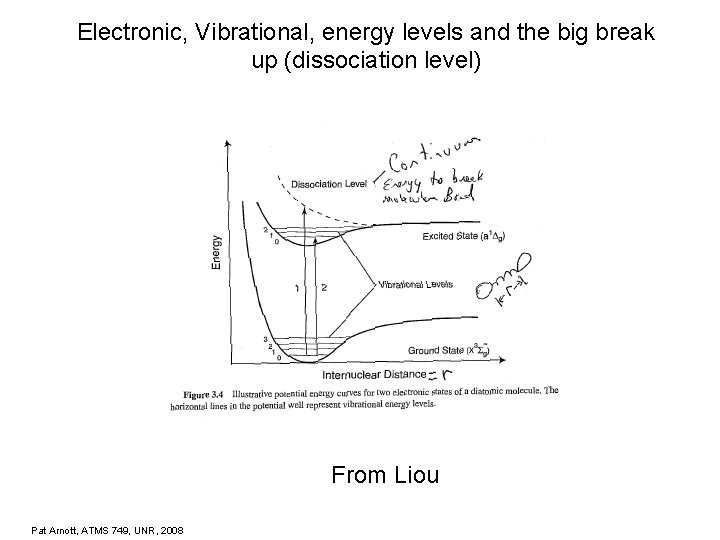
Electronic, Vibrational, energy levels and the big break up (dissociation level) From Liou Pat Arnott, ATMS 749, UNR, 2008
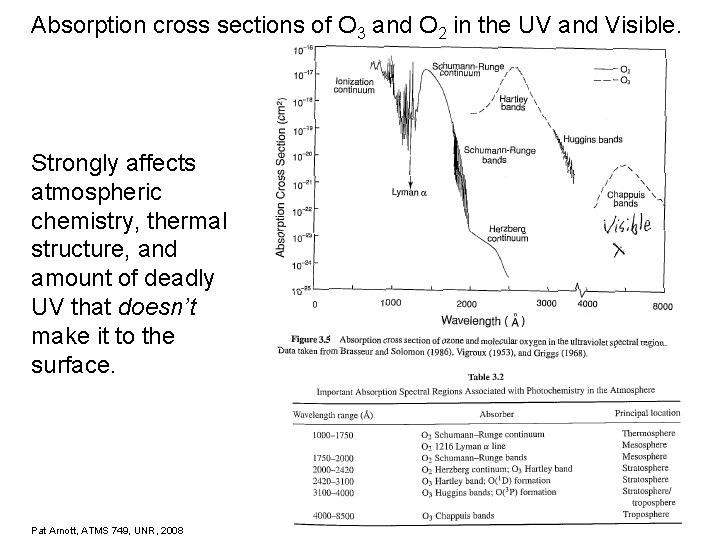
Absorption cross sections of O 3 and O 2 in the UV and Visible. Strongly affects atmospheric chemistry, thermal structure, and amount of deadly UV that doesn’t make it to the surface. Pat Arnott, ATMS 749, UNR, 2008
![Depth for abs=[Babs (Ztoa-H)]=1 as a function of wavelength, and the gases responsible for Depth for abs=[Babs (Ztoa-H)]=1 as a function of wavelength, and the gases responsible for](http://slidetodoc.com/presentation_image_h/36d3a7e888bd91a330b474ad3d819ee5/image-29.jpg)
Depth for abs=[Babs (Ztoa-H)]=1 as a function of wavelength, and the gases responsible for absorption. H (km) Pat Arnott, ATMS 749, UNR, 2008
