Chapter 8Part 2 Basics of ocean structure The
Chapter 8—Part 2 Basics of ocean structure The Inorganic Carbon Cycle/ Marine Organic Carbon Cycle
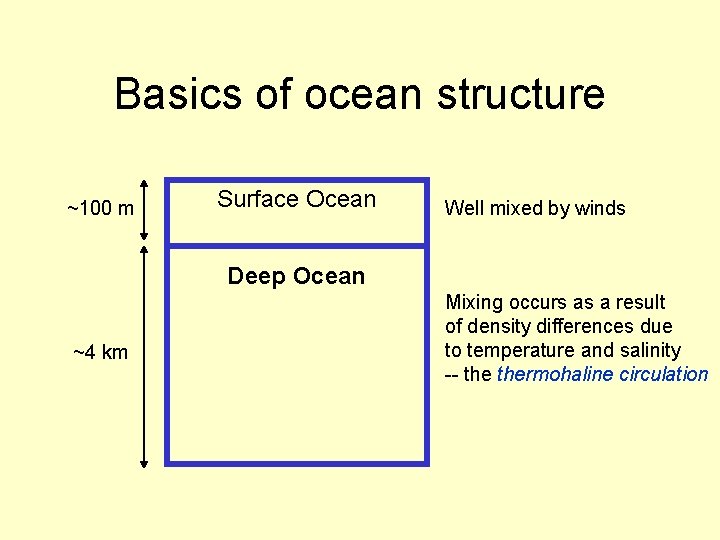
Basics of ocean structure ~100 m Surface Ocean Well mixed by winds Deep Ocean ~4 km Mixing occurs as a result of density differences due to temperature and salinity -- thermohaline circulation
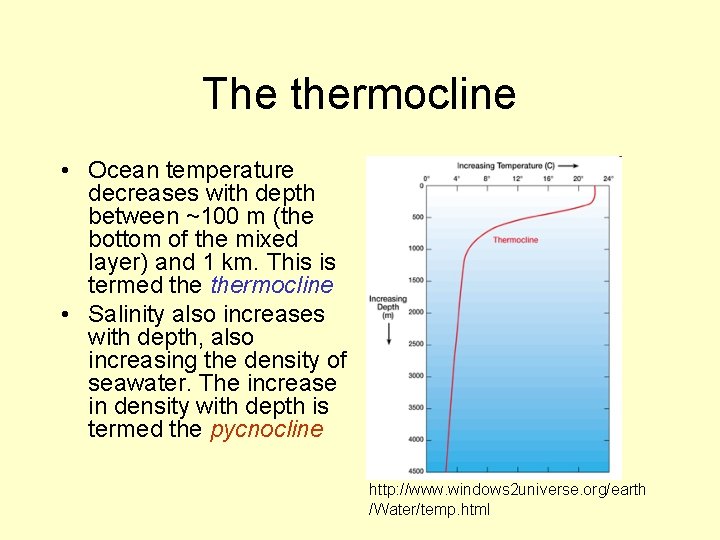
The thermocline • Ocean temperature decreases with depth between ~100 m (the bottom of the mixed layer) and 1 km. This is termed thermocline • Salinity also increases with depth, also increasing the density of seawater. The increase in density with depth is termed the pycnocline http: //www. windows 2 universe. org/earth /Water/temp. html
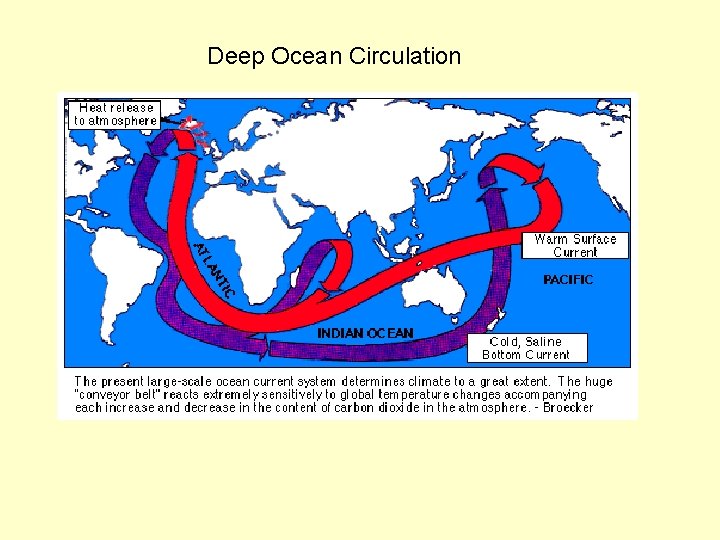
Deep Ocean Circulation
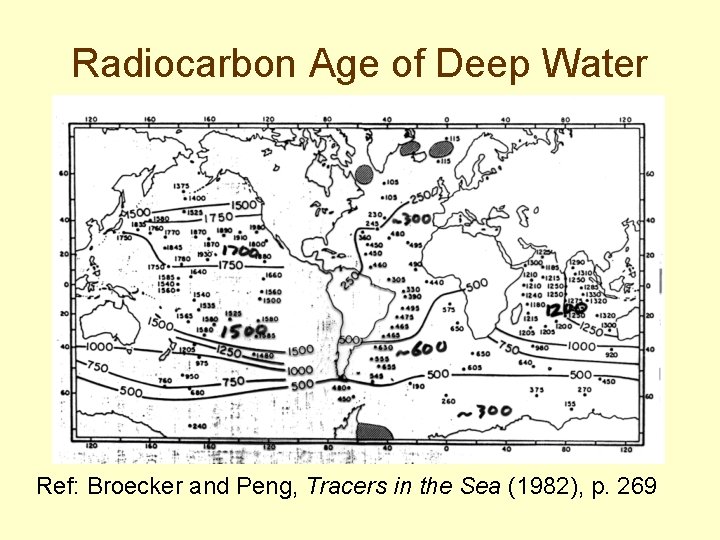
Radiocarbon Age of Deep Water Ref: Broecker and Peng, Tracers in the Sea (1982), p. 269
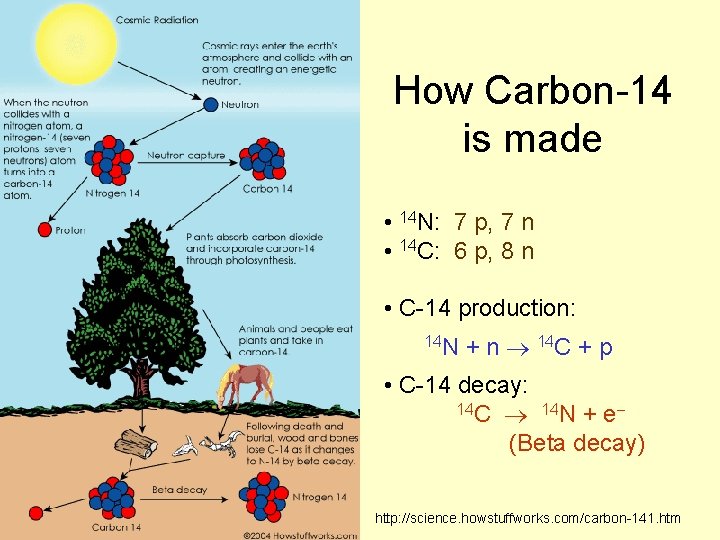
