CHAPTER 8 SALTS Meaning and uses of Salts
















- Slides: 128

CHAPTER 8 : SALTS

Meaning and uses of Salts A salt is an ionic compound formed when the hydrogen ion, from an acid is replaced by a metal ion or an ammonium ion l Example of salts : (i) sodium chloride (ii) potassium carbonate (iii) copper(II) sulphate l

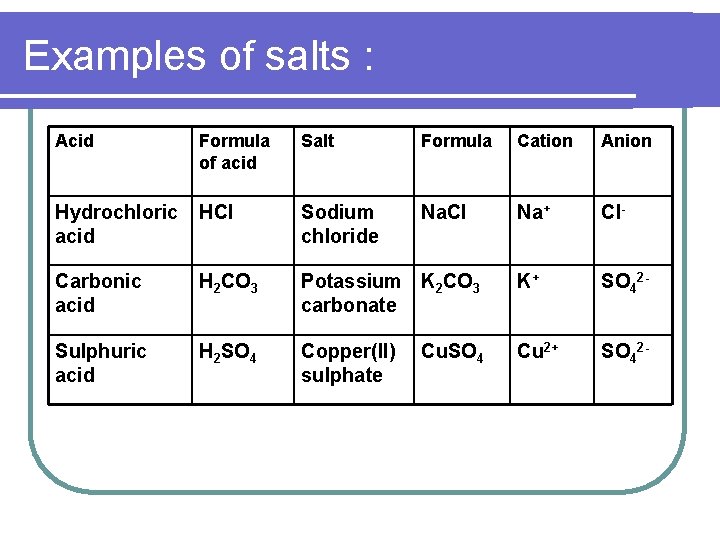
Examples of salts : Acid Formula of acid Salt Formula Cation Anion Hydrochloric acid HCl Sodium chloride Na. Cl Na+ Cl- Carbonic acid H 2 CO 3 Potassium K 2 CO 3 carbonate K+ SO 42 - Sulphuric acid H 2 SO 4 Copper(II) sulphate Cu 2+ SO 42 - Cu. SO 4

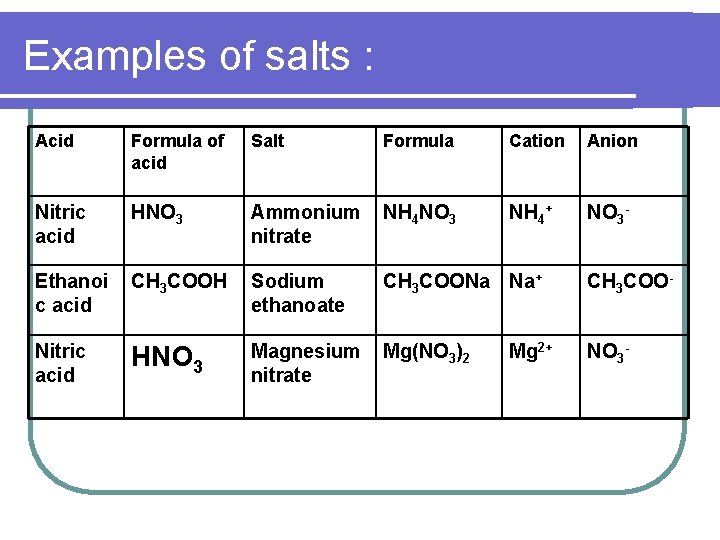
Examples of salts : Acid Formula of acid Salt Formula Cation Anion Nitric acid HNO 3 Ammonium nitrate NH 4 NO 3 NH 4+ NO 3 - Ethanoi c acid CH 3 COOH Sodium ethanoate CH 3 COONa Na+ CH 3 COO- HNO 3 Magnesium nitrate Mg(NO 3)2 NO 3 - Nitric acid Mg 2+

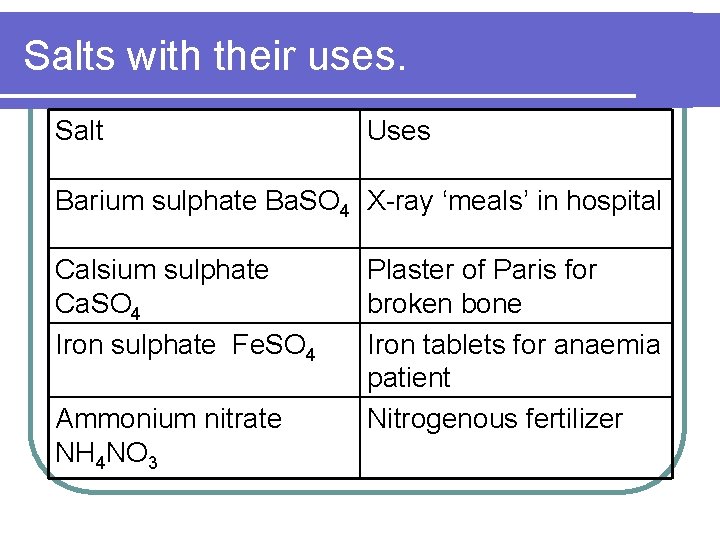
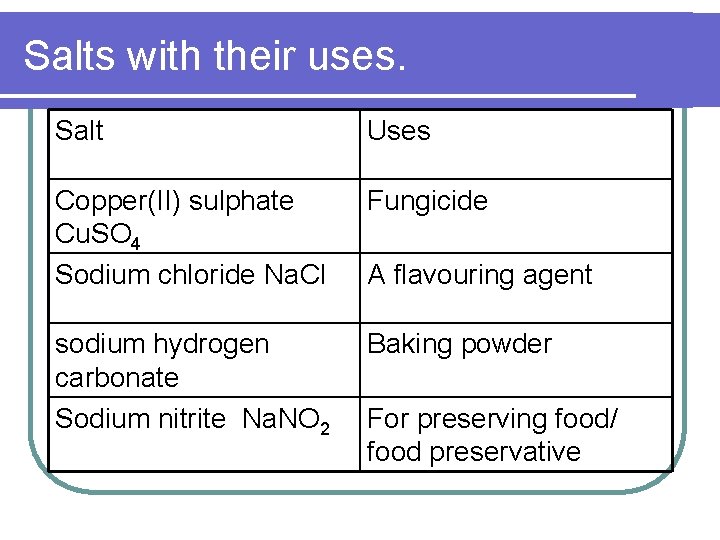
Salts with their uses. Salt Uses Barium sulphate Ba. SO 4 X-ray ‘meals’ in hospital Calsium sulphate Ca. SO 4 Iron sulphate Fe. SO 4 Ammonium nitrate NH 4 NO 3 Plaster of Paris for broken bone Iron tablets for anaemia patient Nitrogenous fertilizer

Salts with their uses. Salt Uses Copper(II) sulphate Cu. SO 4 Sodium chloride Na. Cl Fungicide sodium hydrogen carbonate Sodium nitrite Na. NO 2 Baking powder A flavouring agent For preserving food/ food preservative

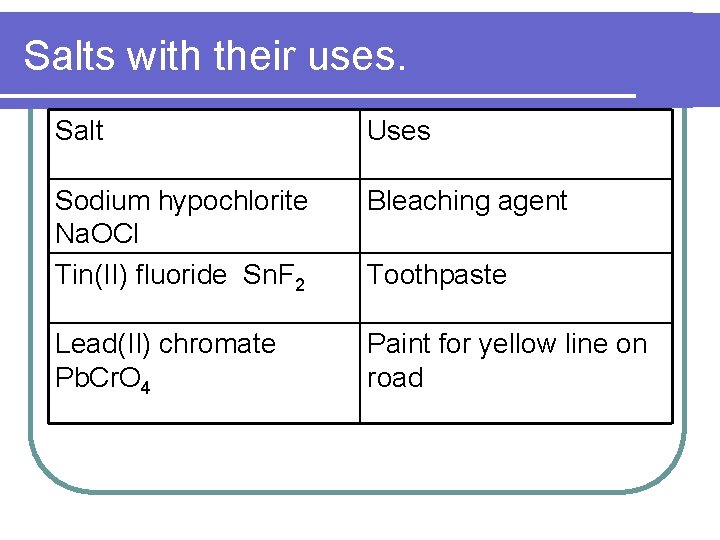
Salts with their uses. Salt Uses Sodium hypochlorite Na. OCl Tin(II) fluoride Sn. F 2 Bleaching agent Lead(II) chromate Pb. Cr. O 4 Paint for yellow line on road Toothpaste

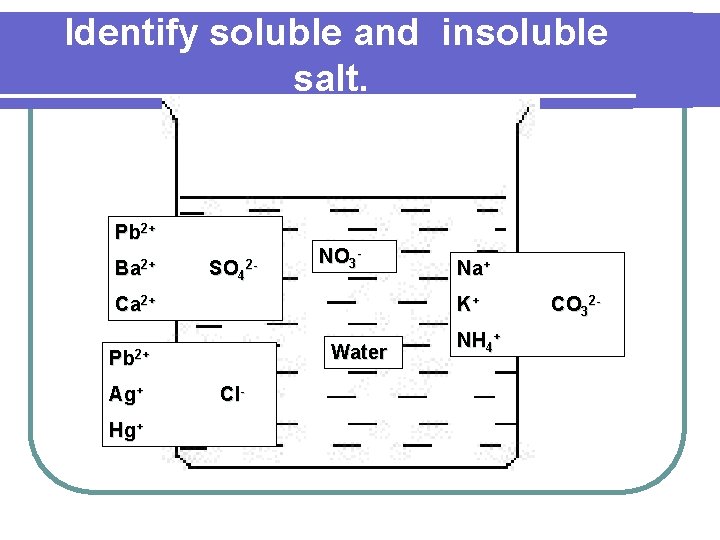
Identify soluble and insoluble salt. Pb 2+ Ba 2+ SO 42 - NO 3 - Ca 2+ K+ Water Pb 2+ Ag+ Hg+ Na+ Cl- NH 4+ CO 32 -

l l l All sodium, potassium and ammonium salts are soluble in water. All nitrate salts are soluble in water. All sulphate salts are soluble in water except lead(II) sulphate, barium sulphate and calcium sulphate. All chloride salts are soluble in water except lead(II) chloride, silver chloride and mercury chloride. All carbonate salts are insoluble in water except sodium carbonate, potassium carbonate and ammonium carbonate

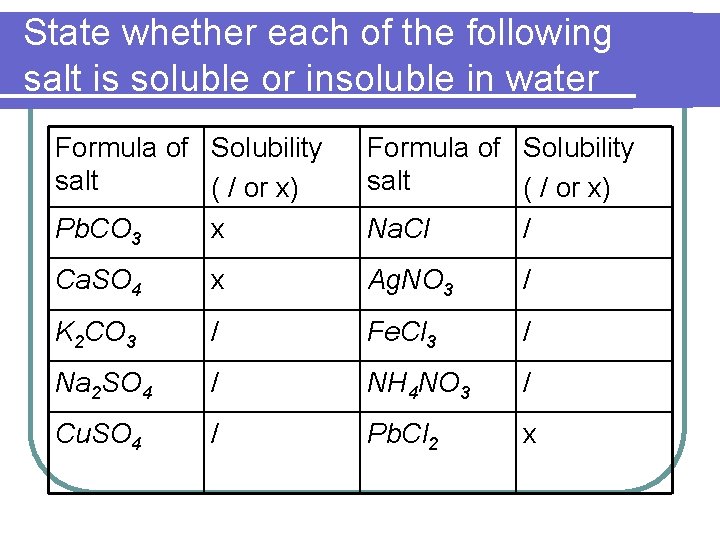
State whether each of the following salt is soluble or insoluble in water Formula of Solubility salt ( / or x) Pb. CO 3 x Formula of Solubility salt ( / or x) Na. Cl / Ca. SO 4 x Ag. NO 3 / K 2 CO 3 / Fe. Cl 3 / Na 2 SO 4 / NH 4 NO 3 / Cu. SO 4 / Pb. Cl 2 x

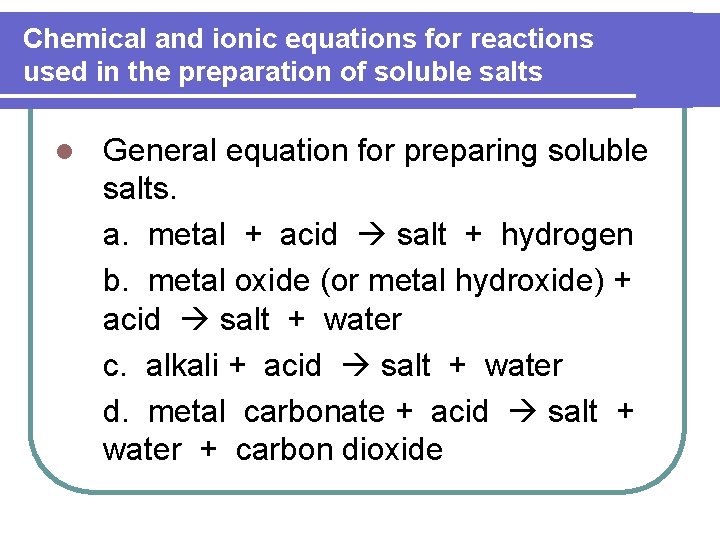
Chemical and ionic equations for reactions used in the preparation of soluble salts l General equation for preparing soluble salts. a. metal + acid salt + hydrogen b. metal oxide (or metal hydroxide) + acid salt + water c. alkali + acid salt + water d. metal carbonate + acid salt + water + carbon dioxide

