Chapter 9 4 Colligative Properties and Brief Ch

Chapter 9. 4 Colligative Properties and Brief Ch 8 -9 Review CHM 1111 Section 04 Instructor: Dr. Jules Carlson Class Time: M/W/F 1: 30 -2: 20 Wednesday, November 23 rd

Announcements • Exam in Manitoba 4 M 47 at 9 AM, Monday, December 9 th • Exam is 3 hours • Exam will have 30 Multiple Choice + 5 Long Answers for a total of 55 Marks • Final exam is worth 45 % of your grade • As a result of different classes being in different places, Ch. 10 will not be included in the exam (unless there is high demand to) and we will do review all next week Also, we will do course evaluations today!
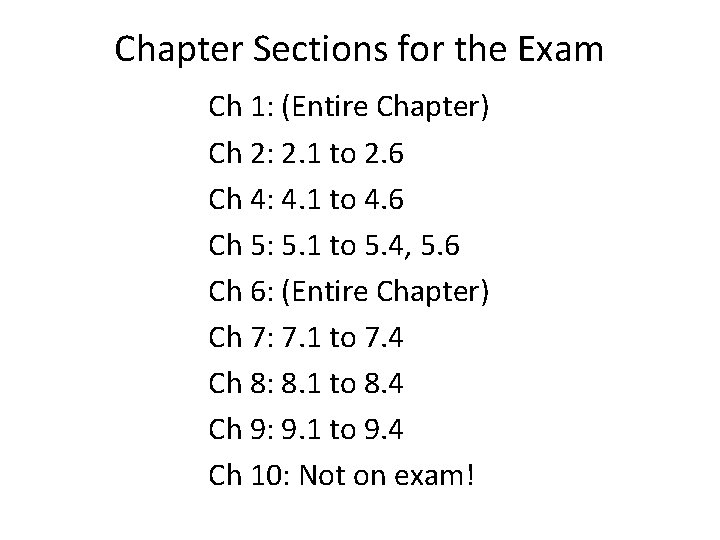
Chapter Sections for the Exam Ch 1: (Entire Chapter) Ch 2: 2. 1 to 2. 6 Ch 4: 4. 1 to 4. 6 Ch 5: 5. 1 to 5. 4, 5. 6 Ch 6: (Entire Chapter) Ch 7: 7. 1 to 7. 4 Ch 8: 8. 1 to 8. 4 Ch 9: 9. 1 to 9. 4 Ch 10: Not on exam!
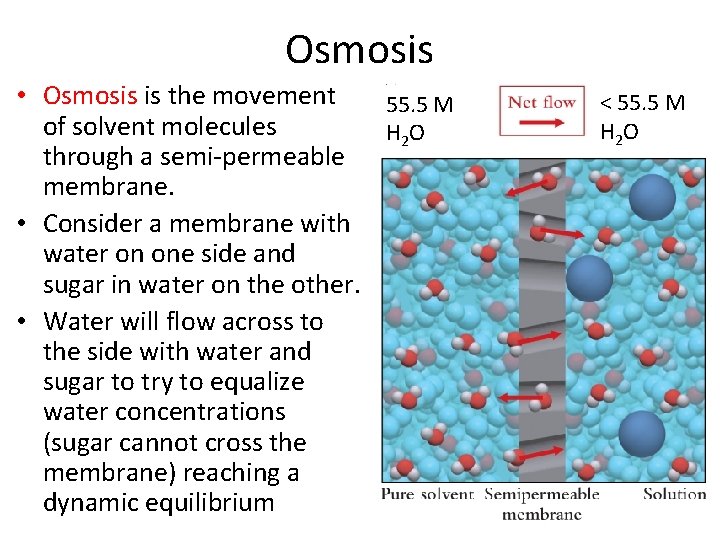
Osmosis • Osmosis is the movement 55. 5 M of solvent molecules H 2 O through a semi-permeable membrane. • Consider a membrane with water on one side and sugar in water on the other. • Water will flow across to the side with water and sugar to try to equalize water concentrations (sugar cannot cross the membrane) reaching a dynamic equilibrium < 55. 5 M H 2 O
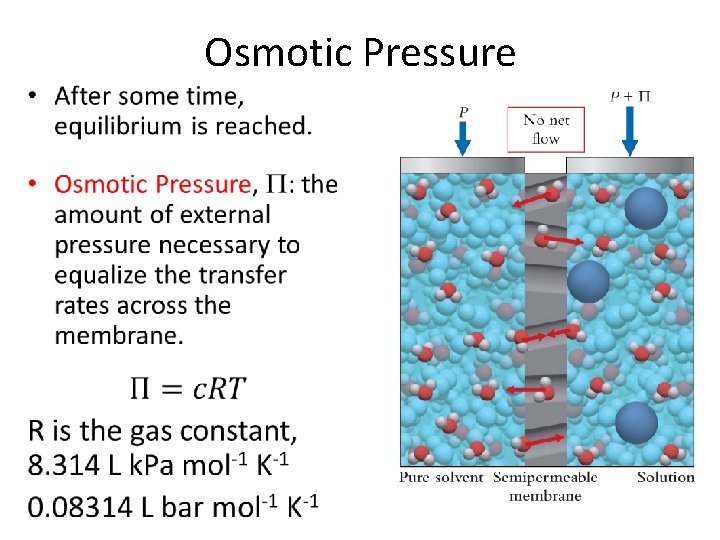
Osmotic Pressure •
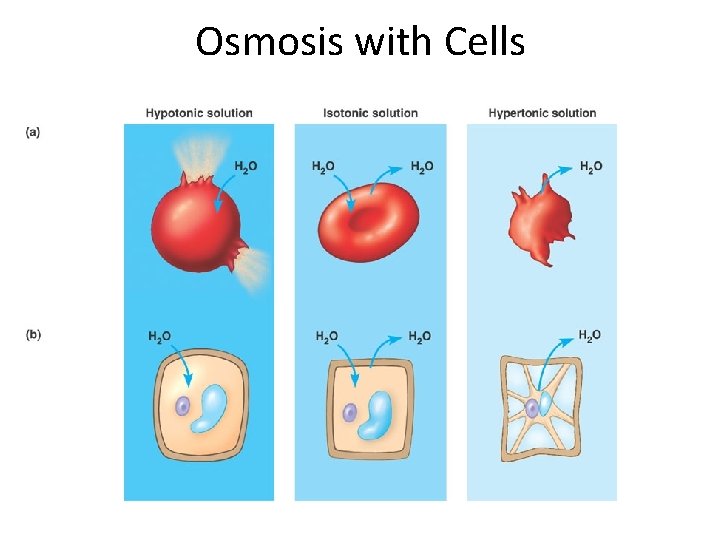
Osmosis with Cells
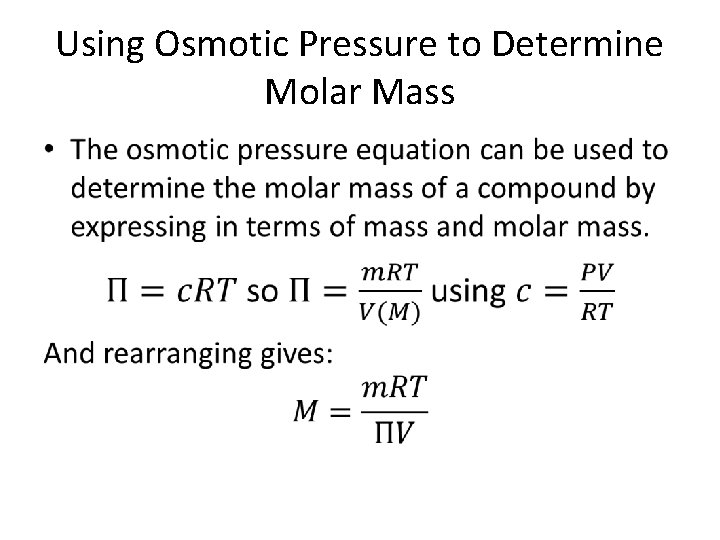
Using Osmotic Pressure to Determine Molar Mass •

