Chapter 8 Section 5 Electronegativity and Polarity Electron





- Slides: 10

Chapter 8 Section 5 Electronegativity and Polarity

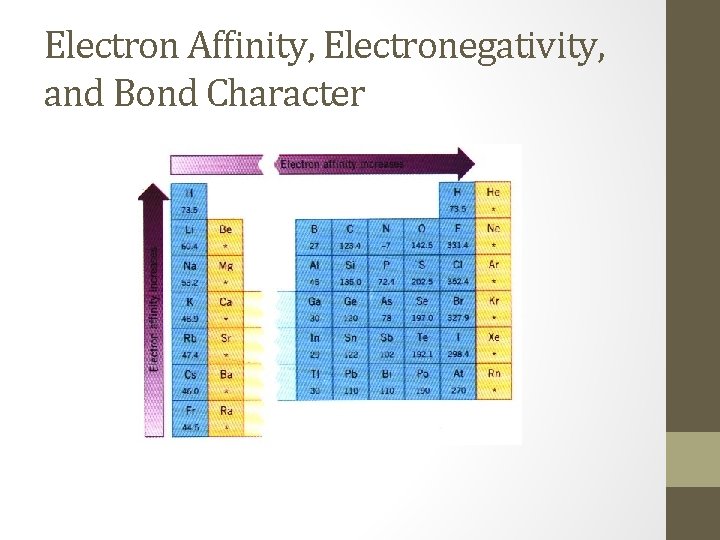
Electron Affinity, Electronegativity, and Bond Character • The type of bond formed during a reaction is related to each atom’s attraction for electrons. • Electron affinity is a measure of the tendency of an atom to accept an electron. • Excluding noble gases, electron affinity increases left to right across a period and bottom to top within a group.

Electron Affinity, Electronegativity, and Bond Character

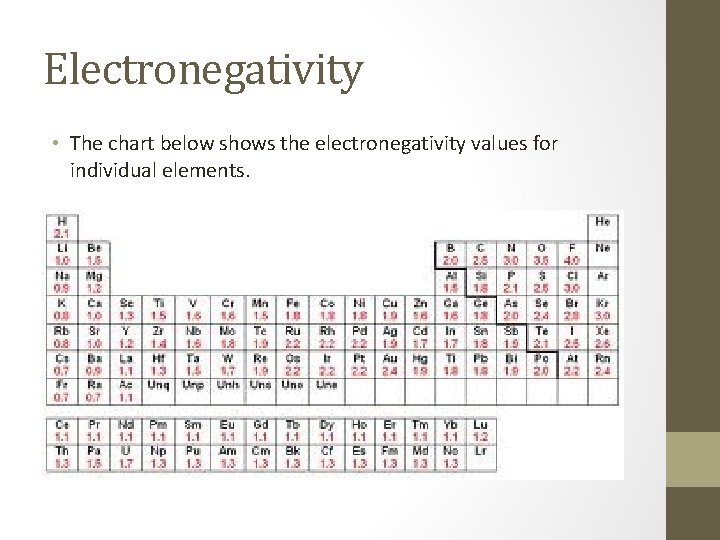
Electronegativity • The chart below shows the electronegativity values for individual elements.

Bond Character • A chemical bond between two different elements is never completely ionic or covalent. • The character of the bond depends on how strongly each of the bonded atoms attracts electrons. • The character and type of chemical bond can be predicted using the electronegativity difference of the elements in the bond.

Bond Character • Electrons in bonds between identical atoms have an electronegativity difference of zero – meaning that the electrons are equally shared between the two atoms. • This type of bond is considered nonpolar covalent, or simply covalent. • Electrons pairs in most other covalent bonds are not equally shared based on their different electronegativities. • Unequal sharing results in a polar covalent bond.

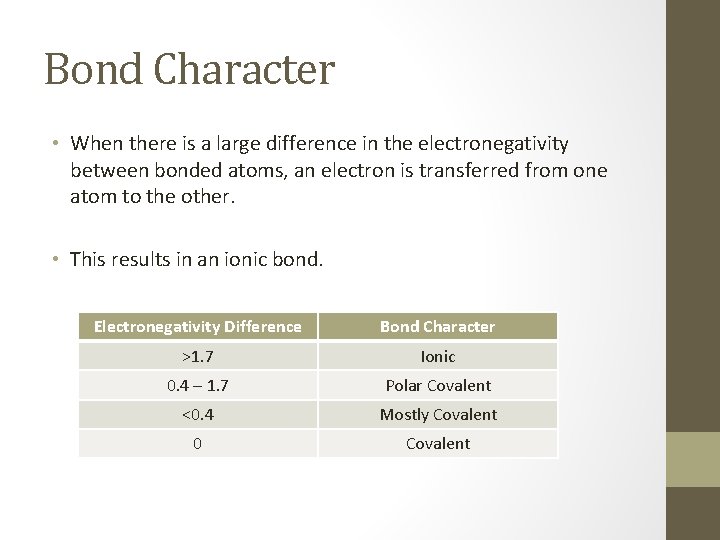
Bond Character • When there is a large difference in the electronegativity between bonded atoms, an electron is transferred from one atom to the other. • This results in an ionic bond. Electronegativity Difference Bond Character >1. 7 Ionic 0. 4 – 1. 7 Polar Covalent <0. 4 Mostly Covalent 0 Covalent

Bond Character • Calculate the electronegativity difference and determine the bond character of the following: • CH 4 • NH 3 • HF

Polar Covalent Bonds • A polar covalent bond is similar to a tug of war in which the two teams are not of equal strength. • When a polar bond forms, the shared electron pair is pulled toward one of the atoms. • Therefore, the electrons spend more of their time around one atom than the other. • This results in partial charges at the ends of the bonds.

Polar Covalent Bonds • The Greek letter delta (δ) is used to represent a partial charge. • An atom can have a partial positive or a partial negative charge. • The more electronegative atom is at the partially negative end, while the less electronegative atom is at the partially positive end. • The resulting polar bond is often referred to as a dipole (two poles).