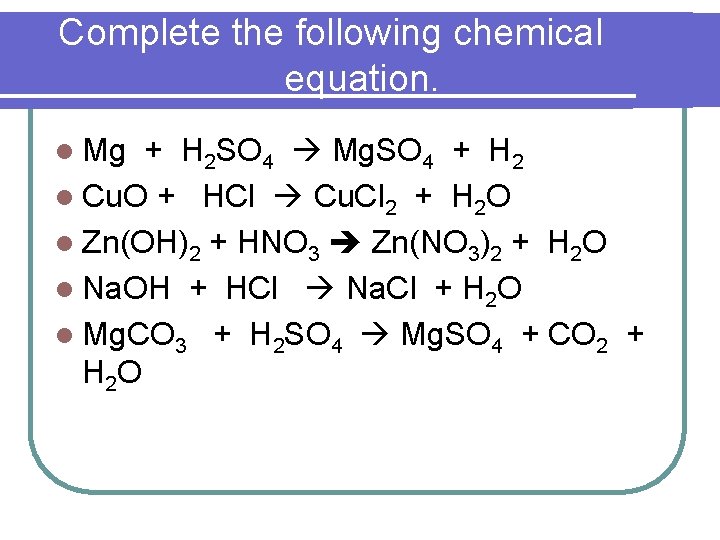
Complete the following chemical equation. l Mg + H 2 SO 4 Mg. SO 4 + H 2 l Cu. O + HCl Cu. Cl 2 + H 2 O l Zn(OH)2 + HNO 3 Zn(NO 3)2 + H 2 O l Na. OH + HCl Na. Cl + H 2 O l Mg. CO 3 + H 2 SO 4 Mg. SO 4 + CO 2 + H 2 O

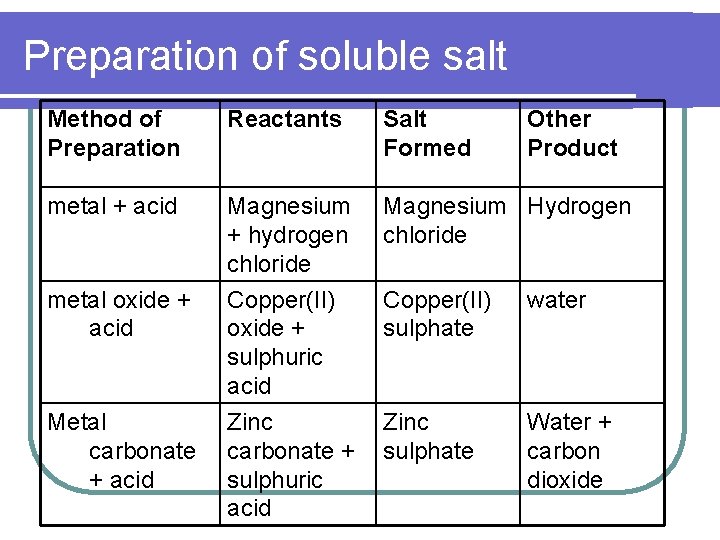
Preparation of soluble salt Method of Preparation Reactants Salt Formed metal + acid Magnesium + hydrogen chloride Magnesium Hydrogen chloride metal oxide + acid Copper(II) oxide + sulphuric acid Zinc carbonate + sulphuric acid Copper(II) sulphate water Zinc sulphate Water + carbon dioxide Metal carbonate + acid Other Product

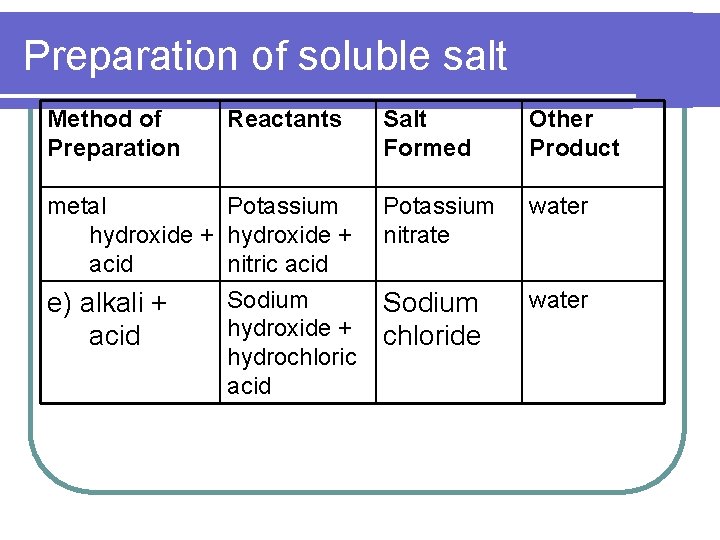
Preparation of soluble salt Method of Preparation Reactants metal Potassium hydroxide + acid nitric acid Sodium e) alkali + hydroxide + acid hydrochloric acid Salt Formed Other Product Potassium nitrate water Sodium chloride water

The reactants which are needed to prepare the following soluble salts: l l l Copper(II) sulphate : Copper(II) oxide / hydroxide / carbonate + sulphuric acid Zinc chloride : Zinc / (zinc oxide / hydroxide / carbonate) + hydrochloric acid Potassium nitrate : potassium hydroxide + nitric acid Ammonium sulphate : aqueous ammonia + sulphuric acid Magnesium nitrate : Magnesium / (magnesium oxide / hydroxide / carbonate) + nitric acid

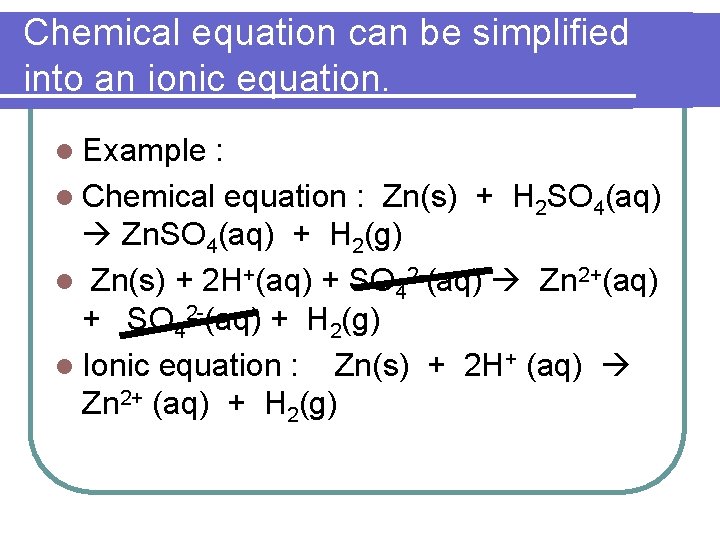
Chemical equation can be simplified into an ionic equation. l Example : l Chemical equation : Zn(s) + H 2 SO 4(aq) Zn. SO 4(aq) + H 2(g) l Zn(s) + 2 H+(aq) + SO 42 -(aq) Zn 2+(aq) + SO 42 -(aq) + H 2(g) l Ionic equation : Zn(s) + 2 H+ (aq) Zn 2+ (aq) + H 2(g)

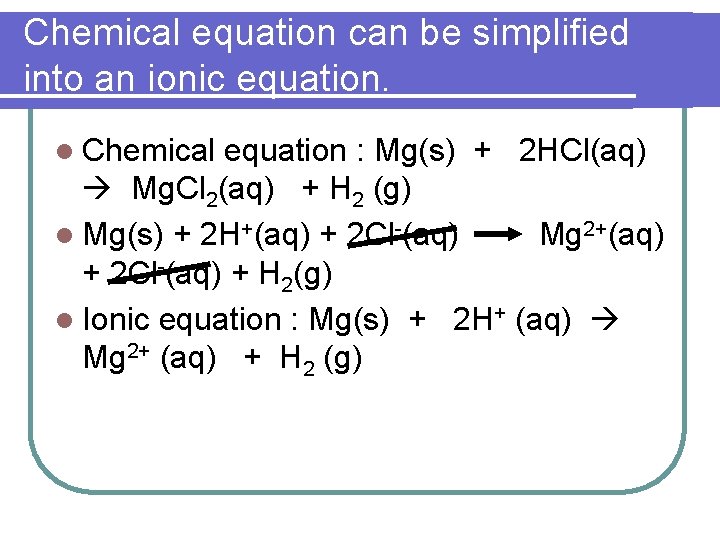
Chemical equation can be simplified into an ionic equation. l Chemical equation : Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 (g) l Mg(s) + 2 H+(aq) + 2 Cl-(aq) Mg 2+(aq) + 2 Cl-(aq) + H 2(g) l Ionic equation : Mg(s) + 2 H+ (aq) Mg 2+ (aq) + H 2 (g)

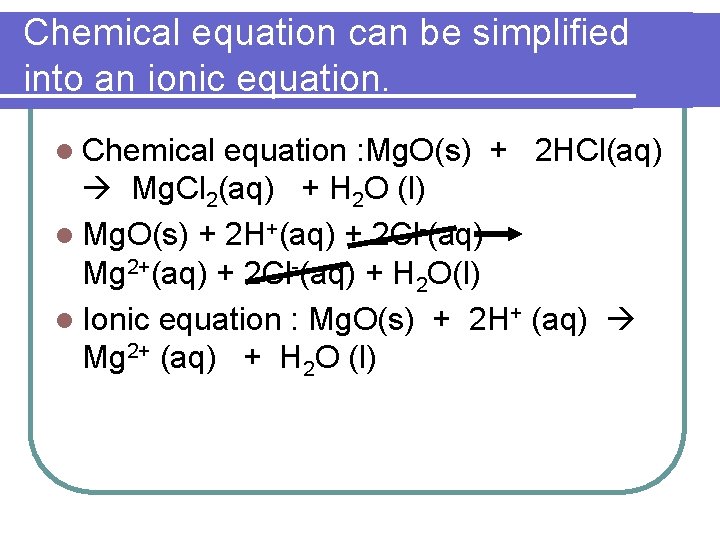
Chemical equation can be simplified into an ionic equation. l Chemical equation : Mg. O(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 O (l) l Mg. O(s) + 2 H+(aq) + 2 Cl-(aq) Mg 2+(aq) + 2 Cl-(aq) + H 2 O(l) l Ionic equation : Mg. O(s) + 2 H+ (aq) Mg 2+ (aq) + H 2 O (l)

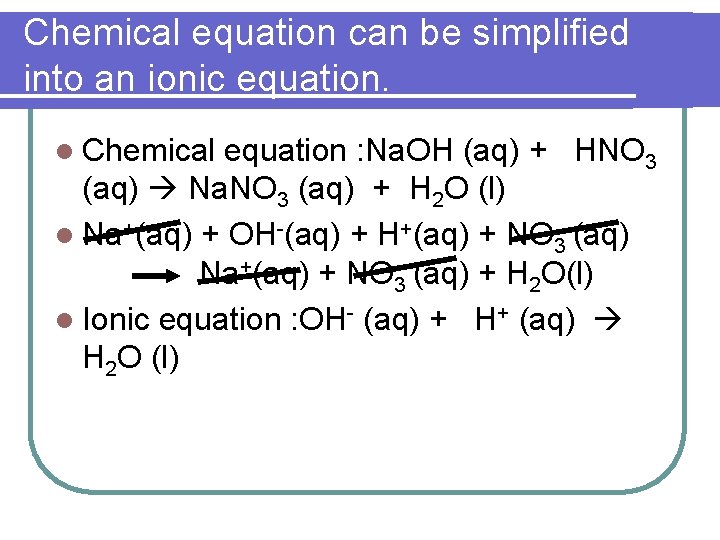
Chemical equation can be simplified into an ionic equation. l Chemical equation : Na. OH (aq) + HNO 3 (aq) Na. NO 3 (aq) + H 2 O (l) l Na+(aq) + OH-(aq) + H+(aq) + NO 3 -(aq) Na+(aq) + NO 3 -(aq) + H 2 O(l) l Ionic equation : OH- (aq) + H+ (aq) H 2 O (l)

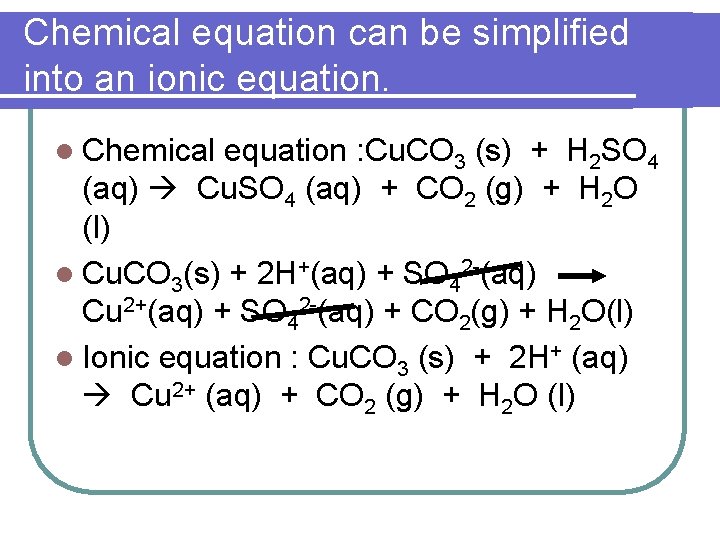
Chemical equation can be simplified into an ionic equation. l Chemical equation : Cu. CO 3 (s) + H 2 SO 4 (aq) Cu. SO 4 (aq) + CO 2 (g) + H 2 O (l) l Cu. CO 3(s) + 2 H+(aq) + SO 42 -(aq) Cu 2+(aq) + SO 42 -(aq) + CO 2(g) + H 2 O(l) l Ionic equation : Cu. CO 3 (s) + 2 H+ (aq) Cu 2+ (aq) + CO 2 (g) + H 2 O (l)