How Carbon-14 is made • 14 N: 7 p, 7 n • 14 C: 6 p, 8 n • C-14 production: 14 N + n 14 C + p • C-14 decay: 14 C 14 N + e (Beta decay) http: //science. howstuffworks. com/carbon-141. htm

The Inorganic Carbon Cycle Carbon Uptake by the Oceans: 1. The biological pump 2. Air-sea gas exchange
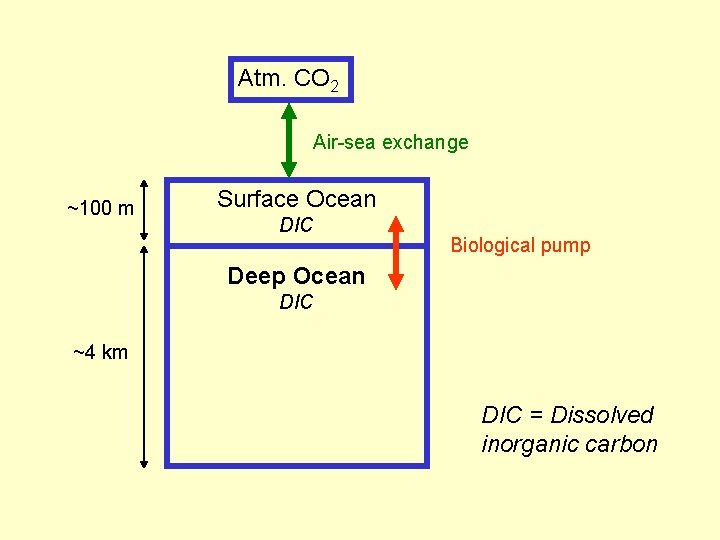
Atm. CO 2 Air-sea exchange ~100 m Surface Ocean DIC Biological pump Deep Ocean DIC ~4 km DIC = Dissolved inorganic carbon

The Biological Pump transfer of CO 2 to the deep ocean: Photosynthesis creates organic matter; this sinks to the deep ocean, where it decays back to CO 2
http: //www. liv. ac. uk/~ric/
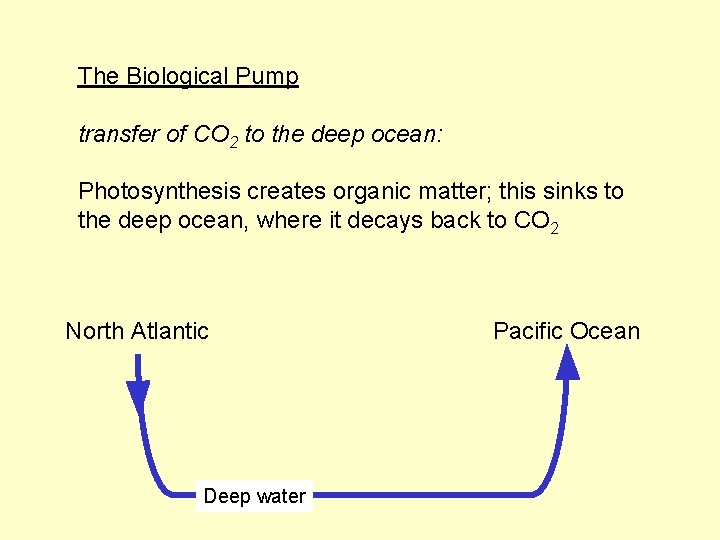
The Biological Pump transfer of CO 2 to the deep ocean: Photosynthesis creates organic matter; this sinks to the deep ocean, where it decays back to CO 2 North Atlantic Deep water Pacific Ocean
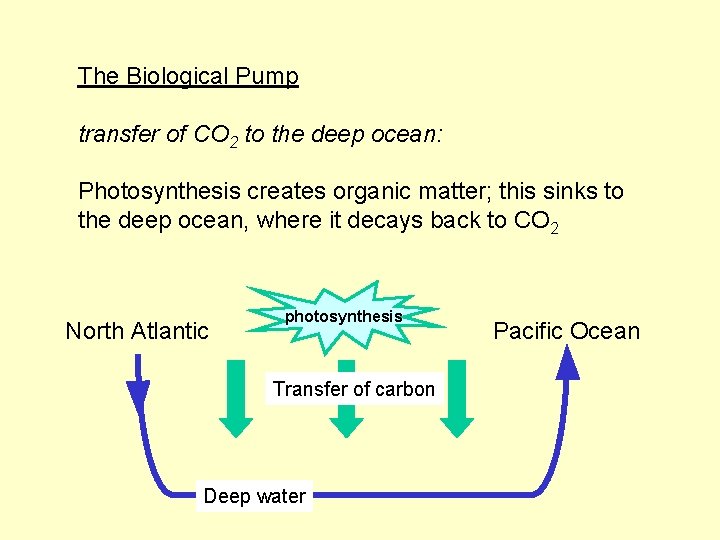
The Biological Pump transfer of CO 2 to the deep ocean: Photosynthesis creates organic matter; this sinks to the deep ocean, where it decays back to CO 2 North Atlantic photosynthesis Transfer of carbon Deep water Pacific Ocean
