Chapter 8 Section 2 Types of Chemical Reactions





- Slides: 12

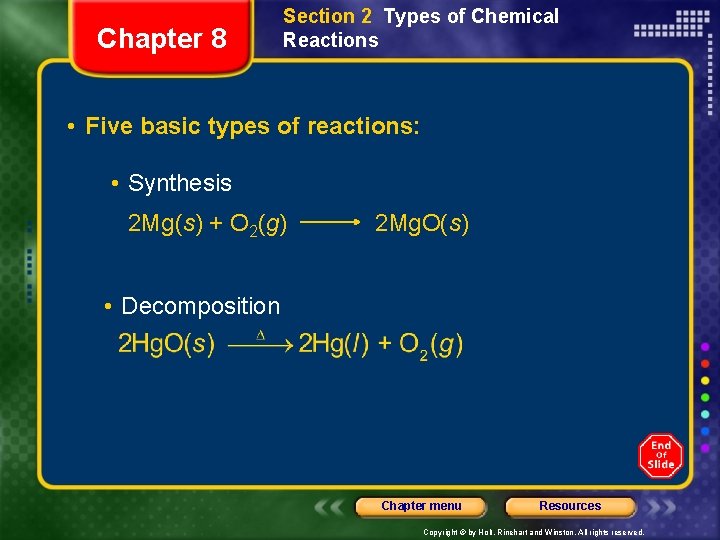
Chapter 8 Section 2 Types of Chemical Reactions • Five basic types of reactions: • Synthesis 2 Mg(s) + O 2(g) 2 Mg. O(s) • Decomposition Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Section 2 Types of Chemical Reactions • Five basic types of reactions: • Single-displacement 2 Al(s) + 3 Pb(NO 3)2(aq) 3 Pb(s) + 2 Al(NO 3)3(aq) • Double-displacement 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq) • Combustion reactions C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

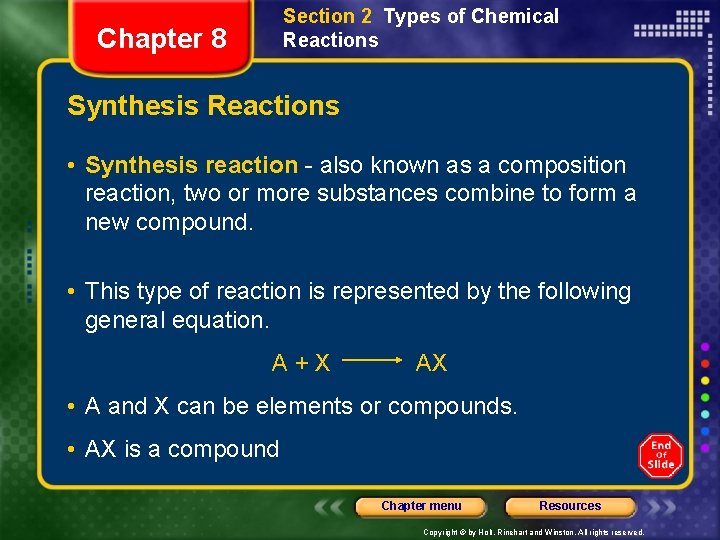
Section 2 Types of Chemical Reactions Chapter 8 Synthesis Reactions • Synthesis reaction - also known as a composition reaction, two or more substances combine to form a new compound. • This type of reaction is represented by the following general equation. A+X AX • A and X can be elements or compounds. • AX is a compound Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

