CHAPTER 8 SEC 8 4 Chemical Bonds CH

CHAPTER 8 SEC 8. 4 Chemical Bonds
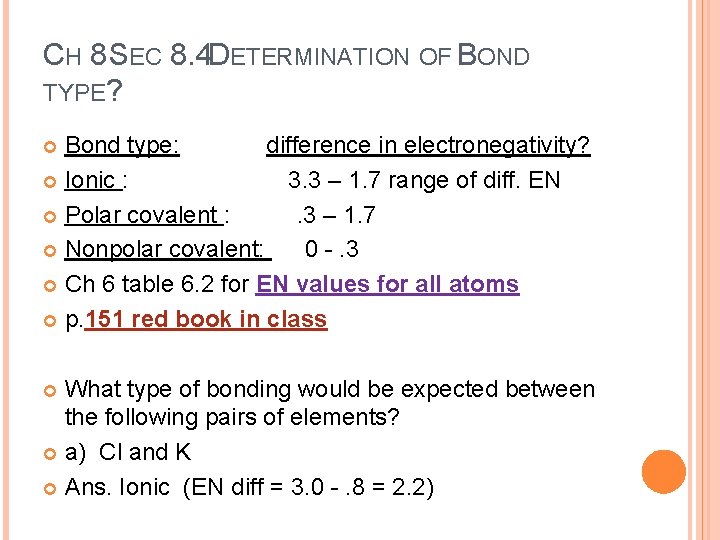
CH 8 SEC 8. 4 DETERMINATION OF BOND TYPE? Bond type: difference in electronegativity? Ionic : 3. 3 – 1. 7 range of diff. EN Polar covalent : . 3 – 1. 7 Nonpolar covalent: 0 -. 3 Ch 6 table 6. 2 for EN values for all atoms p. 151 red book in class What type of bonding would be expected between the following pairs of elements? a) Cl and K Ans. Ionic (EN diff = 3. 0 -. 8 = 2. 2)

b) O and H Ans. 3. 5 – 2. 1 = 1. 4 polar covalent c) O and O Ans. 3. 5 – 3. 5 = 0 nonpolar covalent
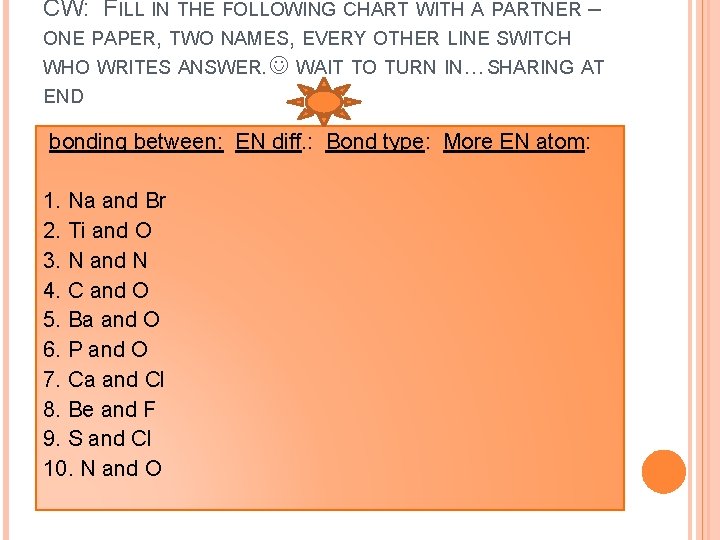
CW: FILL IN THE FOLLOWING CHART WITH A PARTNER – ONE PAPER, TWO NAMES, EVERY OTHER LINE SWITCH WHO WRITES ANSWER. WAIT TO TURN IN…SHARING AT END bonding between: EN diff. : Bond type: More EN atom: 1. Na and Br 2. Ti and O 3. N and N 4. C and O 5. Ba and O 6. P and O 7. Ca and Cl 8. Be and F 9. S and Cl 10. N and O

INTERMOLECULAR FORCES Forces of attraction between molecules (weaker than either ionic or covalent bonds) van der Waals forces are the weakest attractions between molecules (two types: dispersion forces & dipole interactions) Hydrogen bonding – “Not a true bond” The intermolecular force in which a hydrogen atom that is bonded to a highly electronegative atom is attracted to an unshared pair of electrons of an electronegative atom in a nearby molecule. Ex/ water molecules:

dipole-interactions: Forces of attraction between polar molecules. Ex/ammonia London Dispersion forces: forces of attraction between nonpolar atoms or molecules that are created by temporary dipoles. ex/ Noble gases