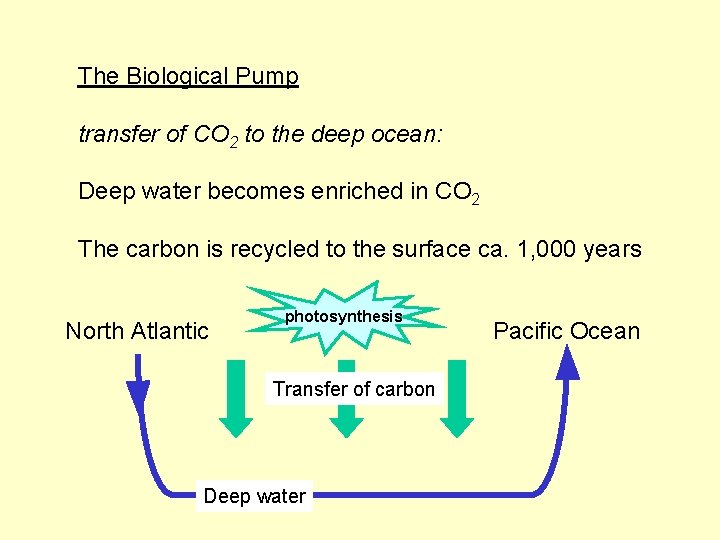
The Biological Pump transfer of CO 2 to the deep ocean: Deep water becomes enriched in CO 2 The carbon is recycled to the surface ca. 1, 000 years North Atlantic photosynthesis Transfer of carbon Deep water Pacific Ocean
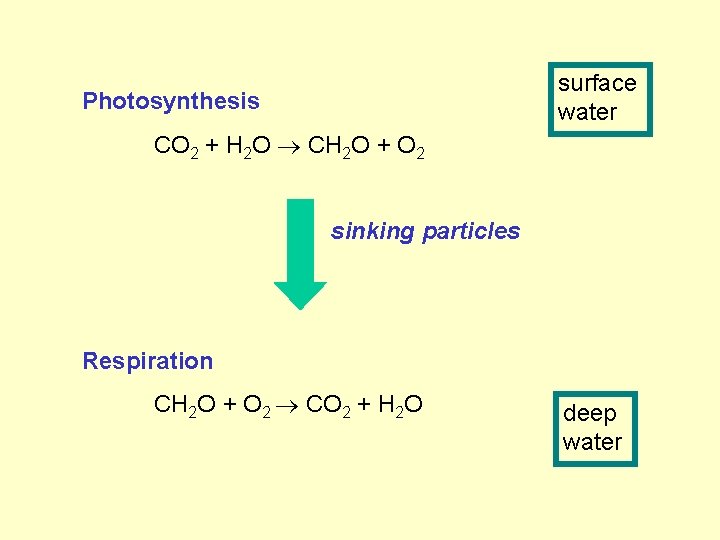
surface water Photosynthesis CO 2 + H 2 O CH 2 O + O 2 sinking particles Respiration CH 2 O + O 2 CO 2 + H 2 O deep water
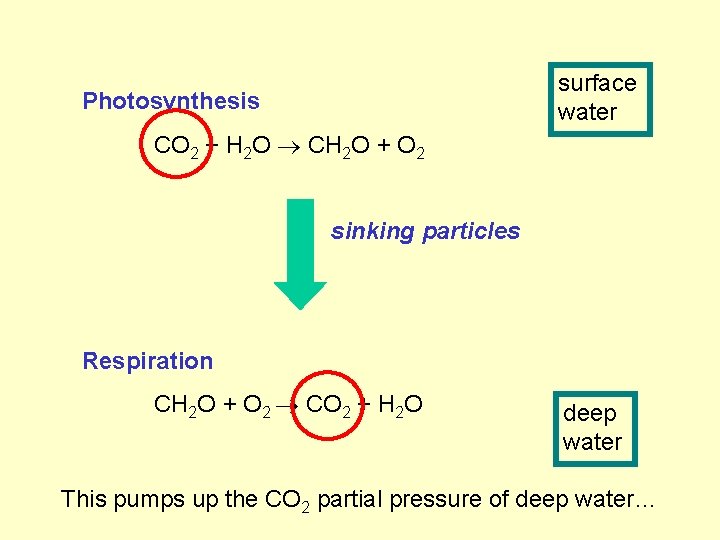
surface water Photosynthesis CO 2 + H 2 O CH 2 O + O 2 sinking particles Respiration CH 2 O + O 2 CO 2 + H 2 O deep water This pumps up the CO 2 partial pressure of deep water…
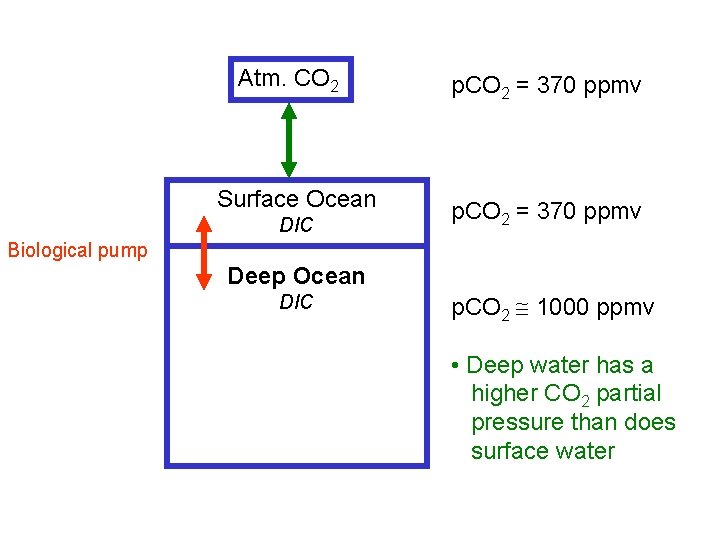
Atm. CO 2 Surface Ocean DIC p. CO 2 = 370 ppmv Biological pump Deep Ocean DIC p. CO 2 1000 ppmv • Deep water has a higher CO 2 partial pressure than does surface water
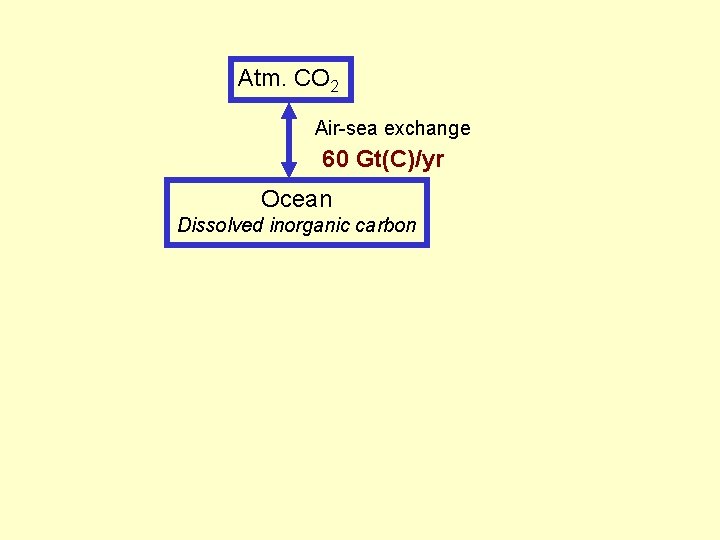
Atm. CO 2 Air-sea exchange 60 Gt(C)/yr Ocean Dissolved inorganic carbon
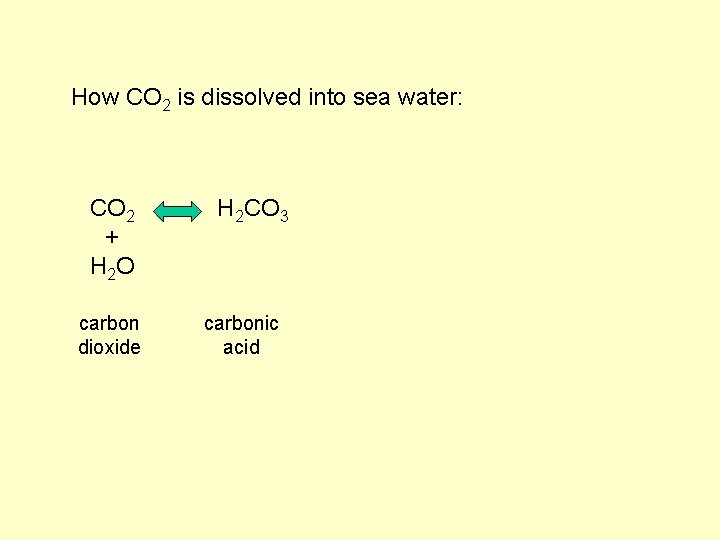
How CO 2 is dissolved into sea water: CO 2 + H 2 O carbon dioxide H 2 CO 3 carbonic acid