Osmotic Pressure Problem What concentration of sodium chloride in water is needed to produce a solution that is isotonic (has the same osmotic pressure) with blood (Π = 7. 80 bar at 25⁰C)
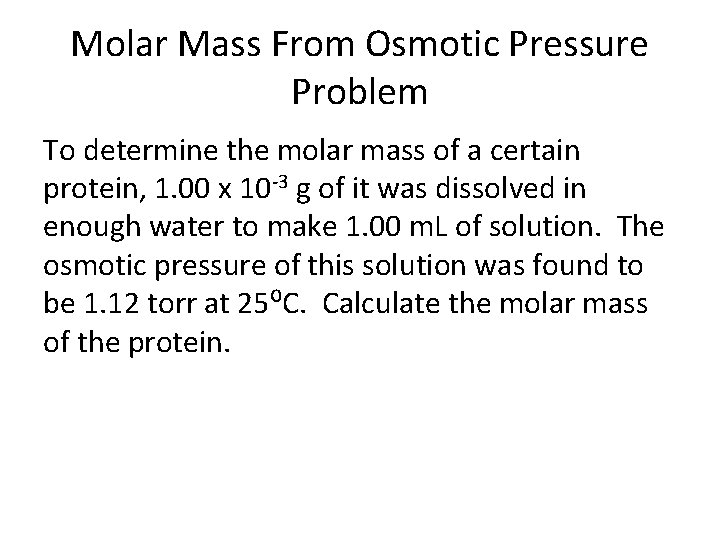
Molar Mass From Osmotic Pressure Problem To determine the molar mass of a certain protein, 1. 00 x 10 -3 g of it was dissolved in enough water to make 1. 00 m. L of solution. The osmotic pressure of this solution was found to be 1. 12 torr at 25⁰C. Calculate the molar mass of the protein.