STRONGEST TO WEAKEST 1. Metallic bonding 2. Covalent bonding 3. Ionic bonding 4. hydrogen bonding (force of attraction, not a bond) 5. dipole-interactions (force of attraction btw polar molecules) 6. London dispersion (force of attraction btw nonpolar atoms – noble gases)
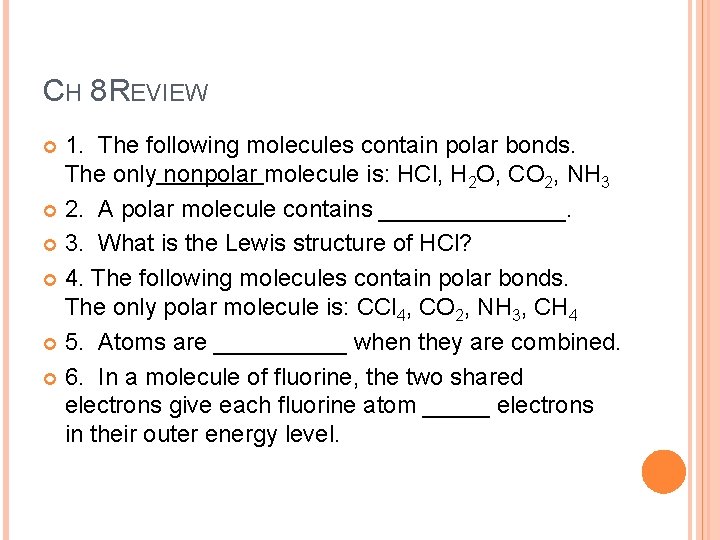
CH 8 REVIEW 1. The following molecules contain polar bonds. The only nonpolar molecule is: HCl, H 2 O, CO 2, NH 3 2. A polar molecule contains _______. 3. What is the Lewis structure of HCl? 4. The following molecules contain polar bonds. The only polar molecule is: CCl 4, CO 2, NH 3, CH 4 5. Atoms are _____ when they are combined. 6. In a molecule of fluorine, the two shared electrons give each fluorine atom _____ electrons in their outer energy level.
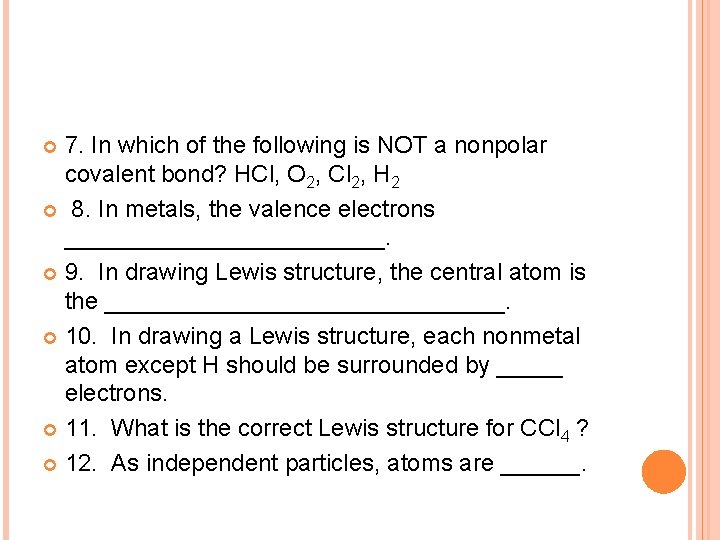
7. In which of the following is NOT a nonpolar covalent bond? HCl, O 2, Cl 2, H 2 8. In metals, the valence electrons ____________. 9. In drawing Lewis structure, the central atom is the _______________. 10. In drawing a Lewis structure, each nonmetal atom except H should be surrounded by _____ electrons. 11. What is the correct Lewis structure for CCl 4 ? 12. As independent particles, atoms are ______.

13. VSEPR theory is a model for predicting_________. 14. If the atoms that share electrons have an unequal attraction for the electrons the bond is called ________. 15. The chemical formula for water is H 2 O. What type of formula is that? 16. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal energies is called ____. 17. The shiny appearance of metals is due to ________.
18. The elements of the ______ group satisfy the octet rule without forming compounds. 19. According to VSEPR theory what is the shape of ammonia, NH 3 ? 20. Use VSEPR theory to predict the shape of HCl? 21. The electrons involved in the formation of a chemical bond are called _________. 22. In the crystal lattice of an ionic compound, each cation is surrounded by ________. 23. Malleability and ductility are properties of ____ bonds.

24. Shifting layers in an ionic compound causes it to __________. 25. A chemical bond results from the mutual attraction of the nuclei of atoms and _____. 26. A covalent bond results when ____ are shared. 27. The B-F bond in BF 3 (electronegativity for B is 2. 0 and F is 4. 0) is _________. 28. A neutral group of atoms held together by covalent bonds is a _________. 29. Which of the following is NOT an example of a molecular formula? B, O 2, Cl 2, H 2 O 30. How many double bonds are in the Lewis structure for HF, hydrogen fluoride?

31. How many extra electrons are in the Lewis structure of the sulfate ion, SO 42 - ? 32. A chemical bond that results from the electrostatic attraction between cations and anions is called a(n)__________. 33. An octet is equal to ______ electrons.

The End ☻
- Slides: 14