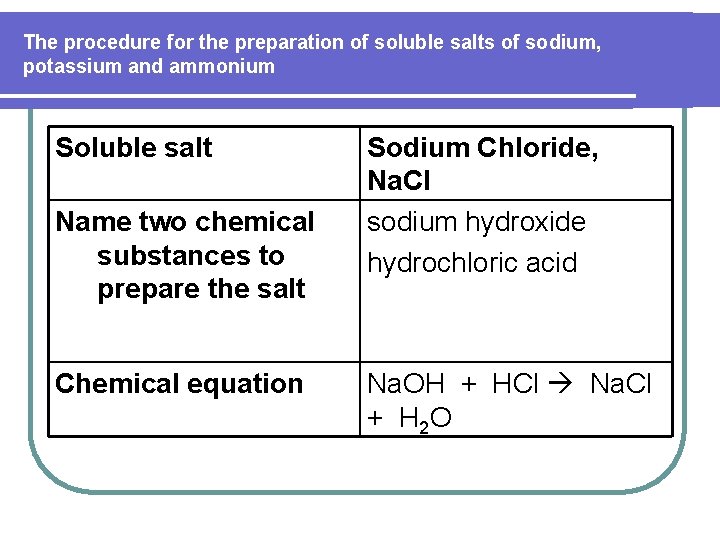
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Soluble salt Name two chemical substances to prepare the salt Chemical equation Sodium Chloride, Na. Cl sodium hydroxide hydrochloric acid Na. OH + HCl Na. Cl + H 2 O

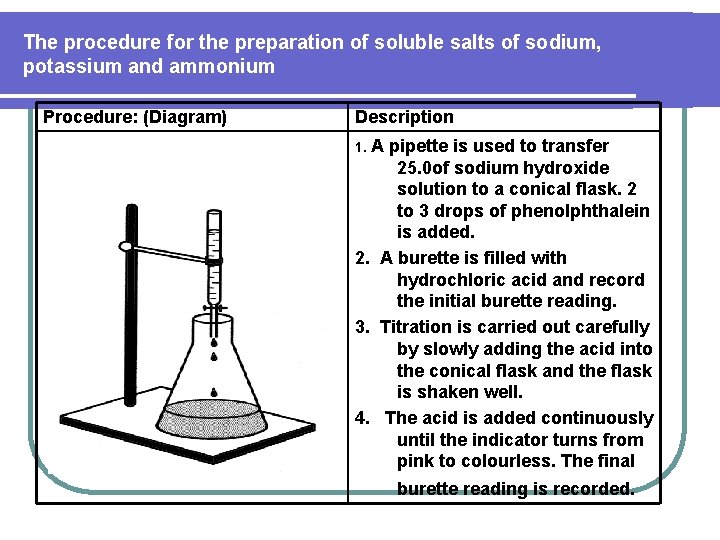
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 1. A pipette is used to transfer 25. 0 of sodium hydroxide solution to a conical flask. 2 to 3 drops of phenolphthalein is added. 2. A burette is filled with hydrochloric acid and record the initial burette reading. 3. Titration is carried out carefully by slowly adding the acid into the conical flask and the flask is shaken well. 4. The acid is added continuously until the indicator turns from pink to colourless. The final burette reading is recorded.

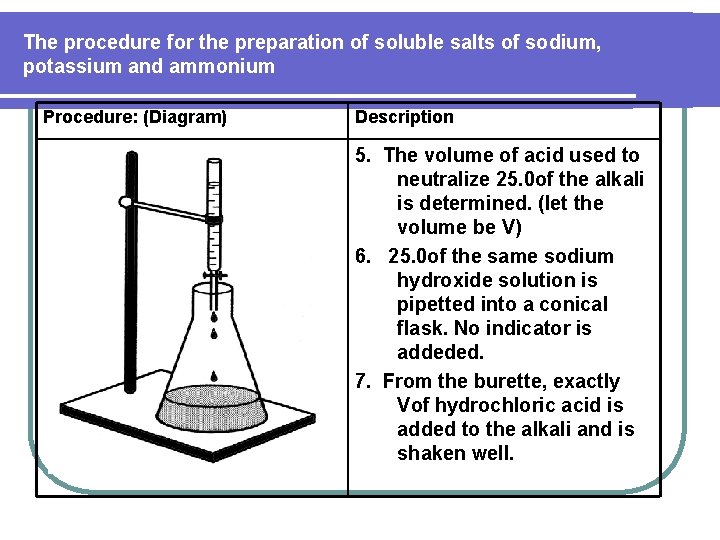
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 5. The volume of acid used to neutralize 25. 0 of the alkali is determined. (let the volume be V) 6. 25. 0 of the same sodium hydroxide solution is pipetted into a conical flask. No indicator is addeded. 7. From the burette, exactly Vof hydrochloric acid is added to the alkali and is shaken well.

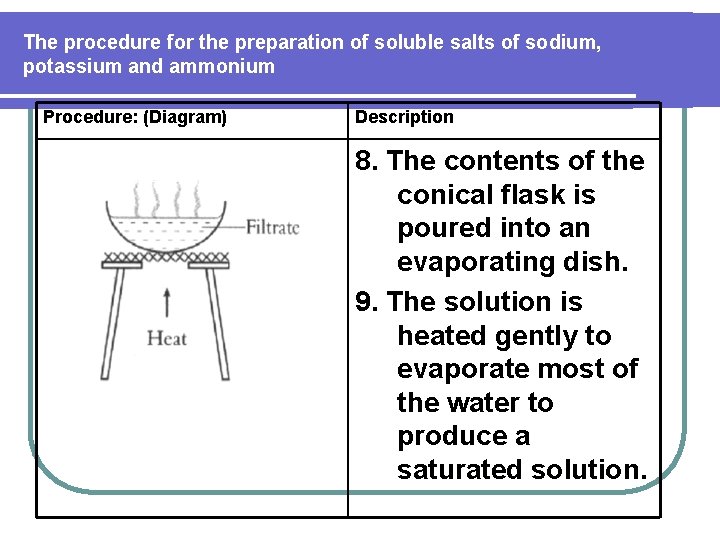
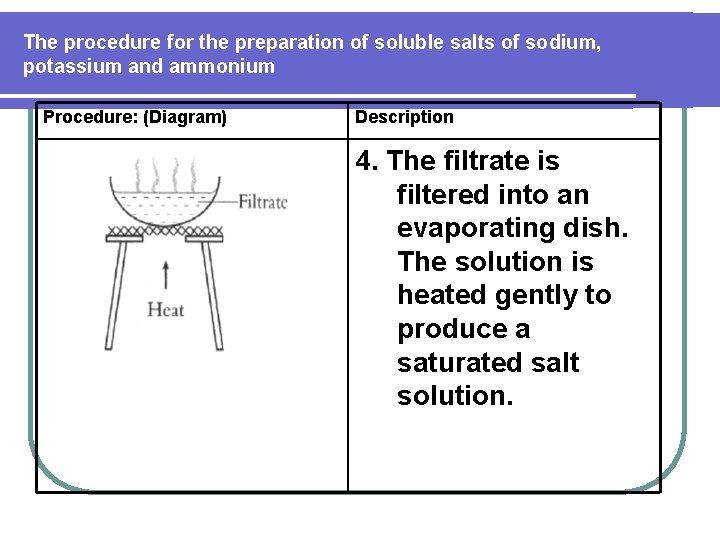
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 8. The contents of the conical flask is poured into an evaporating dish. 9. The solution is heated gently to evaporate most of the water to produce a saturated solution.

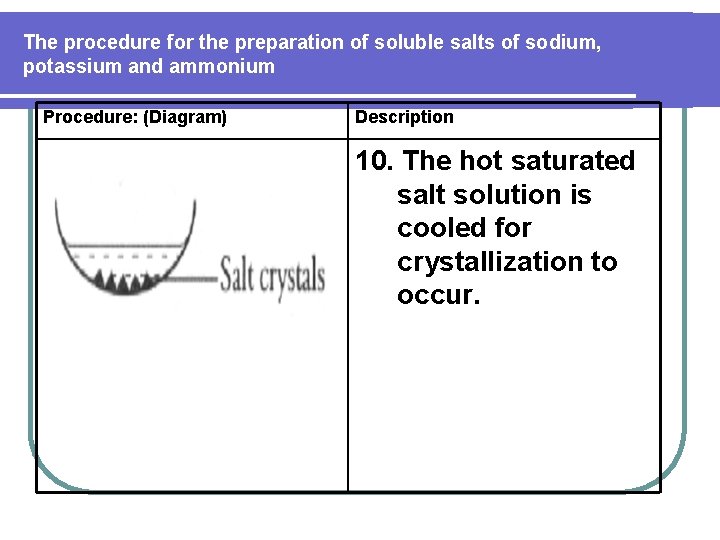
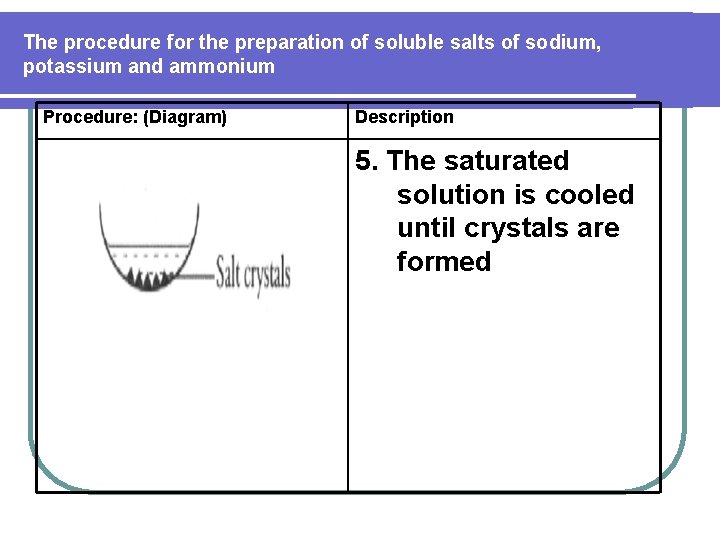
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 10. The hot saturated salt solution is cooled for crystallization to occur.

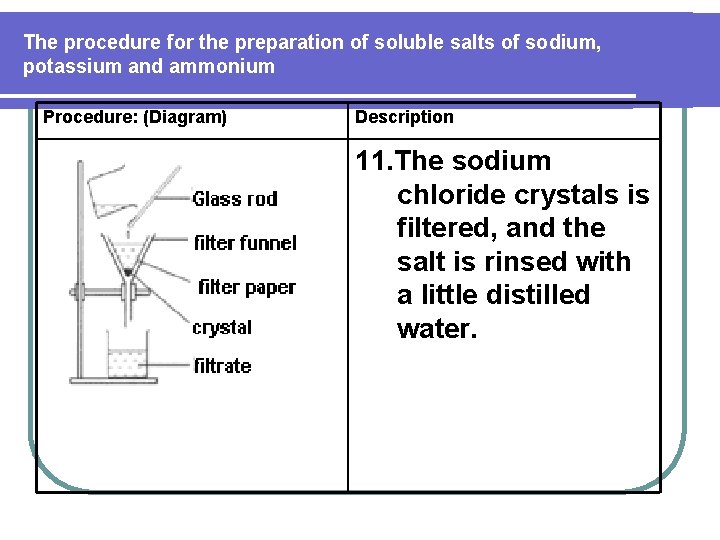
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 11. The sodium chloride crystals is filtered, and the salt is rinsed with a little distilled water.

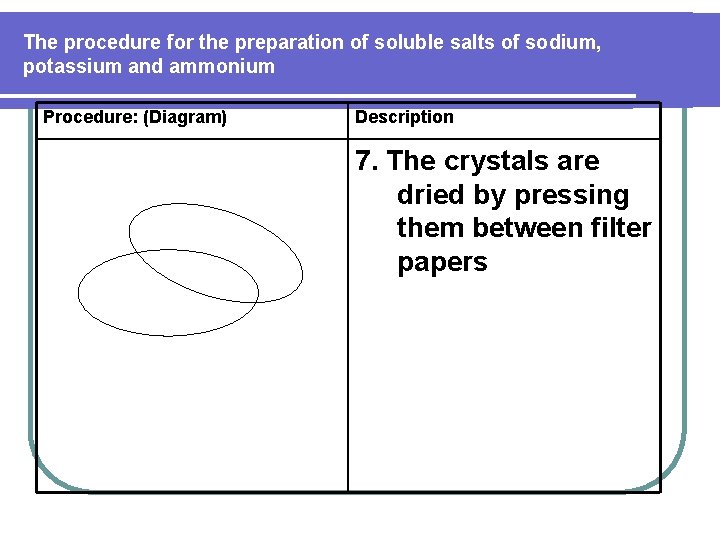
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 12. The crystals are dried by pressing them between filter papers

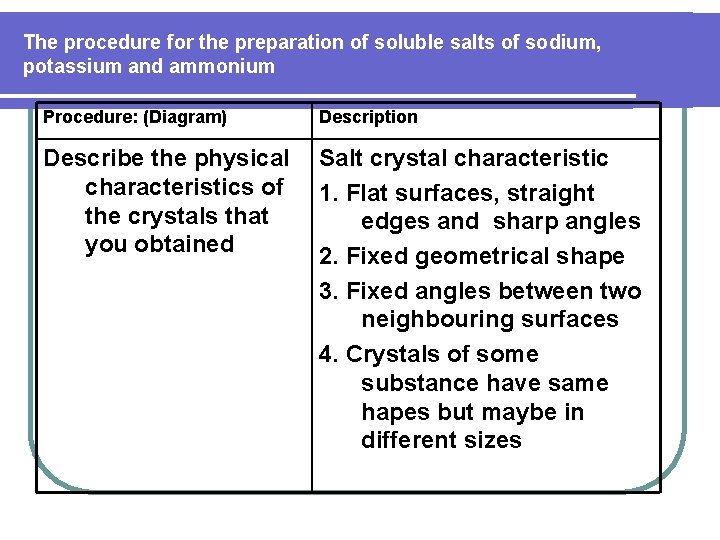
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description Describe the physical characteristics of the crystals that you obtained Salt crystal characteristic 1. Flat surfaces, straight edges and sharp angles 2. Fixed geometrical shape 3. Fixed angles between two neighbouring surfaces 4. Crystals of some substance have same hapes but maybe in different sizes

