Chapter 8 Test Review A salt is dissolved

















- Slides: 20

Chapter 8 Test Review

• A salt is dissolved in water, which has a boiling point of 100 o. C. The boiling point of the solution will be • A. greater than 100 o. C • B. less than 100 o. C • C. equal to 100 o. C • D. equal to 0 o. C A

• In order for a solution to form, • A. one substance must dissolve in another. • B. a solid must dissolve in a liquid. • C. the solvent must be water. • D. a gas must dissolve in a liquid. A

• A solution that contains more solute than it would normally hold at that temperature is said to be • A. saturated • B. unsaturated • C. supersaturated • D. concentrated C

• During the formation of a solution, energy is • A. either released or absorbed. • B. neither released or absorbed. • C. released only. • D. absorbed only. A

• One way to determine the degree of saturation of a solid-liquid solution is to drop a crystal of the solute into the solution. If the crystal sits at the bottom of the container, the solution is • A. saturated. • B. unsaturated. • C. supersaturated. • D. concentrated. A

• In an exothermic reaction, the amount of energy required to break the attractions among the solute particles and among the solvent particles is • A. greater than the energy released as attractions form between solute and solvent particles. • B. less than the energy released as attractions form between solute and solvent particles. • C. equal to the energy released as attractions form between solute and solvent particles. • D. equal to the heat of solution. B

• The solubility of carbon dioxide gas in water can be increased by • A. increasing the temperature. • B. agitating the mixture. • C. increasing the pressure. • D. using more solvent. C

• All materials that are considered to be insoluble will dissolve in a solvent to a very small extent. The rate of dissolving of a rock in a streambed would not be increased by which of the following? • A. breaking the rock into smaller pieces • B. moving the rock to a faster-moving current • C. moving the rock to warmer water • D. reducing the exposure of the rock to the water D

• The maximum amount of a solute that will dissolve in a certain amount of solvent at a given temperature is that solute’s • A. solubility. • B. concentration. • C. degree of saturation. • D. rate of solution. A

• A student dissolved equal amounts of salt in equal amounts of warm water, room-temperature water, and ice water. Which of the following is true? • A. The salt dissolved most quickly in the warm water. • B. The salt dissolved most quickly in the room-temperature water. • C. The salt dissolved most quickly in the ice water. • D. None of the above A

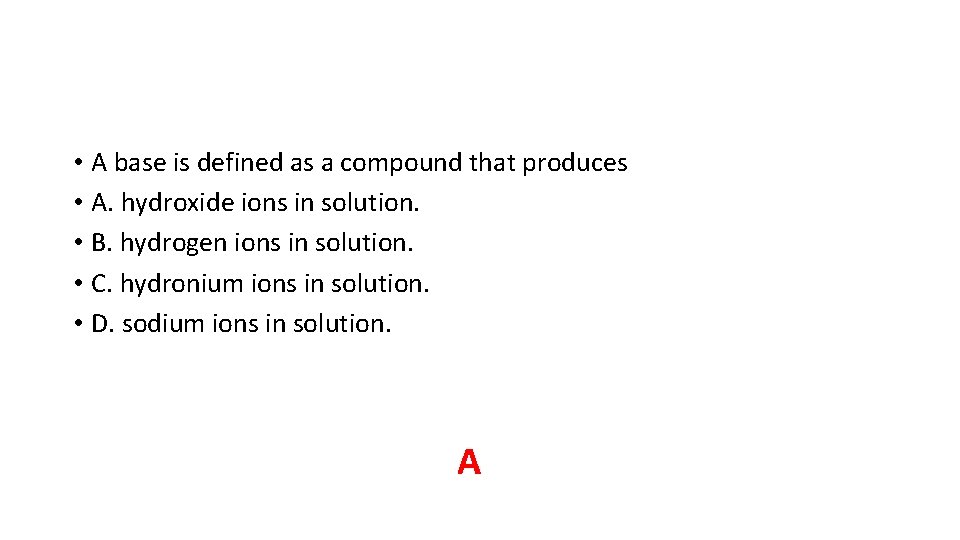
• A base is defined as a compound that produces • A. hydroxide ions in solution. • B. hydrogen ions in solution. • C. hydronium ions in solution. • D. sodium ions in solution. A

• Which of the following is NOT a property of an acid? • A. tastes sour • B. usually reacts with a metal • C. changes the color of an indicator • D. feels slippery D

• Which of the following is NOT a common property of bases? • A. feels slippery • B. tastes bitter • C. reacts with metals • D. changes color of indicators C