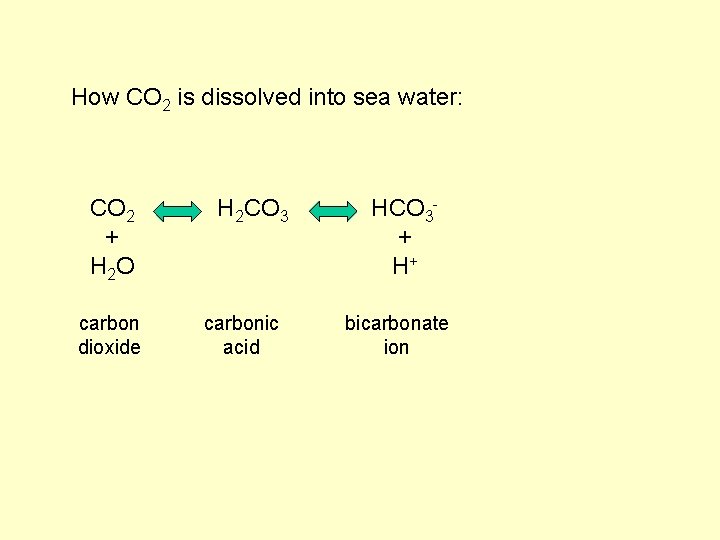
How CO 2 is dissolved into sea water: CO 2 + H 2 O carbon dioxide H 2 CO 3 carbonic acid HCO 3+ H+ bicarbonate ion
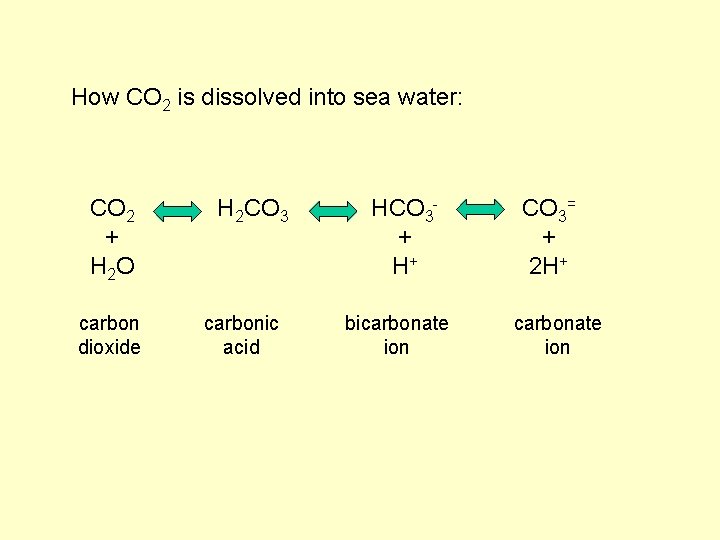
How CO 2 is dissolved into sea water: CO 2 + H 2 O carbon dioxide H 2 CO 3 carbonic acid HCO 3+ H+ bicarbonate ion CO 3= + 2 H+ carbonate ion
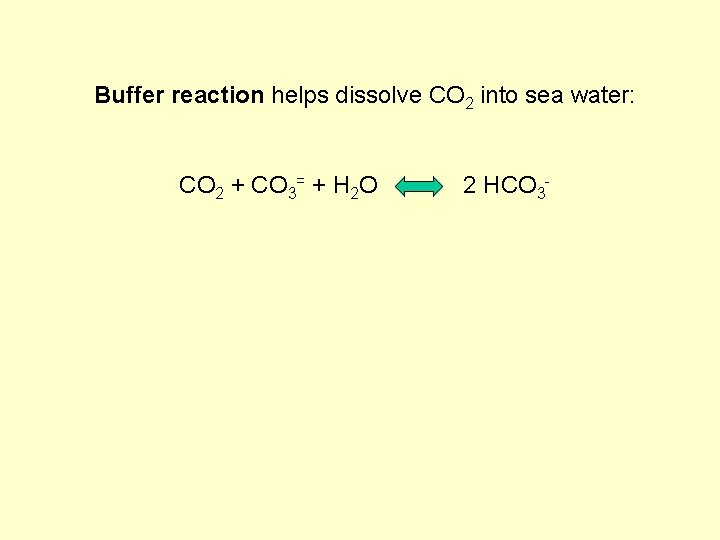
Buffer reaction helps dissolve CO 2 into sea water: CO 2 + CO 3= + H 2 O 2 HCO 3 -
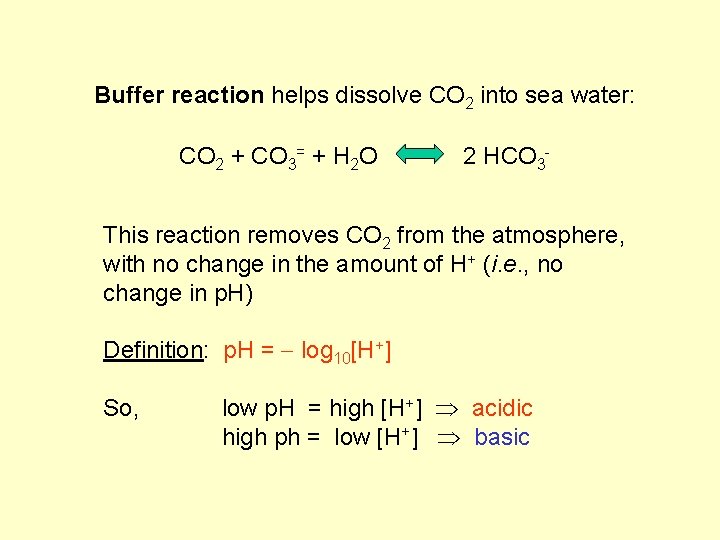
Buffer reaction helps dissolve CO 2 into sea water: CO 2 + CO 3= + H 2 O 2 HCO 3 - This reaction removes CO 2 from the atmosphere, with no change in the amount of H+ (i. e. , no change in p. H) Definition: p. H = log 10[H+] So, low p. H = high [H+] acidic high ph = low [H+] basic
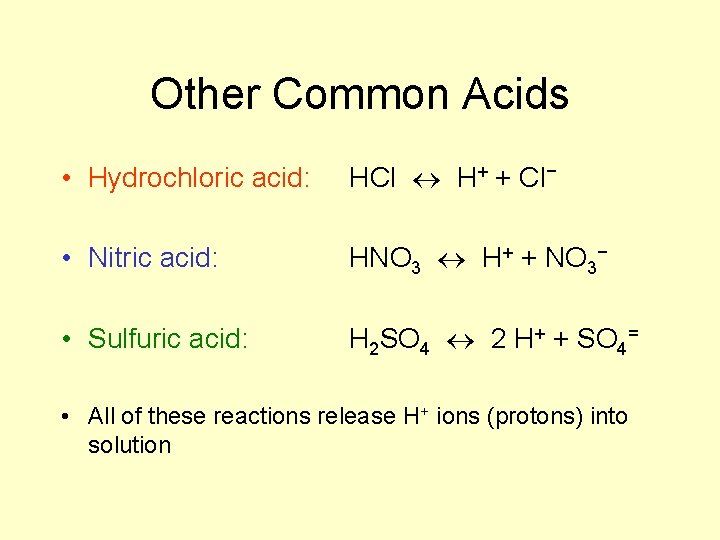
Other Common Acids • Hydrochloric acid: HCl H+ + Cl− • Nitric acid: HNO 3 H+ + NO 3− • Sulfuric acid: H 2 SO 4 2 H+ + SO 4= • All of these reactions release H+ ions (protons) into solution

How much carbon is dissolved in the ocean?