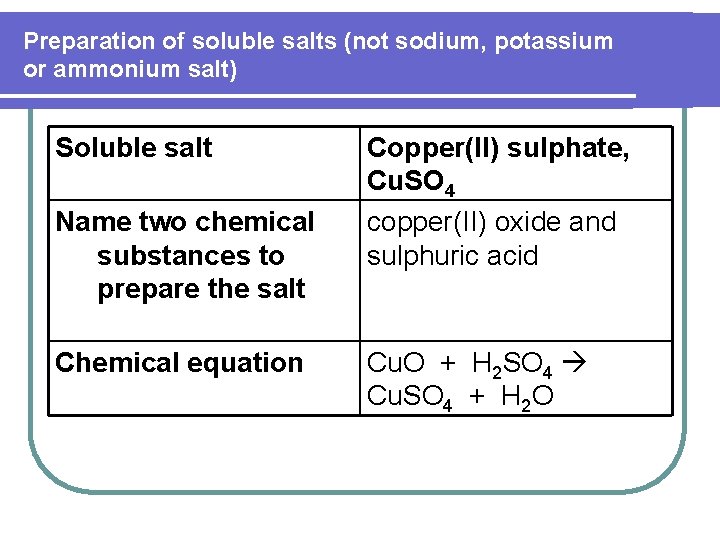
Preparation of soluble salts (not sodium, potassium or ammonium salt) Soluble salt Name two chemical substances to prepare the salt Chemical equation Copper(II) sulphate, Cu. SO 4 copper(II) oxide and sulphuric acid Cu. O + H 2 SO 4 Cu. SO 4 + H 2 O

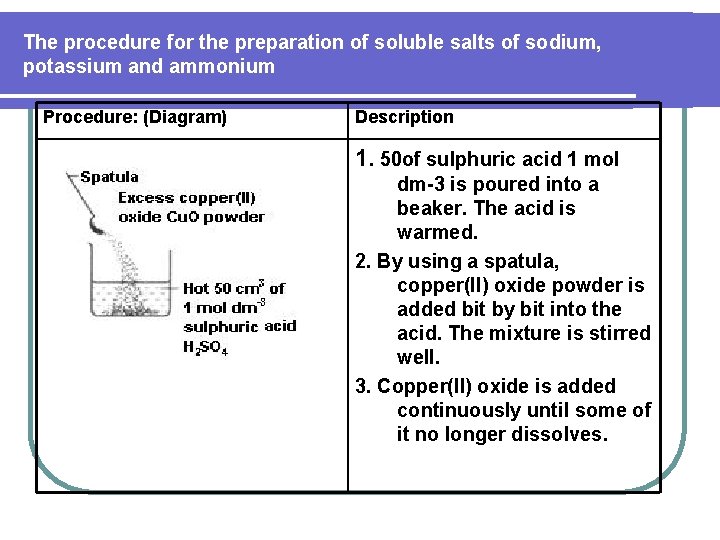
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 1. 50 of sulphuric acid 1 mol dm-3 is poured into a beaker. The acid is warmed. 2. By using a spatula, copper(II) oxide powder is added bit by bit into the acid. The mixture is stirred well. 3. Copper(II) oxide is added continuously until some of it no longer dissolves.

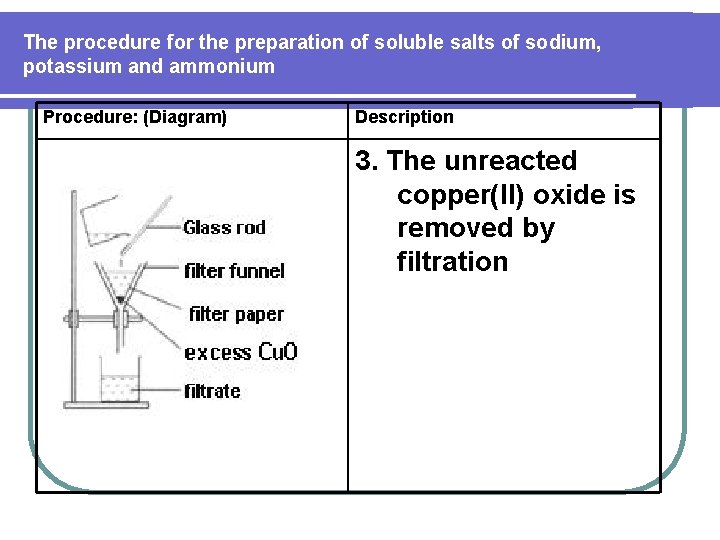
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 3. The unreacted copper(II) oxide is removed by filtration

The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 4. The filtrate is filtered into an evaporating dish. The solution is heated gently to produce a saturated salt solution.

The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 5. The saturated solution is cooled until crystals are formed

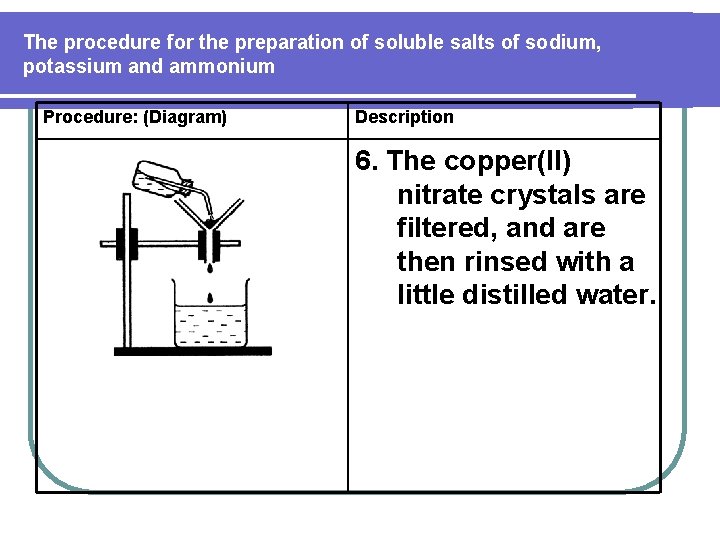
The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 6. The copper(II) nitrate crystals are filtered, and are then rinsed with a little distilled water.

The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description 7. The crystals are dried by pressing them between filter papers

The procedure for the preparation of soluble salts of sodium, potassium and ammonium Procedure: (Diagram) Description Describe the purification process of the crystals Purification process – Recrystallisation 1. The copper(II) sulphate crystals are placed in a beaker. 2. Enough distilled water is added to cover the crystals. The solution is gently heated and stirred with a glass rod. Water is added bit by bit until all the crystals are dissolved. 3. Impurities is removed by filtration and filtrate is poured into an evaporating dish. 4. The solution is heated gently to evaporate most of the water to produce a saturated solution. 5. The hot saturated salt solution is cooled for crystallization to occur. 6. The copper(II) nitrate crystals are filtered, and the salt is rinsed with a little distilled water. 7. The crystals are dried by pressing them between filter papers

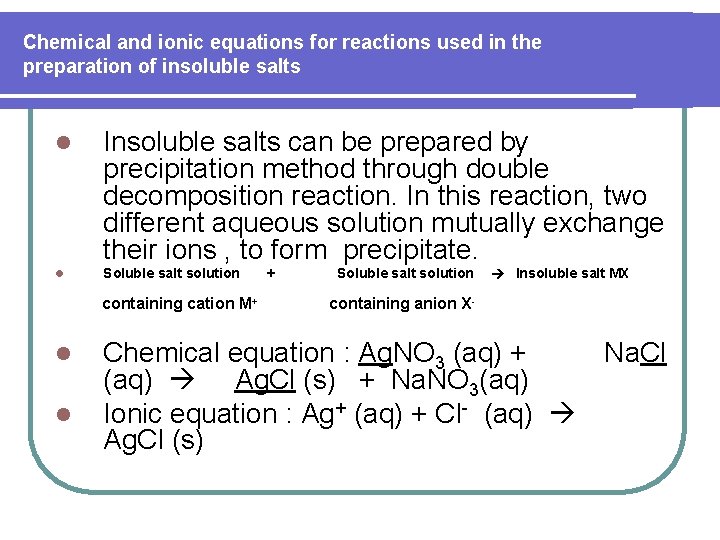
Chemical and ionic equations for reactions used in the preparation of insoluble salts l Insoluble salts can be prepared by precipitation method through double decomposition reaction. In this reaction, two different aqueous solution mutually exchange their ions , to form precipitate. l Soluble salt solution containing cation M+ l l + Soluble salt solution Insoluble salt MX containing anion X- Chemical equation : Ag. NO 3 (aq) + (aq) Ag. Cl (s) + Na. NO 3(aq) Ionic equation : Ag+ (aq) + Cl- (aq) Ag. Cl (s) Na. Cl

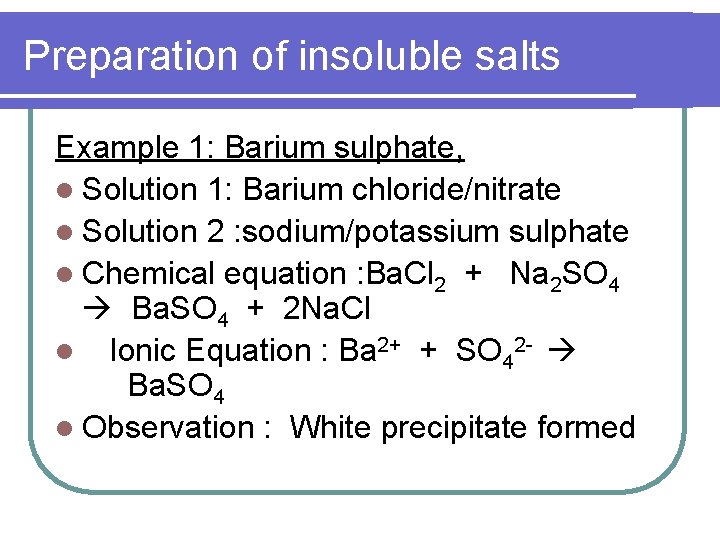
Preparation of insoluble salts Example 1: Barium sulphate, l Solution 1: Barium chloride/nitrate l Solution 2 : sodium/potassium sulphate l Chemical equation : Ba. Cl 2 + Na 2 SO 4 Ba. SO 4 + 2 Na. Cl l Ionic Equation : Ba 2+ + SO 42 - Ba. SO 4 l Observation : White precipitate formed

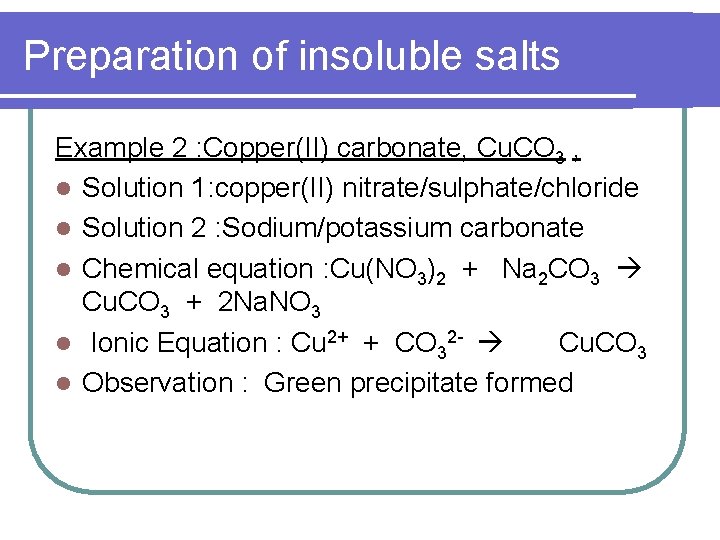
Preparation of insoluble salts Example 2 : Copper(II) carbonate, Cu. CO 3 , l Solution 1: copper(II) nitrate/sulphate/chloride l Solution 2 : Sodium/potassium carbonate l Chemical equation : Cu(NO 3)2 + Na 2 CO 3 Cu. CO 3 + 2 Na. NO 3 l Ionic Equation : Cu 2+ + CO 32 - Cu. CO 3 l Observation : Green precipitate formed

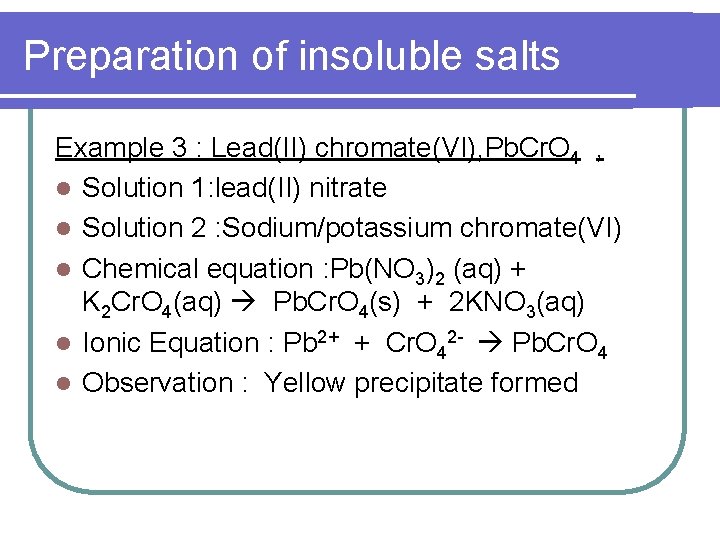
Preparation of insoluble salts Example 3 : Lead(II) chromate(VI), Pb. Cr. O 4 , l Solution 1: lead(II) nitrate l Solution 2 : Sodium/potassium chromate(VI) l Chemical equation : Pb(NO 3)2 (aq) + K 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 KNO 3(aq) l Ionic Equation : Pb 2+ + Cr. O 42 - Pb. Cr. O 4 l Observation : Yellow precipitate formed

The preparation of insoluble salts Insoluble salt Name Lead(II) iodide, Pb. I 2 Two chemical (i) Lead(II) nitrate substances to prepare (ii) Sodium/potas the salt sium iodide

The preparation of insoluble salts Chemical equation Pb(NO 3)2 (aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3 (aq) Ionic equation Pb 2+(aq) + 2 I-(aq) Pb. I 2(s)