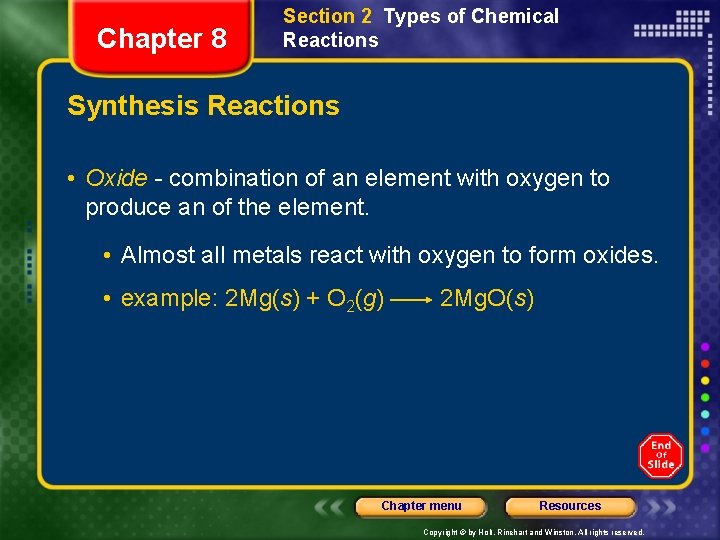
Chapter 8 Section 2 Types of Chemical Reactions Synthesis Reactions • Oxide - combination of an element with oxygen to produce an of the element. • Almost all metals react with oxygen to form oxides. • example: 2 Mg(s) + O 2(g) 2 Mg. O(s) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Section 2 Types of Chemical Reactions • Decomposition reaction - a single compound undergoes a reaction that produces two or more simpler substances. • Decomposition reactions are the opposite of synthesis reactions. • They are represented by the following general equation. AX A+X • AX is a compound. • A and X can be elements or compounds. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

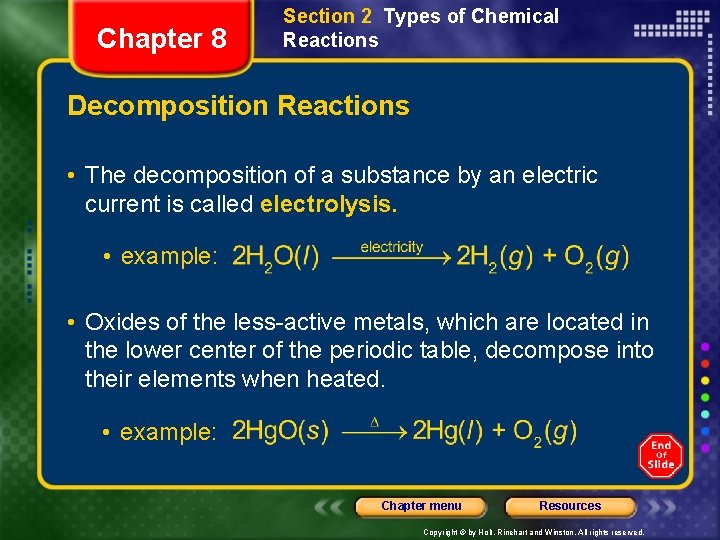
Chapter 8 Section 2 Types of Chemical Reactions Decomposition Reactions • The decomposition of a substance by an electric current is called electrolysis. • example: • Oxides of the less-active metals, which are located in the lower center of the periodic table, decompose into their elements when heated. • example: Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Section 2 Types of Chemical Reactions Single-Displacement Reactions • single-displacement reaction - one element replaces a similar element in a compound. • Single-displacement reactions can be represented by the following general equations. A + BX AX + B or Y + BX BY + X • A, B, X, and Y are elements. AX, BX, and BY are compounds. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Section 2 Types of Chemical Reactions Single-Displacement Reactions Displacement of a Metal in a Compound by Another Metal • Aluminum is more active than lead. 2 Al(s) + 3 Pb(NO 3)2(aq) 3 Pb(s) + 2 Al(NO 3)3(aq) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