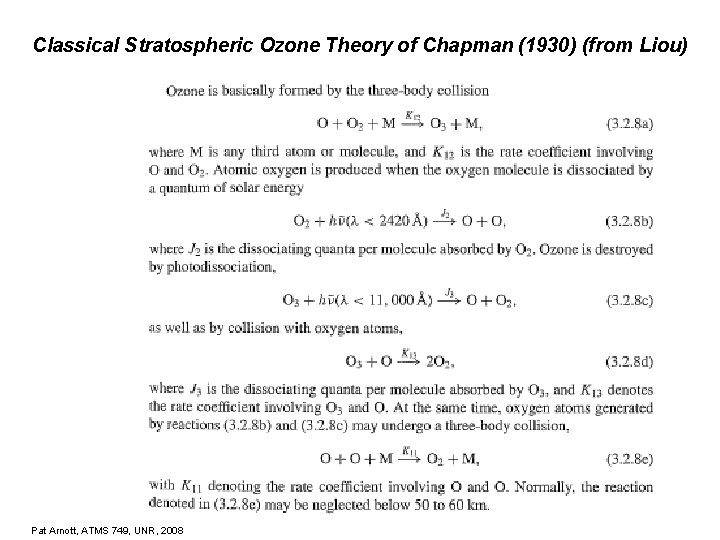
Classical Stratospheric Ozone Theory of Chapman (1930) (from Liou) Pat Arnott, ATMS 749, UNR, 2008
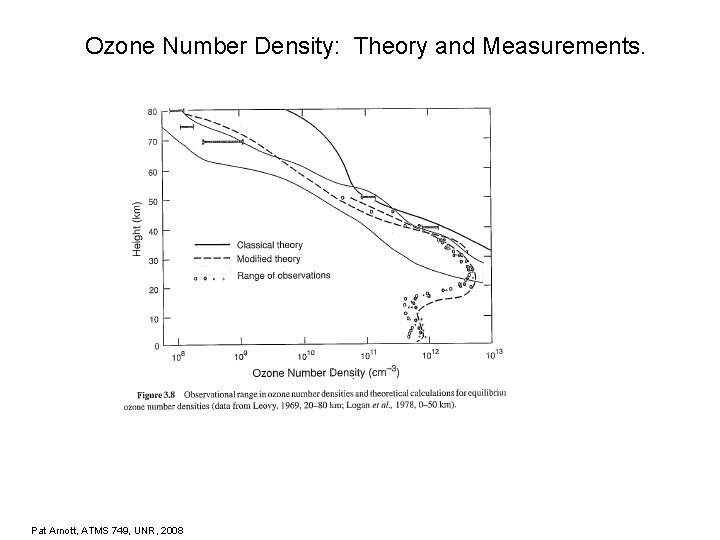
Ozone Number Density: Theory and Measurements. Pat Arnott, ATMS 749, UNR, 2008
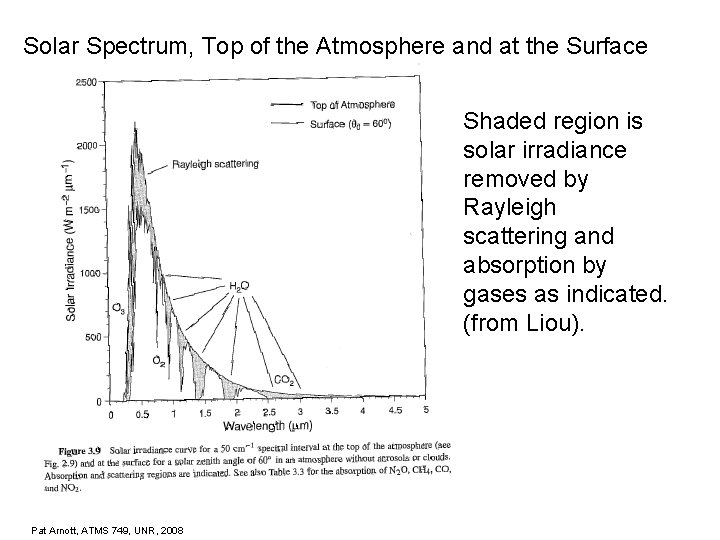
Solar Spectrum, Top of the Atmosphere and at the Surface Shaded region is solar irradiance removed by Rayleigh scattering and absorption by gases as indicated. (from Liou). Pat Arnott, ATMS 749, UNR, 2008
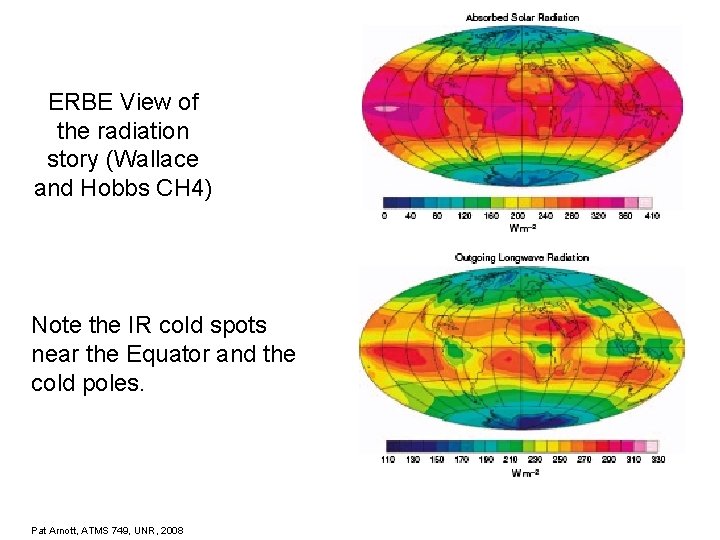
ERBE View of the radiation story (Wallace and Hobbs CH 4) Note the IR cold spots near the Equator and the cold poles. Pat Arnott, ATMS 749, UNR, 2008
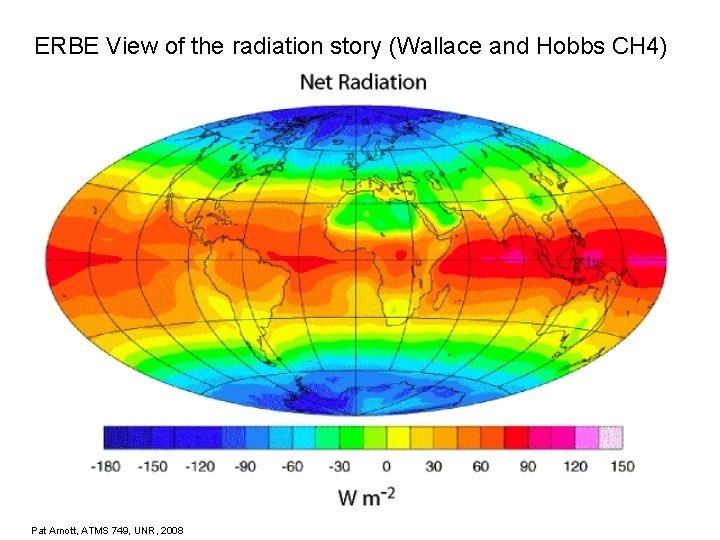
ERBE View of the radiation story (Wallace and Hobbs CH 4) Pat Arnott, ATMS 749, UNR, 2008
- Slides: 34