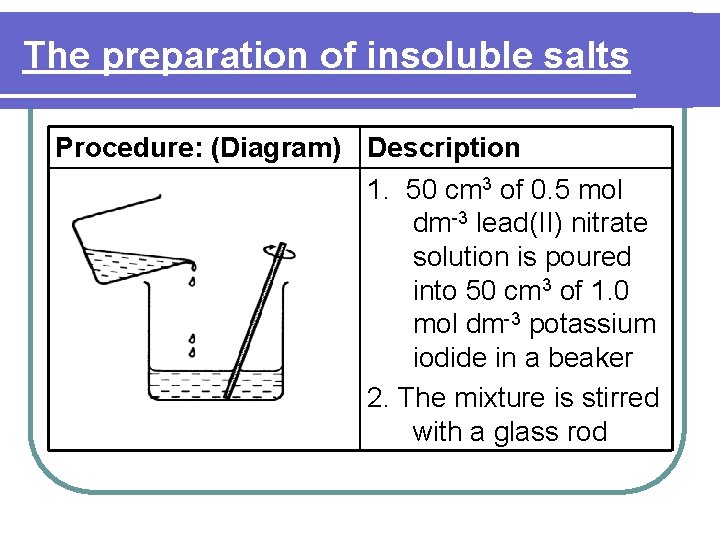
The preparation of insoluble salts Procedure: (Diagram) Description 1. 50 cm 3 of 0. 5 mol dm-3 lead(II) nitrate solution is poured into 50 cm 3 of 1. 0 mol dm-3 potassium iodide in a beaker 2. The mixture is stirred with a glass rod

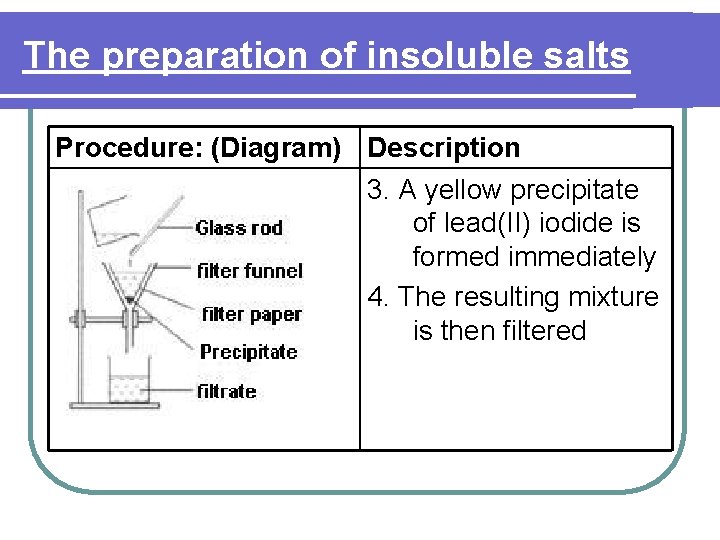
The preparation of insoluble salts Procedure: (Diagram) Description 3. A yellow precipitate of lead(II) iodide is formed immediately 4. The resulting mixture is then filtered

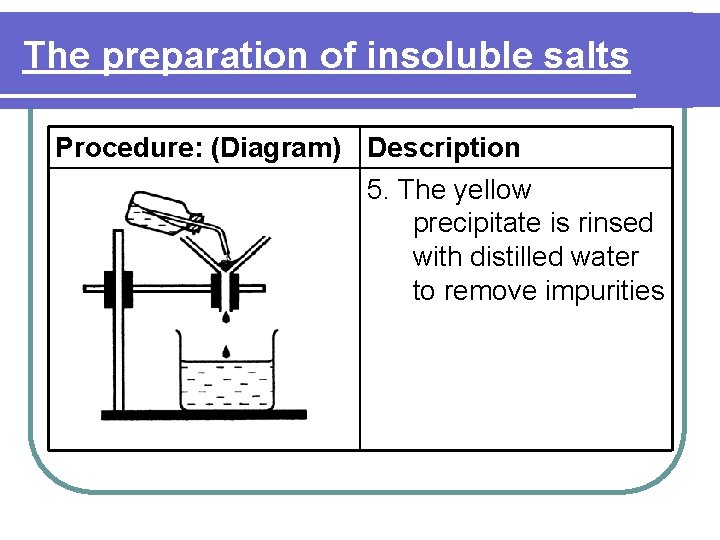
The preparation of insoluble salts Procedure: (Diagram) Description 5. The yellow precipitate is rinsed with distilled water to remove impurities

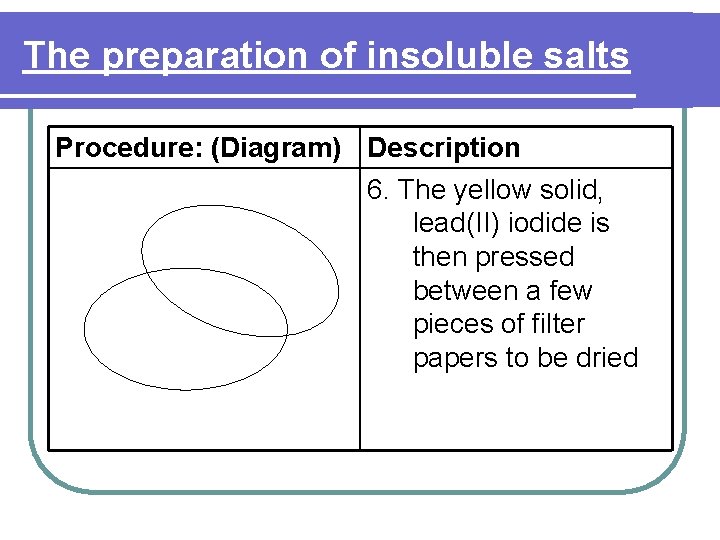
The preparation of insoluble salts Procedure: (Diagram) Description 6. The yellow solid, lead(II) iodide is then pressed between a few pieces of filter papers to be dried

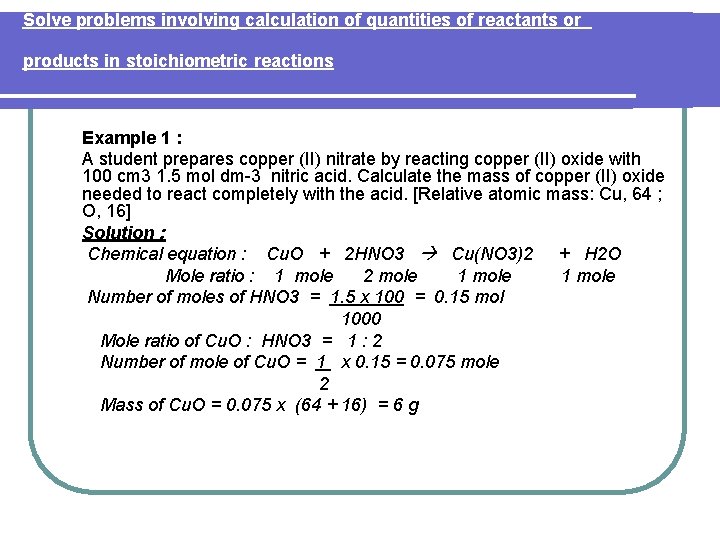
Solve problems involving calculation of quantities of reactants or products in stoichiometric reactions Example 1 : A student prepares copper (II) nitrate by reacting copper (II) oxide with 100 cm 3 1. 5 mol dm-3 nitric acid. Calculate the mass of copper (II) oxide needed to react completely with the acid. [Relative atomic mass: Cu, 64 ; O, 16] Solution : Chemical equation : Cu. O + 2 HNO 3 Cu(NO 3)2 + H 2 O Mole ratio : 1 mole 2 mole 1 mole Number of moles of HNO 3 = 1. 5 x 100 = 0. 15 mol 1000 Mole ratio of Cu. O : HNO 3 = 1 : 2 Number of mole of Cu. O = 1 x 0. 15 = 0. 075 mole 2 Mass of Cu. O = 0. 075 x (64 + 16) = 6 g

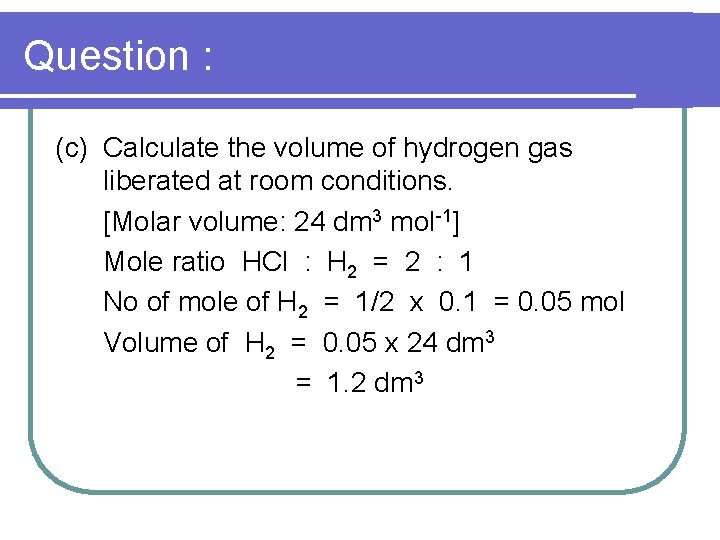
Question : 1. Excess zinc powder is added to react completely with 50 cm 3 of 2. 0 mol dm-3 hydrochloric acid. (a) Write an ionic equation for the reaction between zinc and hydrochloric acid. Zn + 2 HCl Zn. Cl 2 + H 2

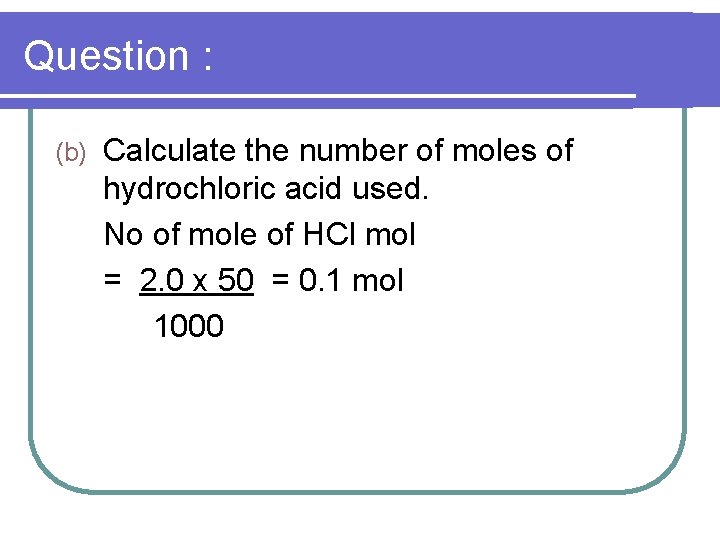
Question : (b) Calculate the number of moles of hydrochloric acid used. No of mole of HCl mol = 2. 0 x 50 = 0. 1 mol 1000

Question : (c) Calculate the volume of hydrogen gas liberated at room conditions. [Molar volume: 24 dm 3 mol-1] Mole ratio HCl : H 2 = 2 : 1 No of mole of H 2 = 1/2 x 0. 1 = 0. 05 mol Volume of H 2 = 0. 05 x 24 dm 3 = 1. 2 dm 3

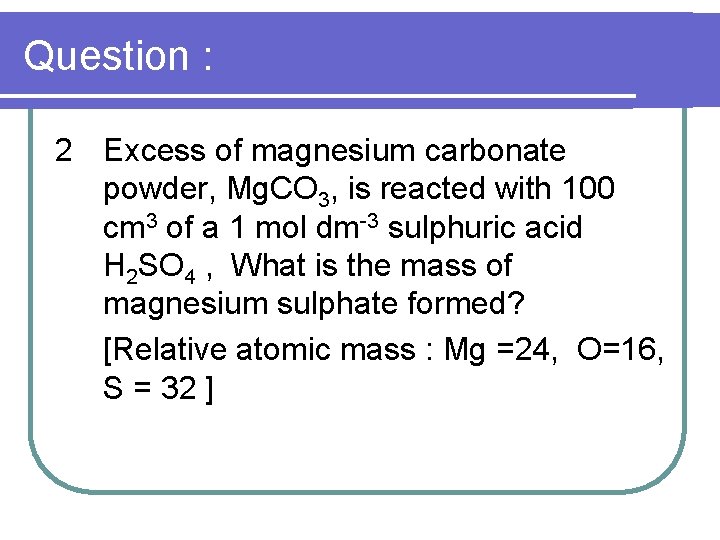
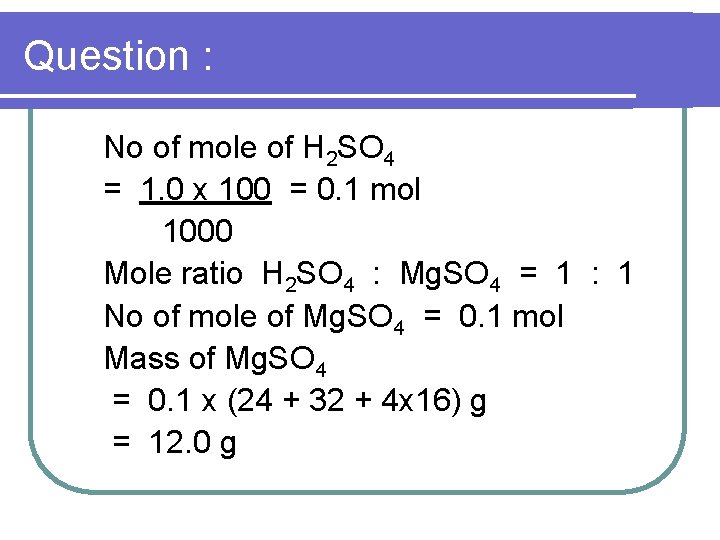
Question : 2 Excess of magnesium carbonate powder, Mg. CO 3, is reacted with 100 cm 3 of a 1 mol dm-3 sulphuric acid H 2 SO 4 , What is the mass of magnesium sulphate formed? [Relative atomic mass : Mg =24, O=16, S = 32 ]