How much carbon is dissolved in the ocean? Gt C Atmospheric CO 2 760

How much carbon is dissolved in the ocean? Gt C Atmospheric CO 2 760 Carbonic acid 740 (H 2 CO 3) Bicarbonate ion 37, 000 (HCO 3 -) Carbonate ion (CO 3=) 1, 300

How much carbon is dissolved in the ocean? Gt C Atmospheric CO 2 760 Carbonic acid 740 (H 2 CO 3) Bicarbonate ion 37, 000 (HCO 3 -) Carbonate ion (CO 3=) 1, 300 39, 040 Gton C
Inorganic Carbon in Marine Sediments: Sea shells Reefs Algae tests Carbonate minerals (Ca. CO 3)

http: //www. cmas-md. org/Images/Sanjay/Univ. Top 4. jpg http: //www. summerclouds. com/Vero/Sea%20 Shells. jpg http: //educate. si. edu/lessons/currkits/ocean/


The Inorganic Carbon Cycle (Carbonate-silicate Cycle) • The rest of this lecture will be done slightly differently on the board. I will leave these slides in, though, for the benefit of anyone who missed the lecture.

Silicate Mineral Weathering: Based on dissolution of calcium silicate minerals (Ca. Si. O 3) Silicate weathering removes CO 2 from the atmosphere. Globally, it removes about 0. 03 Gton C/year