I clicker Question Which of the following statements are true: a) Straight-chained hydrocarbons with larger molecular weights always have lower vapour pressures at the same temperature. b) Na 2 SO 4 will produce a larger freezing point depression of water than Ca(NO 3)2 at the same concentration. c) For two aqueous solutions of the same molality, the solution with a solute of higher density will produce a higher osmotic pressure. d) Both (a) and (c) e) All statements are true
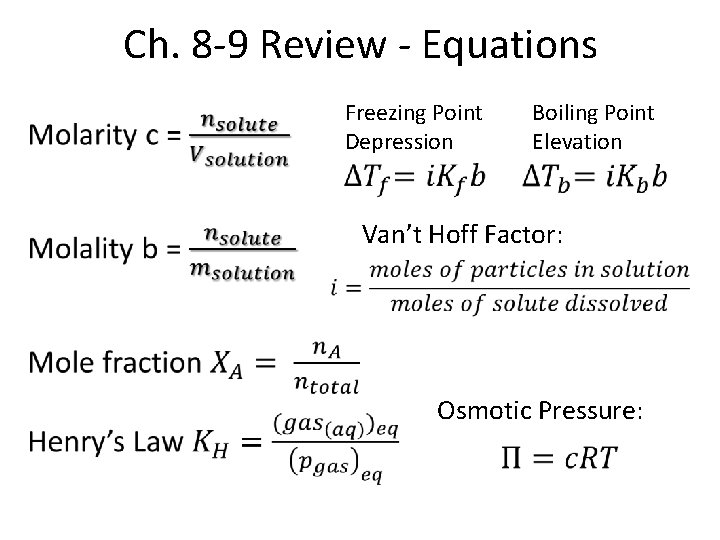
Ch. 8 -9 Review - Equations • Freezing Point Depression Boiling Point Elevation Van’t Hoff Factor: Osmotic Pressure:
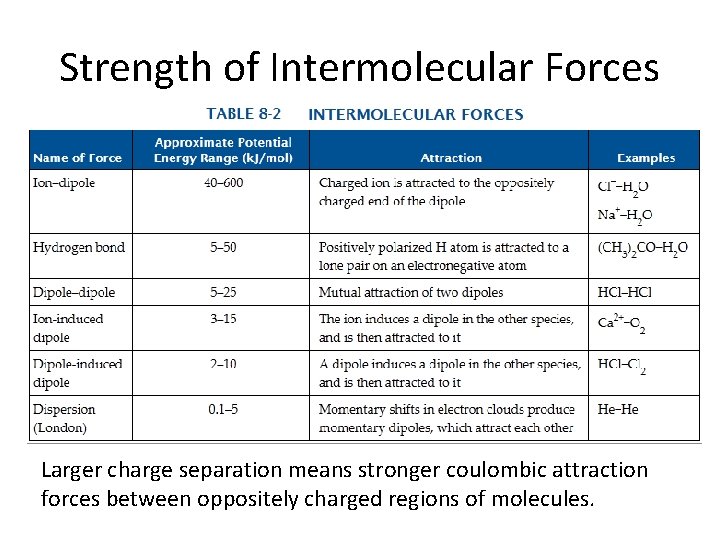
Strength of Intermolecular Forces Larger charge separation means stronger coulombic attraction forces between oppositely charged regions of molecules.
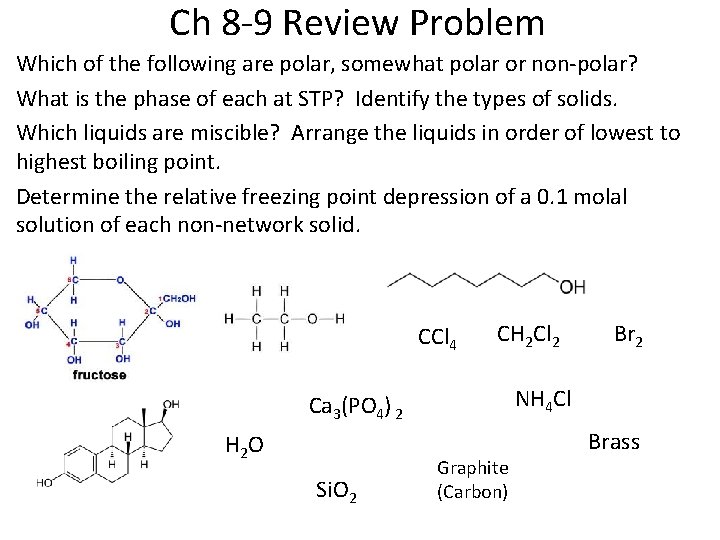
Ch 8 -9 Review Problem Which of the following are polar, somewhat polar or non-polar? What is the phase of each at STP? Identify the types of solids. Which liquids are miscible? Arrange the liquids in order of lowest to highest boiling point. Determine the relative freezing point depression of a 0. 1 molal solution of each non-network solid. CCl 4 CH 2 Cl 2 NH 4 Cl Ca 3(PO 4) 2 H 2 O Si. O 2 Br 2 Graphite (Carbon) Brass

Course Evaluations Need 2 volunteers!!!! I have to leave!!!!!!! Please write in number 2 pencil! 1 Volunteer will return Evaluations to 4 C 68 A in Centennial Hall in DROP BOX. • 1 Volunteer will return pencils to 2 RC 006. • •
- Slides: 14