Question : No of mole of H 2 SO 4 = 1. 0 x 100 = 0. 1 mol 1000 Mole ratio H 2 SO 4 : Mg. SO 4 = 1 : 1 No of mole of Mg. SO 4 = 0. 1 mol Mass of Mg. SO 4 = 0. 1 x (24 + 32 + 4 x 16) g = 12. 0 g

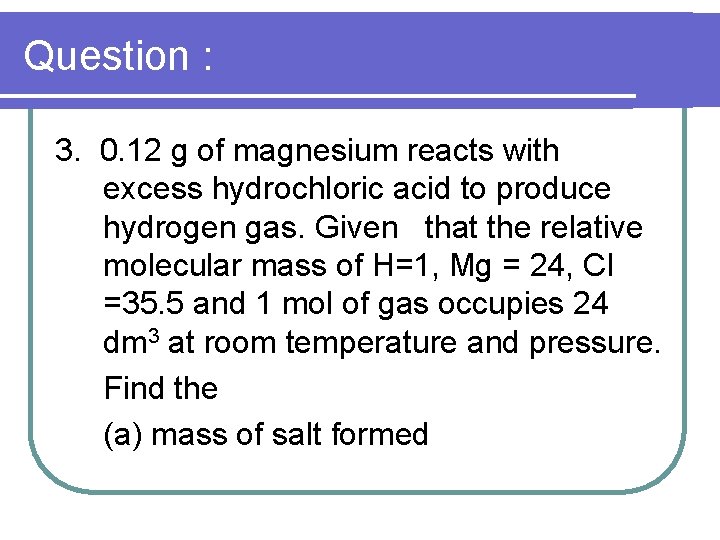
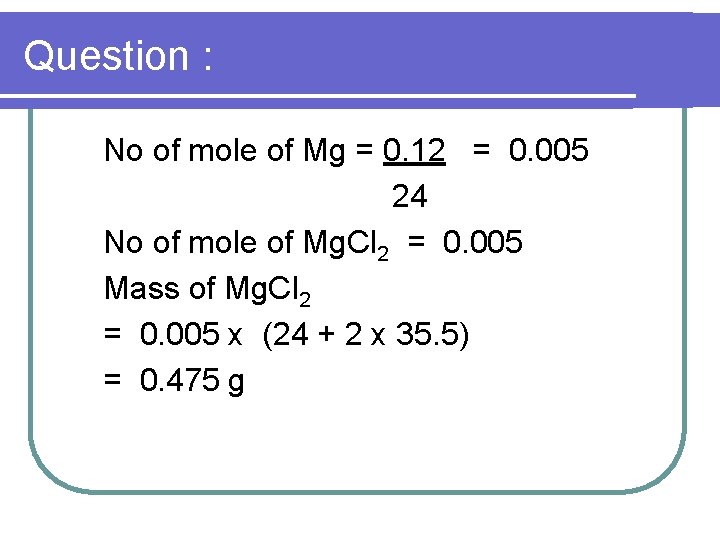
Question : 3. 0. 12 g of magnesium reacts with excess hydrochloric acid to produce hydrogen gas. Given that the relative molecular mass of H=1, Mg = 24, CI =35. 5 and 1 mol of gas occupies 24 dm 3 at room temperature and pressure. Find the (a) mass of salt formed

Question : No of mole of Mg = 0. 12 = 0. 005 24 No of mole of Mg. Cl 2 = 0. 005 Mass of Mg. Cl 2 = 0. 005 x (24 + 2 x 35. 5) = 0. 475 g

Question : (b) volume of gas produced No of mole of H 2 = 0. 005 mole Volume of H 2 = 0. 005 x 24 dm 3 = 0. 12 dm 3 or 120 cm 3

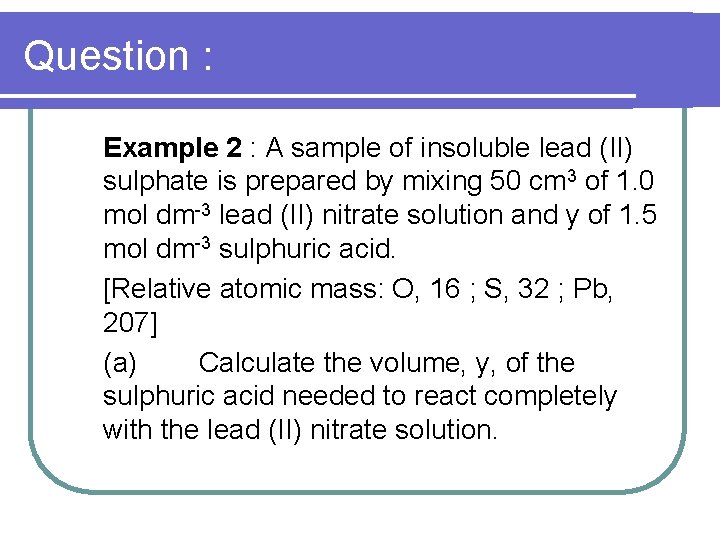
Question : Example 2 : A sample of insoluble lead (II) sulphate is prepared by mixing 50 cm 3 of 1. 0 mol dm-3 lead (II) nitrate solution and y of 1. 5 mol dm-3 sulphuric acid. [Relative atomic mass: O, 16 ; S, 32 ; Pb, 207] (a) Calculate the volume, y, of the sulphuric acid needed to react completely with the lead (II) nitrate solution.

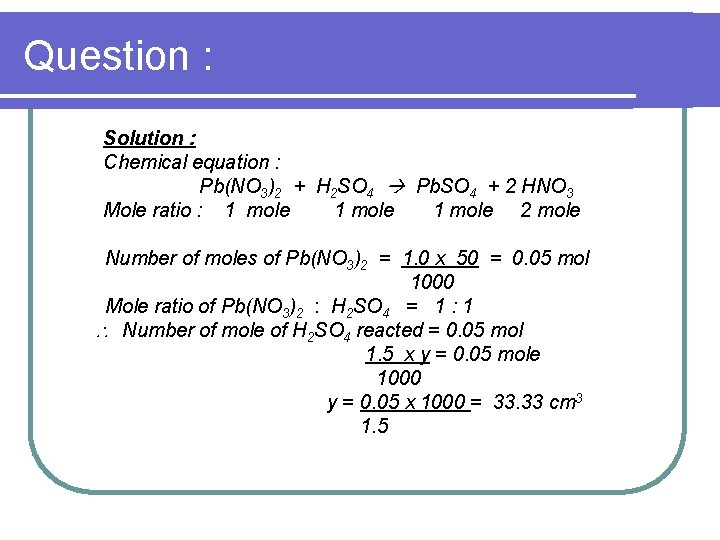
Question : Solution : Chemical equation : Pb(NO 3)2 + H 2 SO 4 Pb. SO 4 + 2 HNO 3 Mole ratio : 1 mole 2 mole Number of moles of Pb(NO 3)2 = 1. 0 x 50 = 0. 05 mol 1000 Mole ratio of Pb(NO 3)2 : H 2 SO 4 = 1 : 1 Number of mole of H 2 SO 4 reacted = 0. 05 mol 1. 5 x y = 0. 05 mole 1000 y = 0. 05 x 1000 = 33. 33 cm 3 1. 5

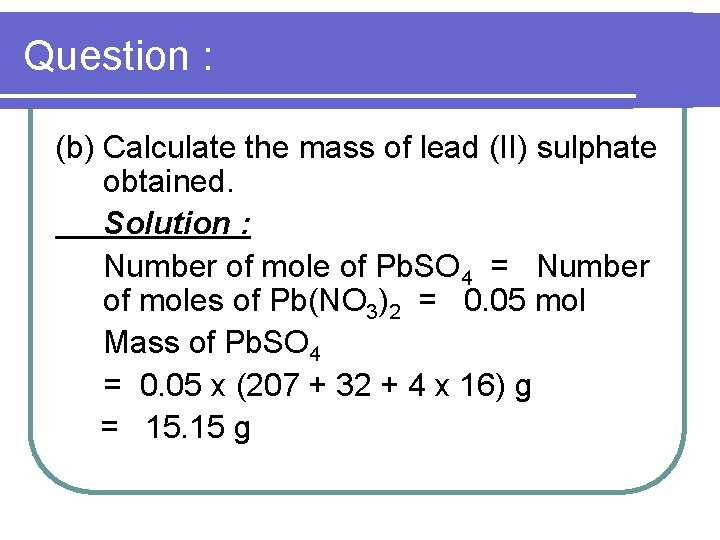
Question : (b) Calculate the mass of lead (II) sulphate obtained. Solution : Number of mole of Pb. SO 4 = Number of moles of Pb(NO 3)2 = 0. 05 mol Mass of Pb. SO 4 = 0. 05 x (207 + 32 + 4 x 16) g = 15. 15 g

Question : 4. A sample of insoluble silver chloride is prepared by mixing 50 cm 3 of 1. 0 mol dm-3 silver nitrate solution and z cm 3 of 0. 5 mol dm-3 sodium chloride solution. [Relative atomic mass: Ag 108; Cl 35. 5] (a) Write the chemical equation for the reaction between silver nitrate and sodium chloride. Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3

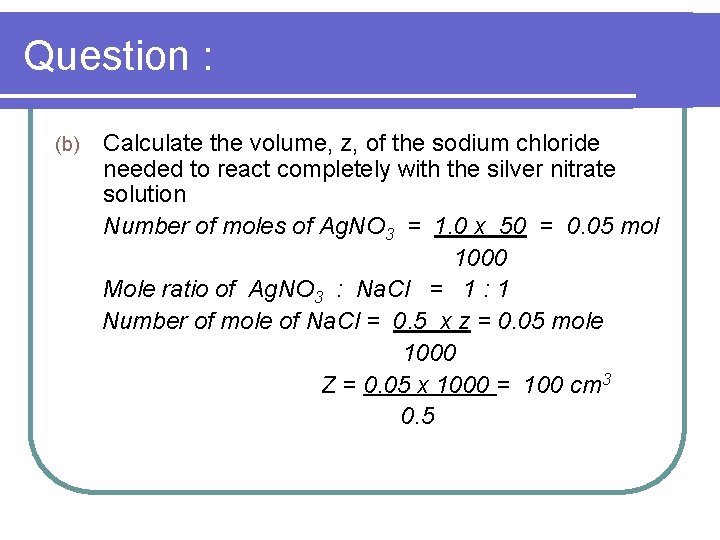
Question : (b) Calculate the volume, z, of the sodium chloride needed to react completely with the silver nitrate solution Number of moles of Ag. NO 3 = 1. 0 x 50 = 0. 05 mol 1000 Mole ratio of Ag. NO 3 : Na. Cl = 1 : 1 Number of mole of Na. Cl = 0. 5 x z = 0. 05 mole 1000 Z = 0. 05 x 1000 = 100 cm 3 0. 5

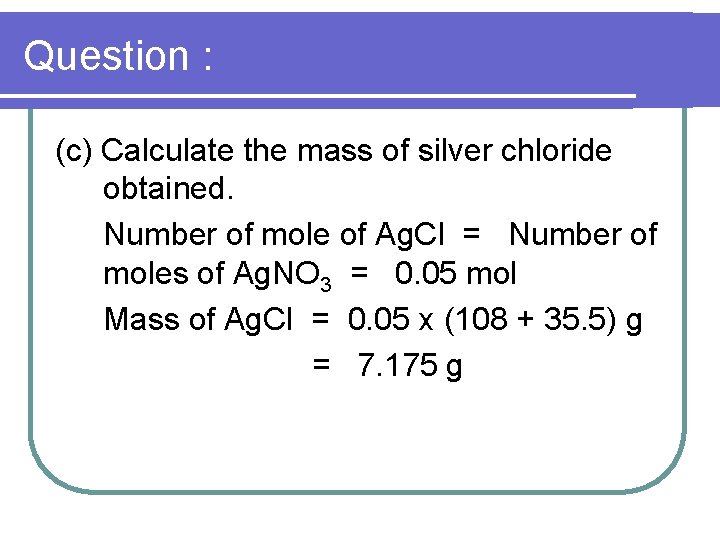
Question : (c) Calculate the mass of silver chloride obtained. Number of mole of Ag. Cl = Number of moles of Ag. NO 3 = 0. 05 mol Mass of Ag. Cl = 0. 05 x (108 + 35. 5) g = 7. 175 g

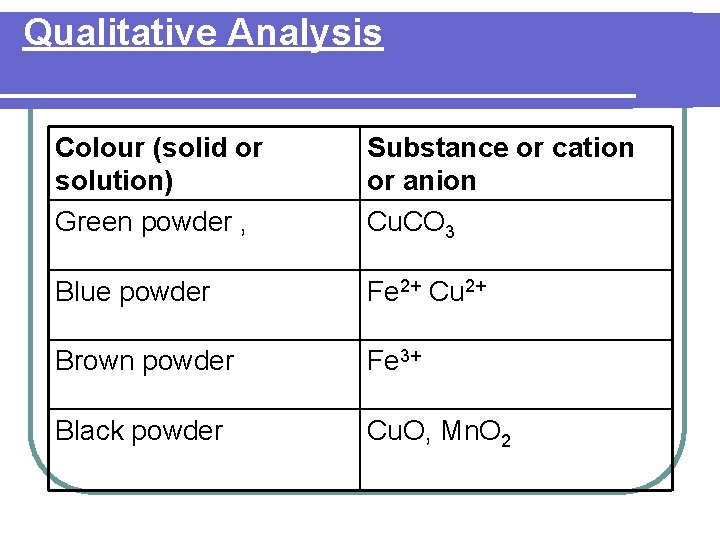
Qualitative Analysis l Qualtitative analysis of a salt is a chemical technique used to identify the ions that are present in a salt by analysing its physical and chemical properties.