• A compound has a p. H of 6 in solution, where very little of it has ionized. The compound is a • A. weak base. • B. strong base. • C. weak acid. • D. strong acid. C

• A small amount of acid is added to a buffer solution. The p. H of the solution will • A. increase. • B. decrease. • C. stay about the same. • D. become neutral. C

• Calcium hydroxide forms very few hydroxide ions in solution, but it is still considered a strong base because • A. calcium hydroxide is insoluble. • B. all the calcium hydroxide in solution ionizes. • C. all the calcium hydroxide in solution dissociates. • D. very little of the calcium hydroxide in solution ionizes. C

• When sugar dissolves in water, water is the _______ and sugar is the _______. Solvent, solute

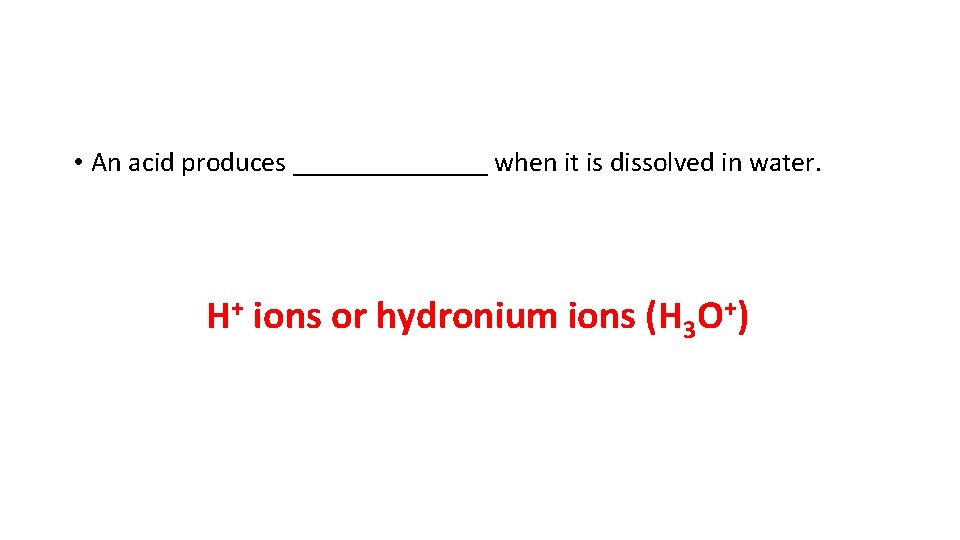
• An acid produces _______ when it is dissolved in water. H+ ions or hydronium ions (H 3 O+)

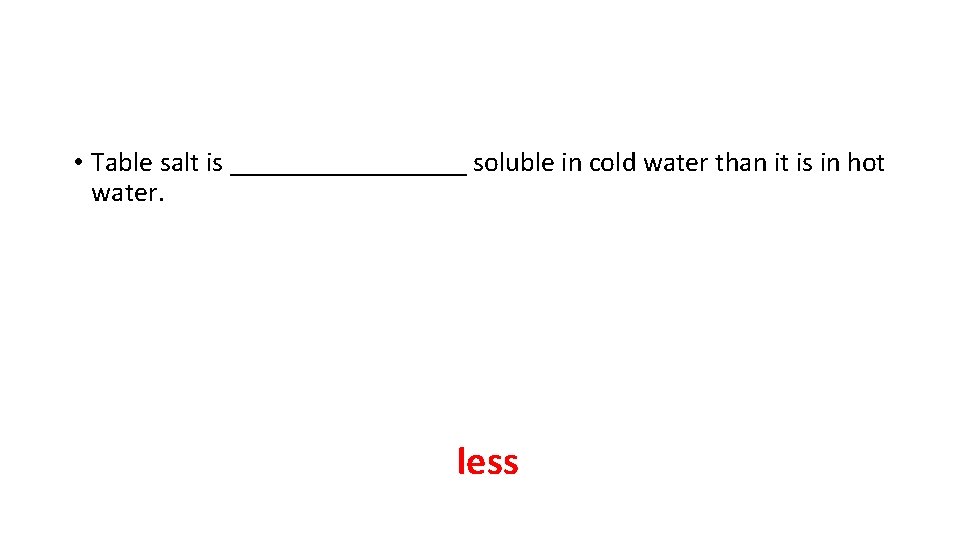
• Table salt is _________ soluble in cold water than it is in hot water. less