Silicate Mineral Weathering: Based on dissolution of calcium silicate minerals (Ca. Si. O 3) Silicate weathering removes CO 2 from the atmosphere. Globally, it removes about 0. 03 Gton C/year http: //144. 173. 212. 218/Granite. jpg http: //www. explorelabrador. nf. ca/Granite. jpg
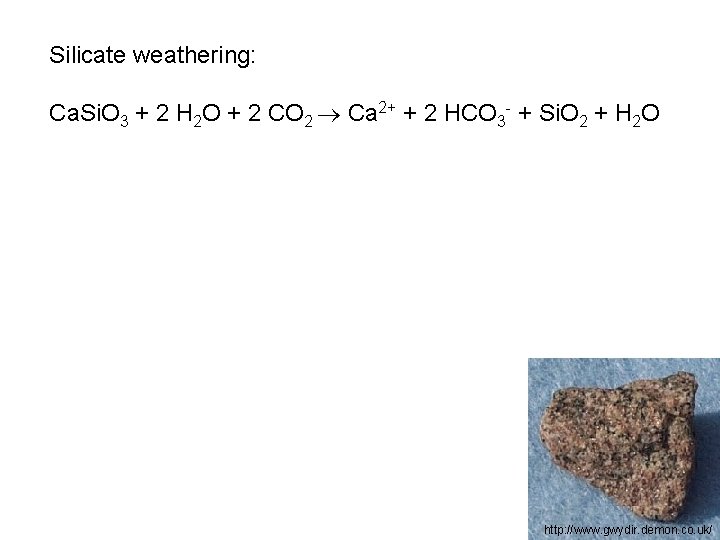
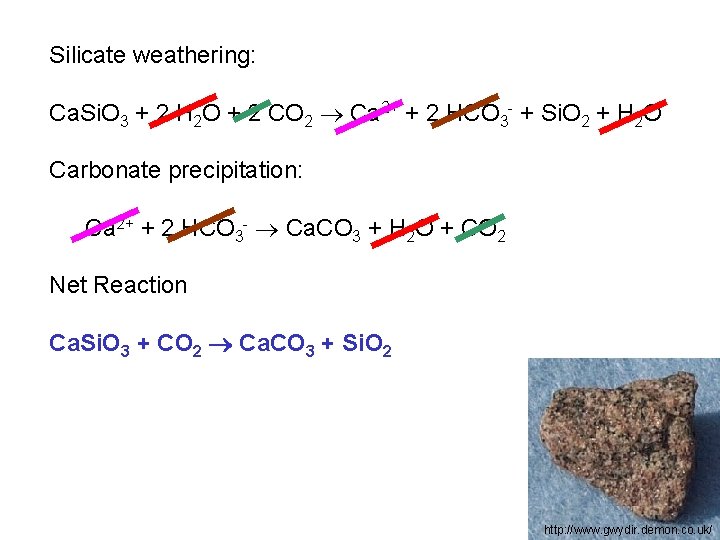
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O http: //www. gwydir. demon. co. uk/
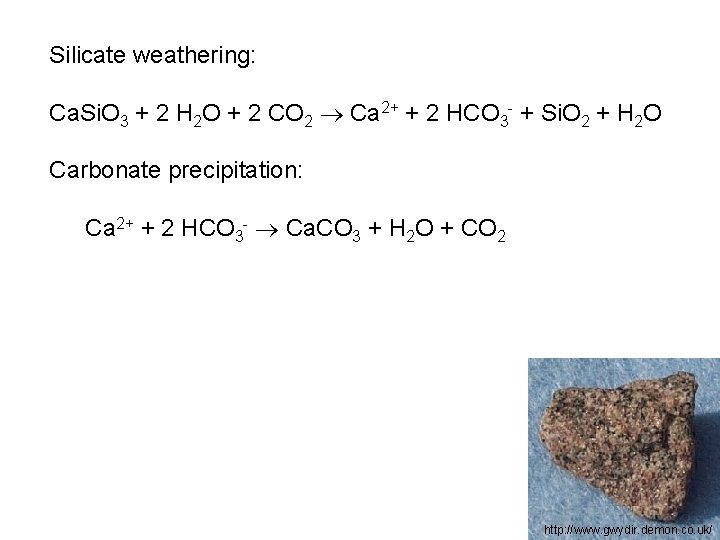
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 http: //www. gwydir. demon. co. uk/
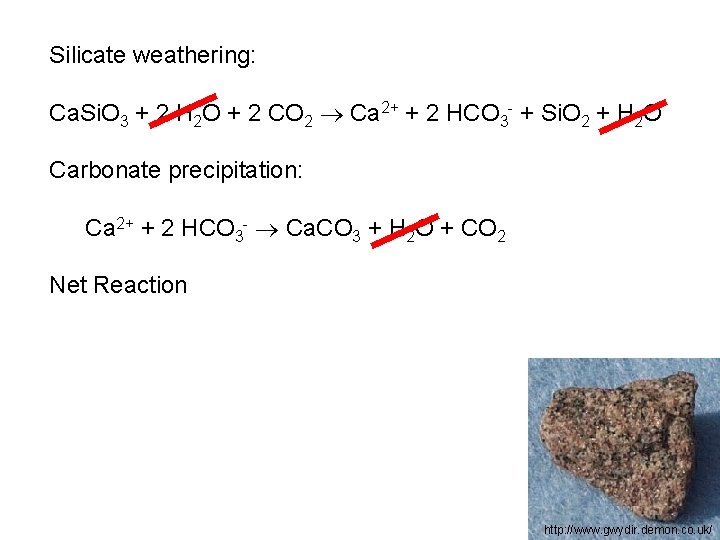
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Net Reaction http: //www. gwydir. demon. co. uk/
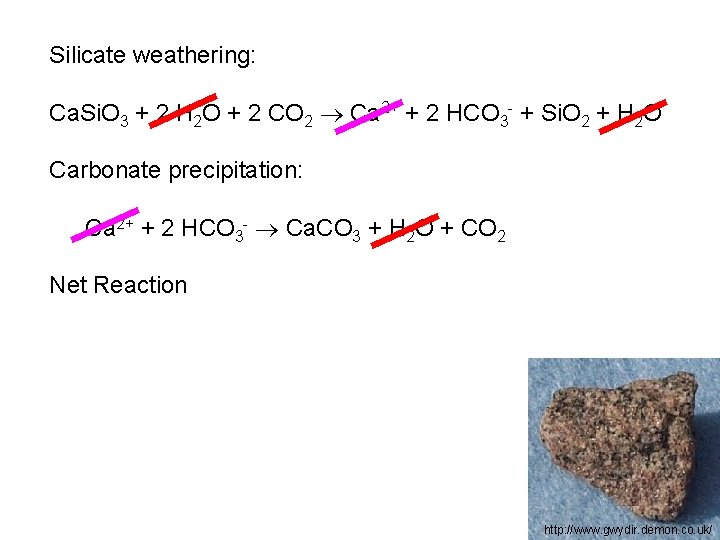
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Net Reaction http: //www. gwydir. demon. co. uk/
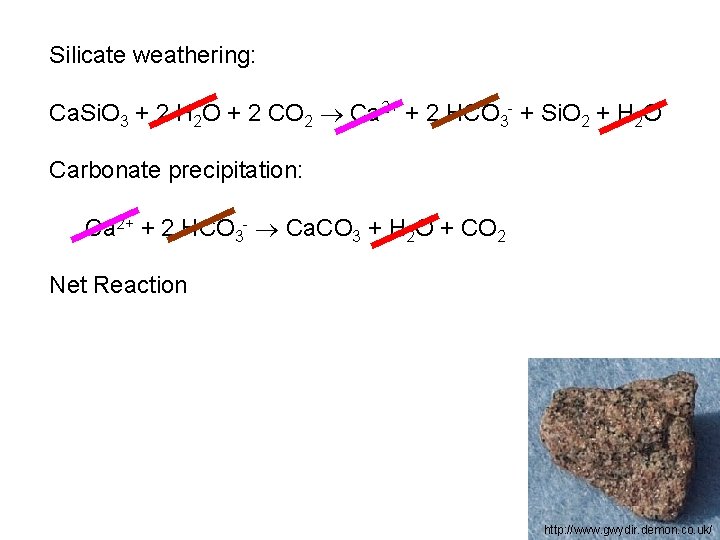
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Net Reaction http: //www. gwydir. demon. co. uk/
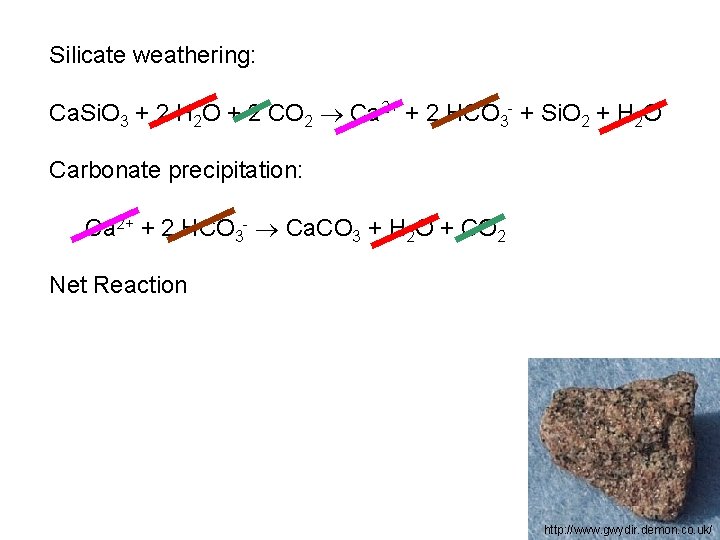
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Net Reaction http: //www. gwydir. demon. co. uk/