Qualitative Analysis Colour (solid or solution) Green powder , Substance or cation or anion Cu. CO 3 Blue powder Fe 2+ Cu 2+ Brown powder Fe 3+ Black powder Cu. O, Mn. O 2

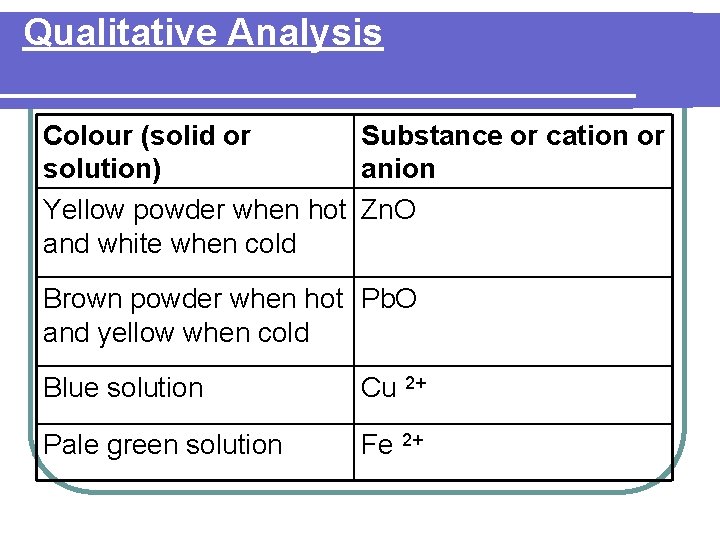
Qualitative Analysis Colour (solid or Substance or cation or solution) anion Yellow powder when hot Zn. O and white when cold Brown powder when hot Pb. O and yellow when cold Blue solution Cu 2+ Pale green solution Fe 2+

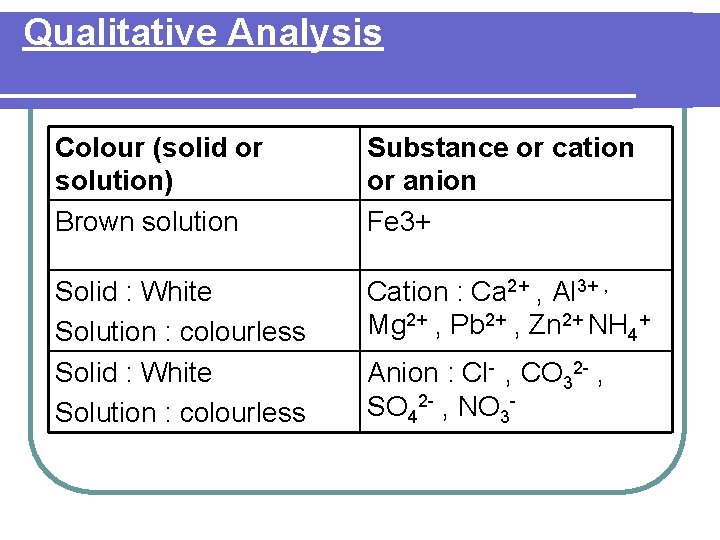
Qualitative Analysis Colour (solid or solution) Brown solution Substance or cation or anion Fe 3+ Solid : White Solution : colourless Cation : Ca 2+ , Al 3+ , Mg 2+ , Pb 2+ , Zn 2+ NH 4+ Anion : Cl- , CO 32 - , SO 42 - , NO 3 -

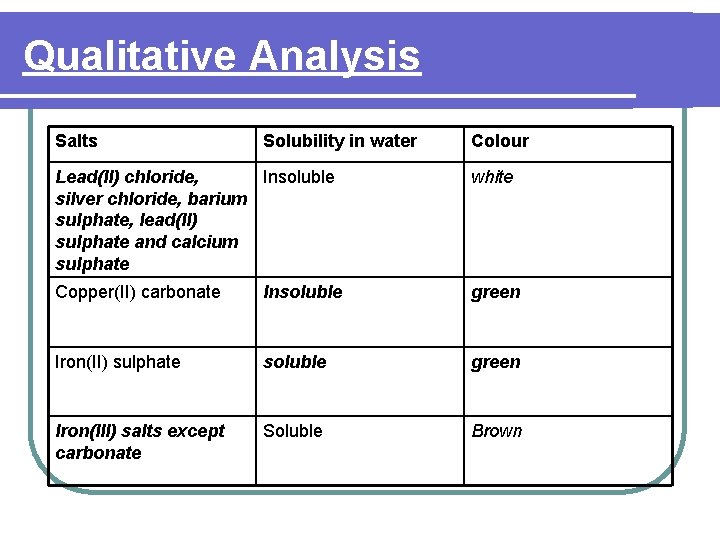
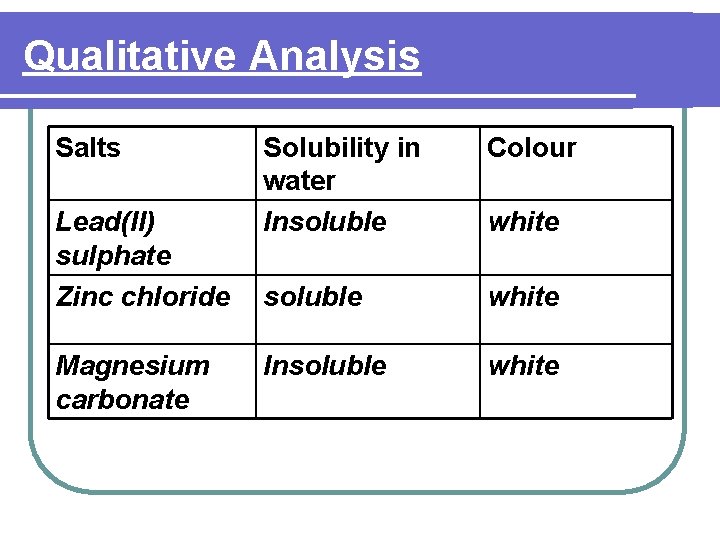
Qualitative Analysis Salts Solubility in water Colour Lead(II) chloride, Insoluble silver chloride, barium sulphate, lead(II) sulphate and calcium sulphate white Copper(II) carbonate Insoluble green Iron(II) sulphate soluble green Iron(III) salts except carbonate Soluble Brown

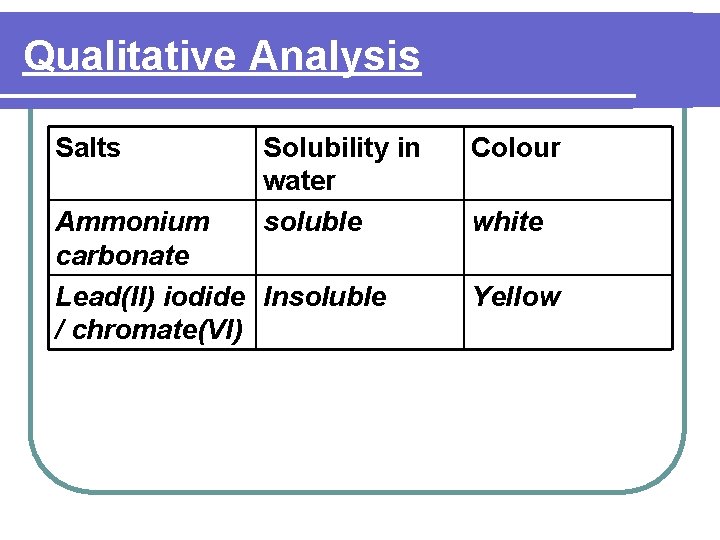
Qualitative Analysis Salts Lead(II) sulphate Zinc chloride Magnesium carbonate Solubility in water Insoluble Colour soluble white Insoluble white

Qualitative Analysis Salts Solubility in water soluble Ammonium carbonate Lead(II) iodide Insoluble / chromate(VI) Colour white Yellow

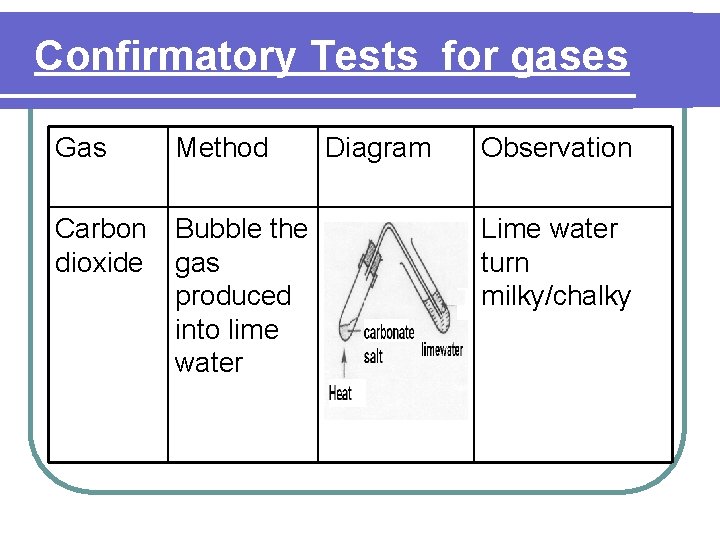
Confirmatory Tests for gases Gas Method Carbon dioxide Bubble the gas produced into lime water Diagram Observation Lime water turn milky/chalky

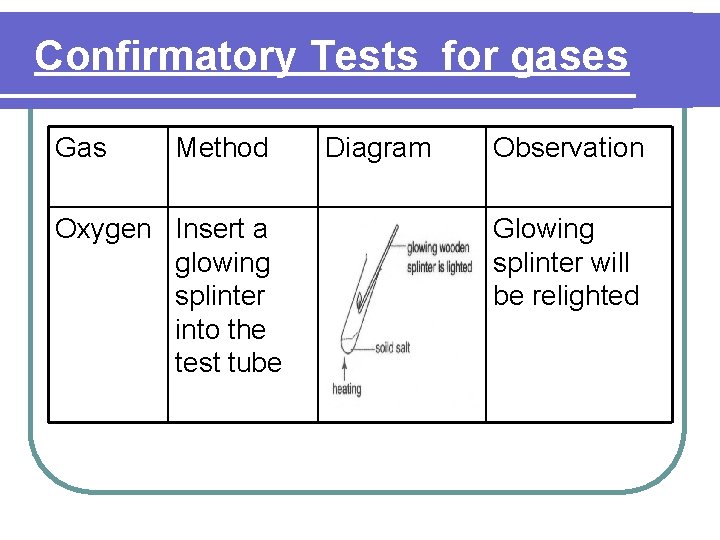
Confirmatory Tests for gases Gas Method Oxygen Insert a glowing splinter into the test tube Diagram Observation Glowing splinter will be relighted

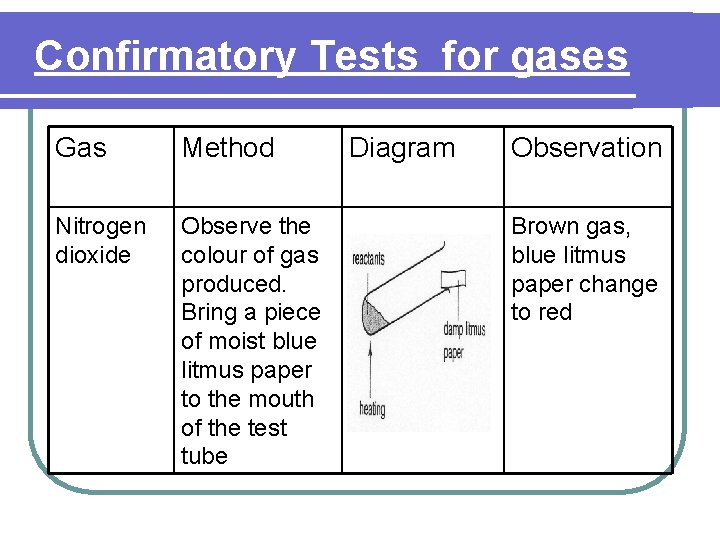
Confirmatory Tests for gases Gas Method Nitrogen dioxide Observe the colour of gas produced. Bring a piece of moist blue litmus paper to the mouth of the test tube Diagram Observation Brown gas, blue litmus paper change to red

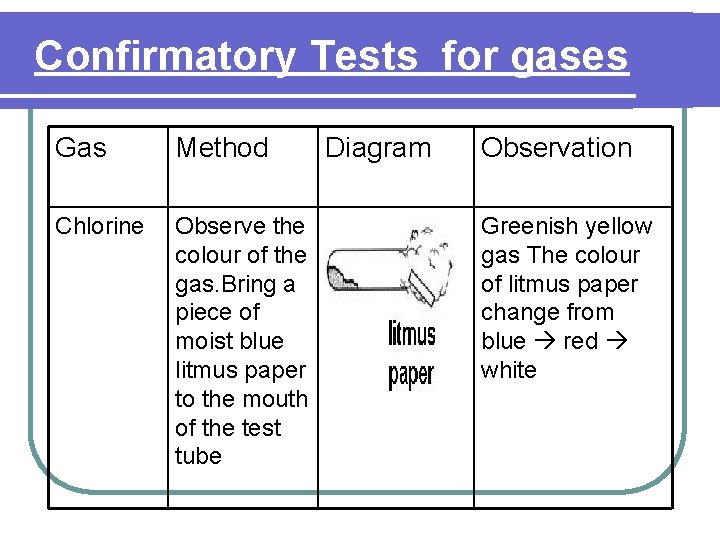
Confirmatory Tests for gases Gas Method Chlorine Observe the colour of the gas. Bring a piece of moist blue litmus paper to the mouth of the test tube Diagram Observation Greenish yellow gas The colour of litmus paper change from blue red white

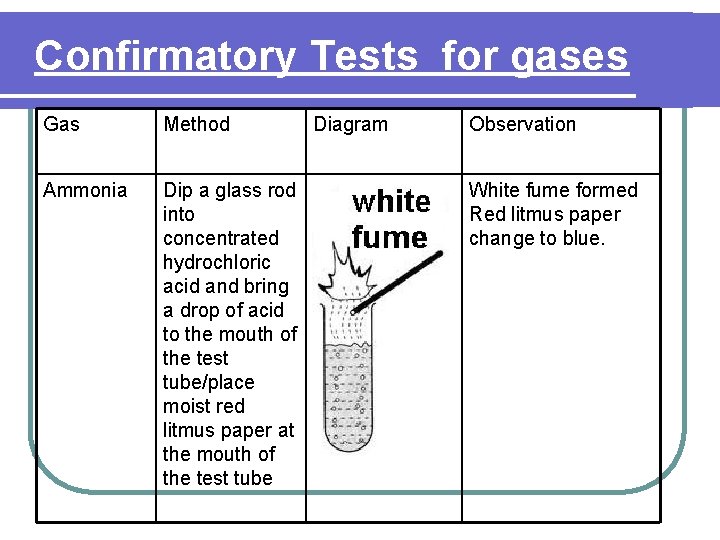
Confirmatory Tests for gases Gas Method Ammonia Dip a glass rod into concentrated hydrochloric acid and bring a drop of acid to the mouth of the test tube/place moist red litmus paper at the mouth of the test tube Diagram Observation White fume formed Red litmus paper change to blue.