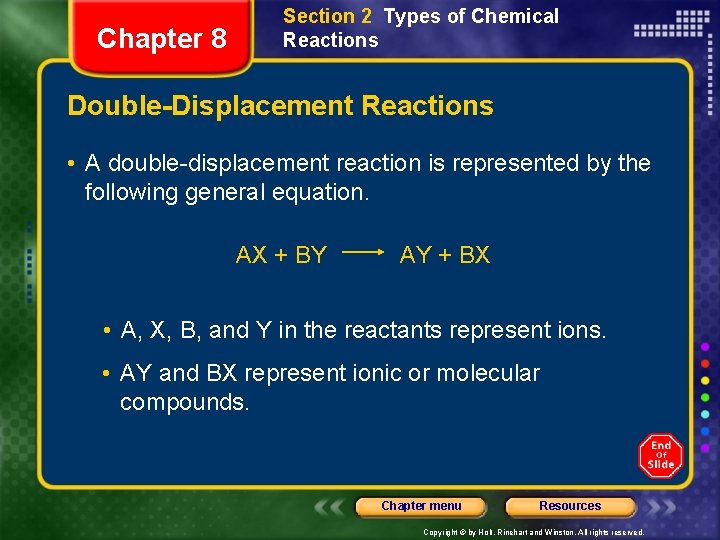
Chapter 8 Section 2 Types of Chemical Reactions Double-Displacement Reactions • double-displacement reactions - ions of two compounds exchange places in an aqueous solution to form two new compounds. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Section 2 Types of Chemical Reactions Double-Displacement Reactions • A double-displacement reaction is represented by the following general equation. AX + BY AY + BX • A, X, B, and Y in the reactants represent ions. • AY and BX represent ionic or molecular compounds. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

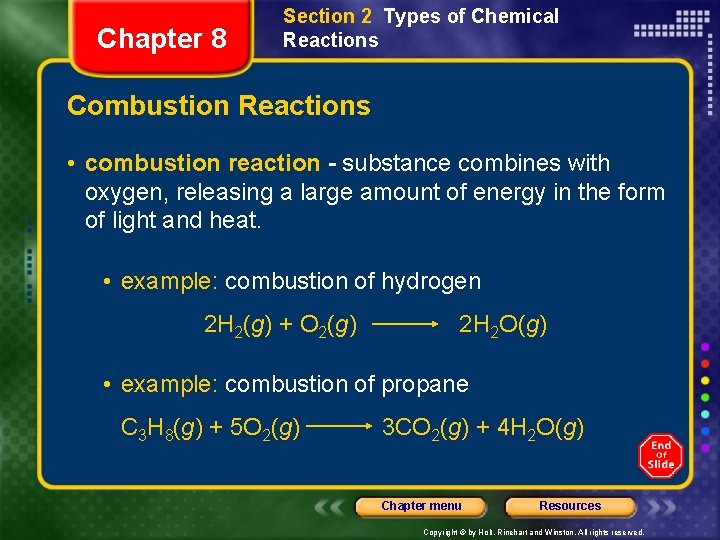
Chapter 8 Section 2 Types of Chemical Reactions Combustion Reactions • combustion reaction - substance combines with oxygen, releasing a large amount of energy in the form of light and heat. • example: combustion of hydrogen 2 H 2(g) + O 2(g) 2 H 2 O(g) • example: combustion of propane C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

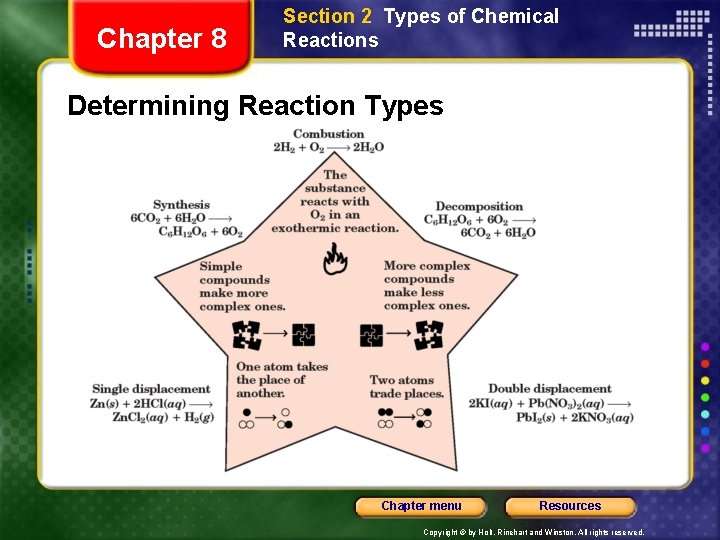
Chapter 8 Section 2 Types of Chemical Reactions Determining Reaction Types Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.