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Net Reaction Ca. Si. O 3 + CO 2 Ca. CO 3 + Si. O 2 http: //www. gwydir. demon. co. uk/
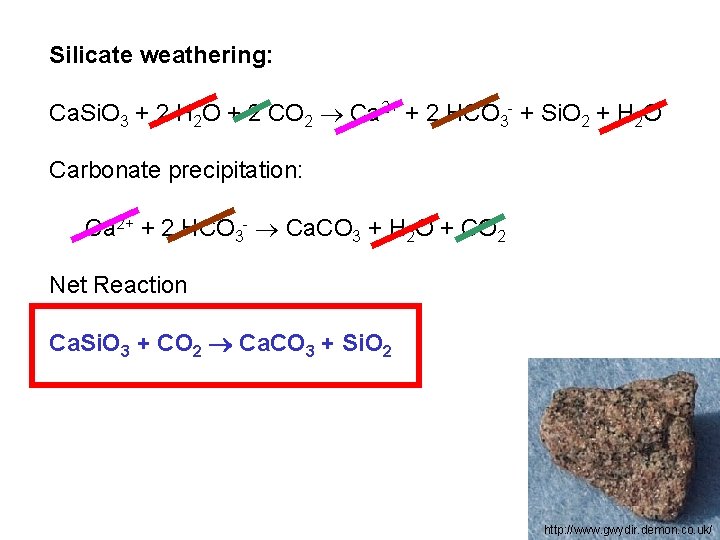
Silicate weathering: Ca. Si. O 3 + 2 H 2 O + 2 CO 2 Ca 2+ + 2 HCO 3 - + Si. O 2 + H 2 O Carbonate precipitation: Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Net Reaction Ca. Si. O 3 + CO 2 Ca. CO 3 + Si. O 2 http: //www. gwydir. demon. co. uk/

Silicate weathering results in a net loss of CO 2 This contrasts with carbonate weathering, which does not remove atmospheric CO 2
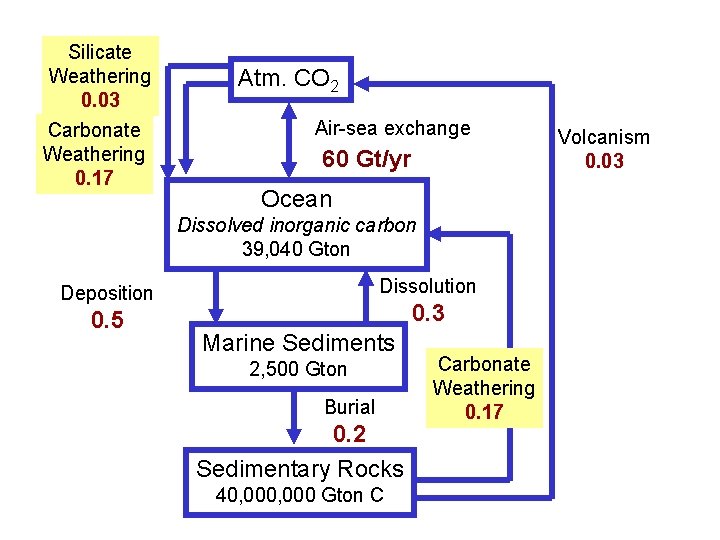
Silicate Weathering 0. 03 Carbonate Weathering 0. 17 Atm. CO 2 Air-sea exchange 60 Gt/yr Ocean Dissolved inorganic carbon 39, 040 Gton Dissolution Deposition 0. 5 0. 3 Marine Sediments 2, 500 Gton Burial 0. 2 Sedimentary Rocks 40, 000 Gton C Carbonate Weathering 0. 17 Volcanism 0. 03
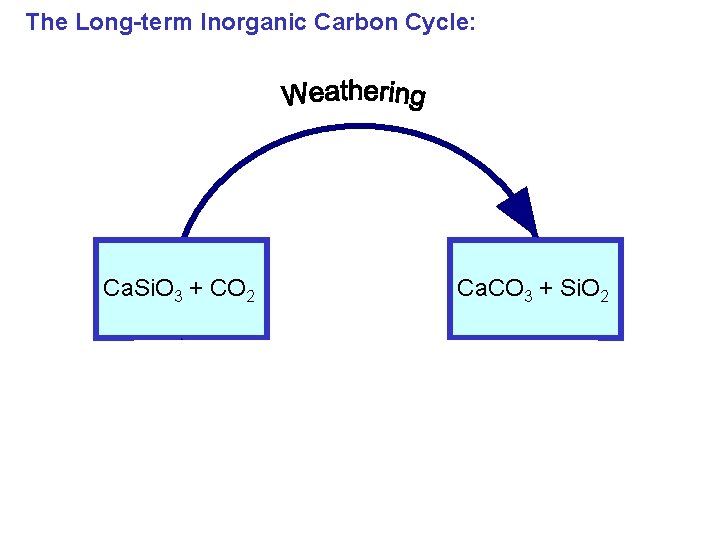
The Long-term Inorganic Carbon Cycle: Ca. Si. O 3 + CO 2 Ca. CO 3 + Si. O 2
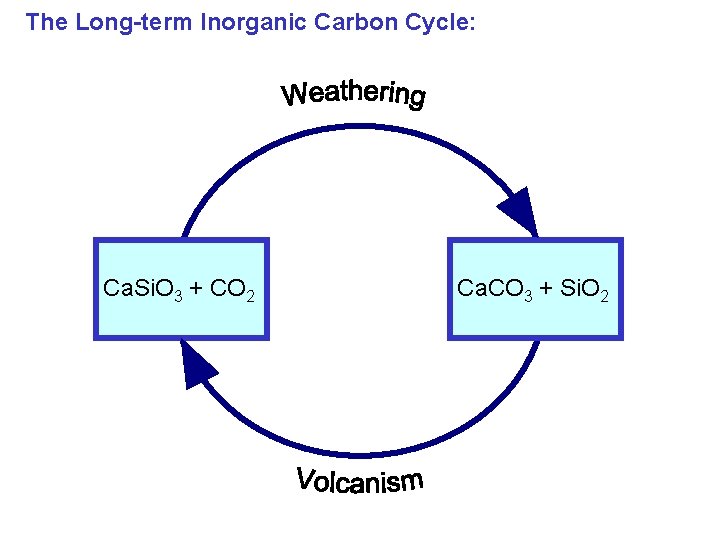
The Long-term Inorganic Carbon Cycle: Ca. Si. O 3 + CO 2 Ca. CO 3 + Si. O 2
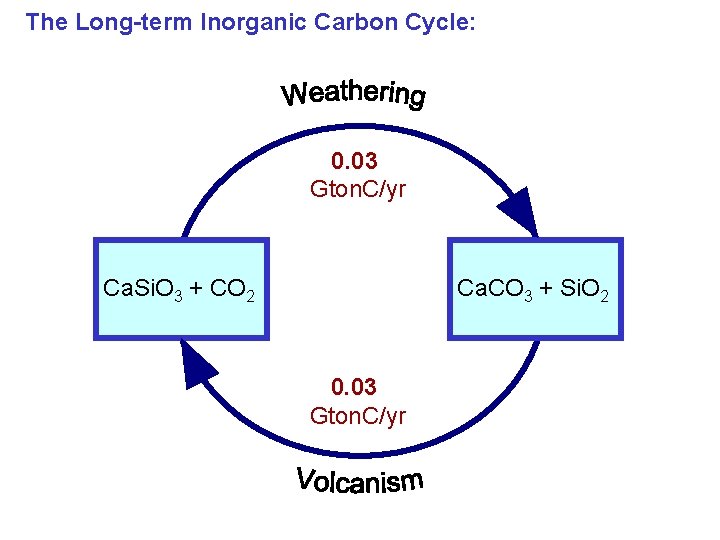
The Long-term Inorganic Carbon Cycle: 0. 03 Gton. C/yr Ca. Si. O 3 + CO 2 Ca. CO 3 + Si. O 2 0. 03 Gton. C/yr

What controls silicate weathering rates? • Time • Temperature • Rainfall • Exposure of fresh rock surfaces • Vegetation (roots provide acid)
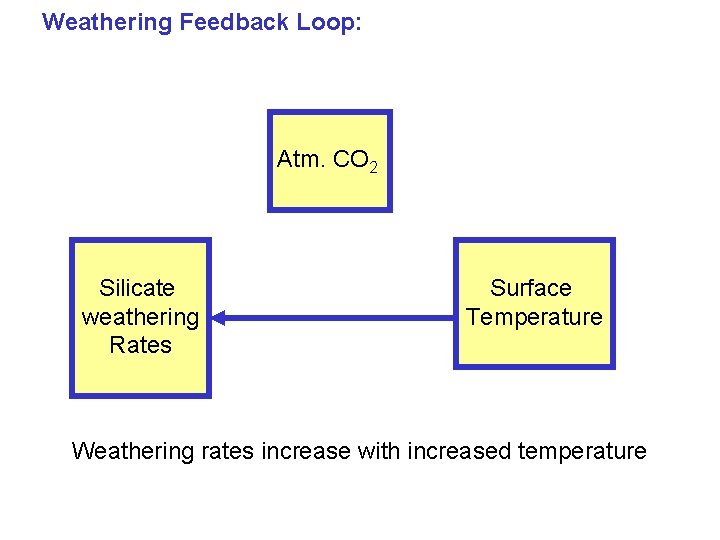
Weathering Feedback Loop: Atm. CO 2 Silicate weathering Rates Surface Temperature Weathering rates increase with increased temperature
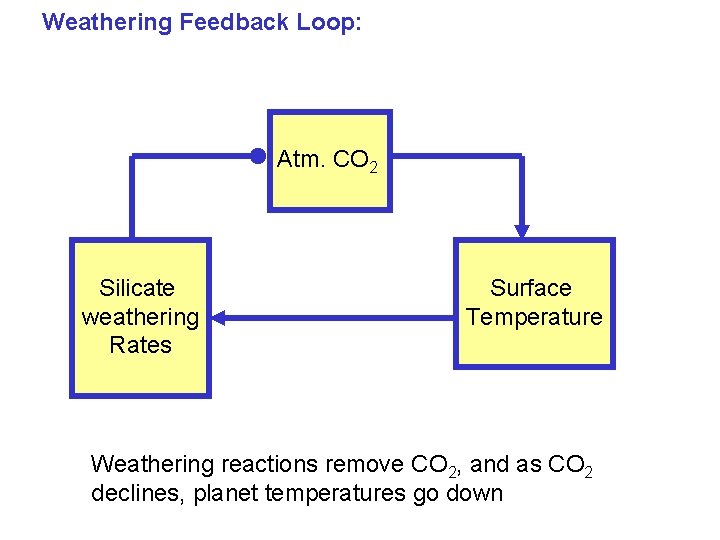
Weathering Feedback Loop: Atm. CO 2 Silicate weathering Rates Surface Temperature Weathering reactions remove CO 2, and as CO 2 declines, planet temperatures go down
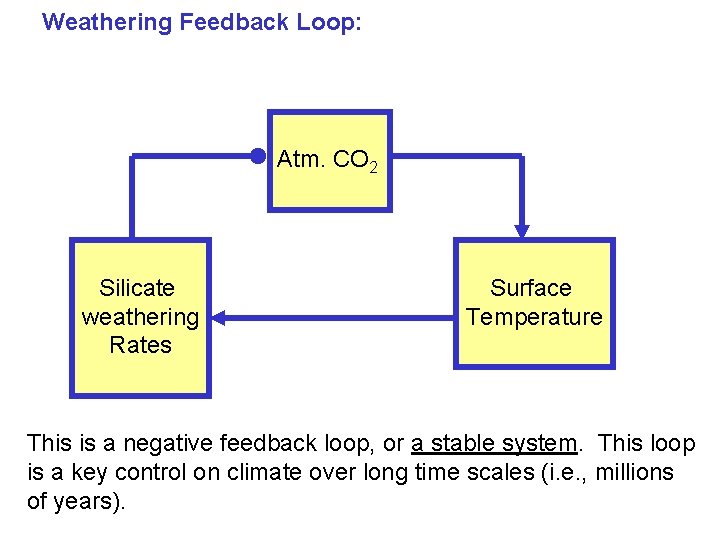
Weathering Feedback Loop: Atm. CO 2 Silicate weathering Rates Surface Temperature This is a negative feedback loop, or a stable system. This loop is a key control on climate over long time scales (i. e. , millions of years).
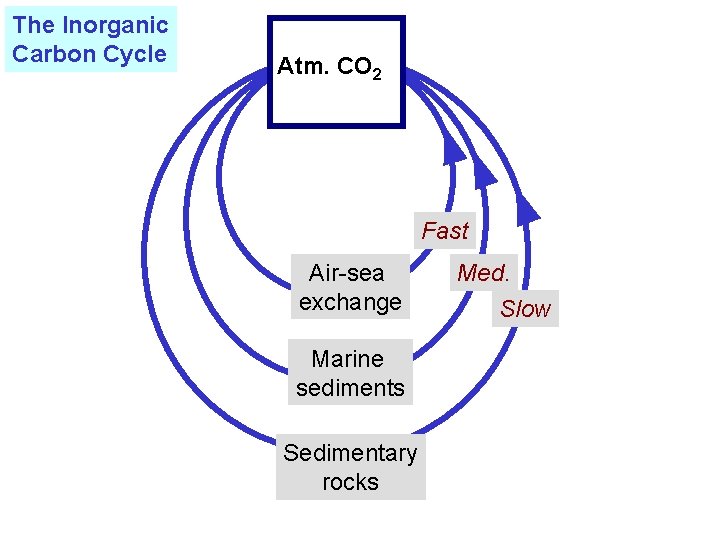
The Inorganic Carbon Cycle Atm. CO 2 Fast Air-sea exchange Marine sediments Sedimentary rocks Med. Slow
- Slides: 51