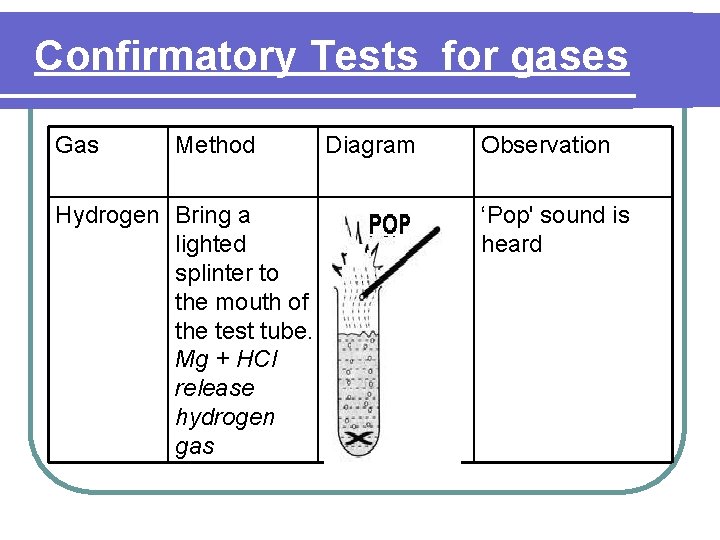
Confirmatory Tests for gases Gas Method Hydrogen Bring a lighted splinter to the mouth of the test tube. Mg + HCl release hydrogen gas Diagram Observation ‘Pop' sound is heard

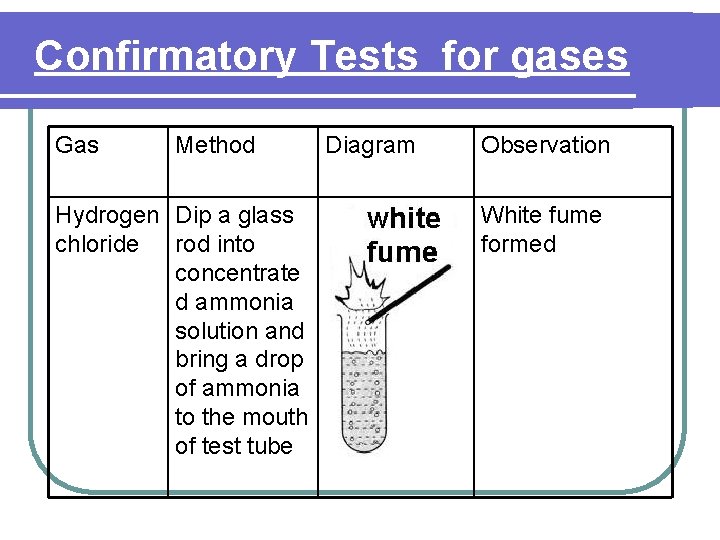
Confirmatory Tests for gases Gas Method Hydrogen Dip a glass chloride rod into concentrate d ammonia solution and bring a drop of ammonia to the mouth of test tube Diagram Observation White fume formed

Action of Heat On Carbonate Salts l Carbonate salts (except Na+ & K+ ) decompose on heating giving off carbon dioxide gas and residue metal oxide

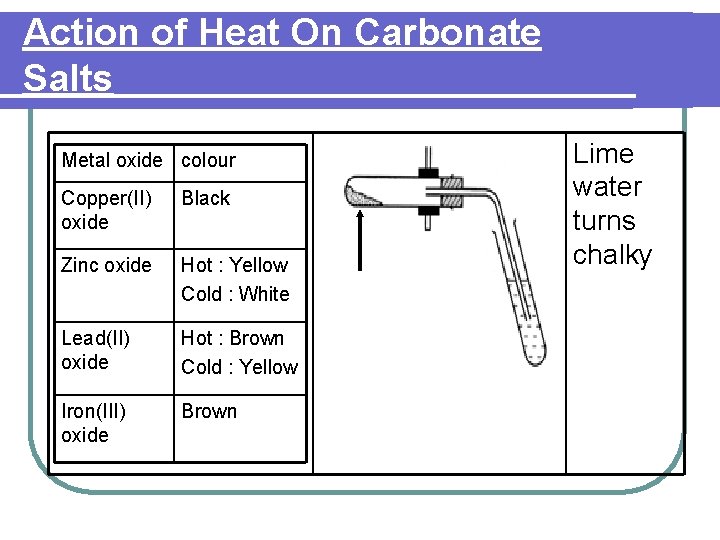
Action of Heat On Carbonate Salts Metal oxide colour Copper(II) oxide Black Zinc oxide Hot : Yellow Cold : White Lead(II) oxide Hot : Brown Cold : Yellow Iron(III) oxide Brown Lime water turns chalky

Action of Heat On Carbonate Salts Carbonate salt Action of heat Potassium Not decompose by heat carbonate K 2 CO 3 , Sodium carbonate Na 2 CO 3

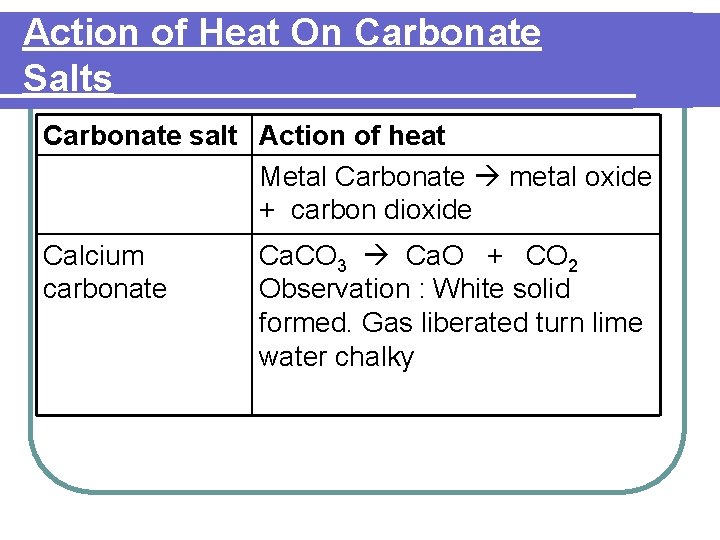
Action of Heat On Carbonate Salts Carbonate salt Action of heat Metal Carbonate metal oxide + carbon dioxide Calcium carbonate Ca. CO 3 Ca. O + CO 2 Observation : White solid formed. Gas liberated turn lime water chalky

Action of Heat On Carbonate Salts Carbonate salt Action of heat Mg. CO 3 Mg. O + CO 2 Magnesium carbonate Observation : White solid formed. Gas liberated turn lime water chalky Al 2(CO 3)3 Al 2 O 3 + 3 CO 2 Aluminium carbonate Observation : White solid formed. Gas liberated turn lime water chalky

Action of Heat On Carbonate Salts Carbonate salt Zinc carbonate Lead(II) carbonate Action of heat Zn. CO 3 Zn. O + CO 2 Observation : The residue is yellow when hot and white when cold. Gas liberated turn lime water chalky Pb. CO 3 Pb. O + CO 2 Observation : The residue is brown when hot and yellow when cold. Gas liberated turn lime water chalky

Action of Heat On Carbonate Salts Carbonate salt Action of heat Cu. CO 3 Cu. O + CO 2 Copper(II) carbonate Observation : Black solid formed. Gas liberated turn lime water chalky

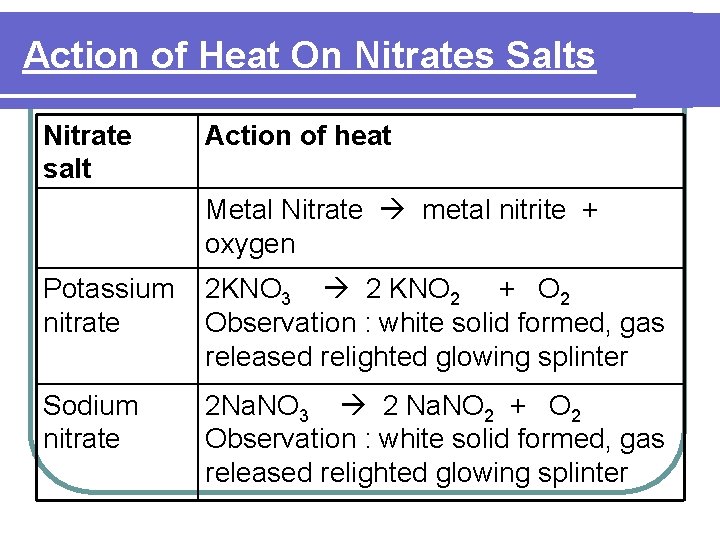
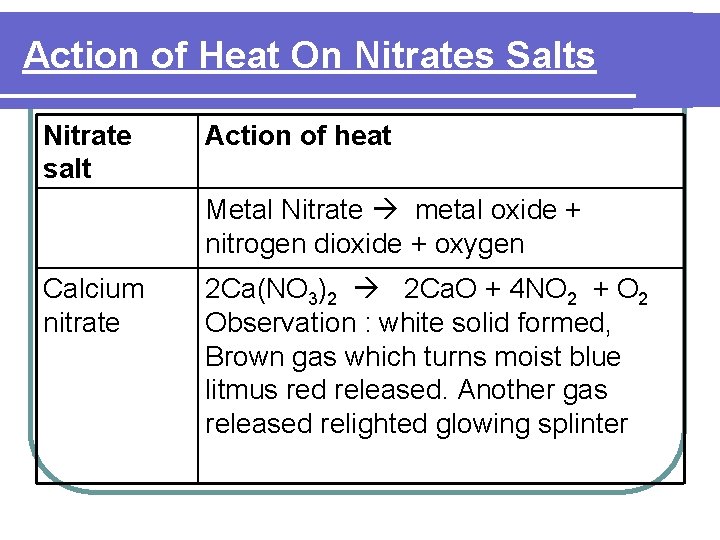
Action of Heat On Nitrate Salts l Nitrates Salts - Decompose on heating liberate nitrogen dioxide gas and oxygen gas except Na. NO 3 and KNO 3 which liberate oxygen gas only.

Action of Heat On Nitrate Salts Heat Brown gas turn moist blue litmus to red (NO 2) Colourless gas relighted glowing splinter (O 2 )

Action of Heat On Nitrates Salts Nitrate salt Action of heat Metal Nitrate metal nitrite + oxygen Potassium nitrate 2 KNO 3 2 KNO 2 + O 2 Observation : white solid formed, gas released relighted glowing splinter Sodium nitrate 2 Na. NO 3 2 Na. NO 2 + O 2 Observation : white solid formed, gas released relighted glowing splinter

Action of Heat On Nitrates Salts Nitrate salt Action of heat Metal Nitrate metal oxide + nitrogen dioxide + oxygen Calcium nitrate 2 Ca(NO 3)2 2 Ca. O + 4 NO 2 + O 2 Observation : white solid formed, Brown gas which turns moist blue litmus red released. Another gas released relighted glowing splinter

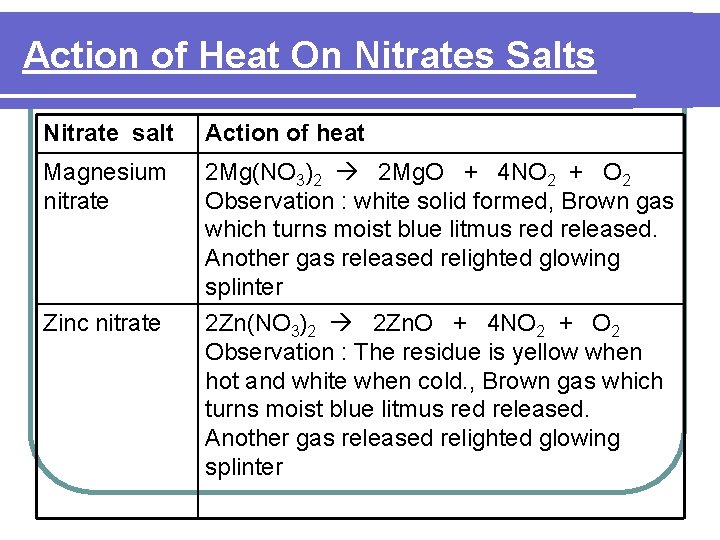
Action of Heat On Nitrates Salts Nitrate salt Action of heat Magnesium nitrate 2 Mg(NO 3)2 2 Mg. O + 4 NO 2 + O 2 Observation : white solid formed, Brown gas which turns moist blue litmus red released. Another gas released relighted glowing splinter 2 Zn(NO 3)2 2 Zn. O + 4 NO 2 + O 2 Observation : The residue is yellow when hot and white when cold. , Brown gas which turns moist blue litmus red released. Another gas released relighted glowing splinter Zinc nitrate

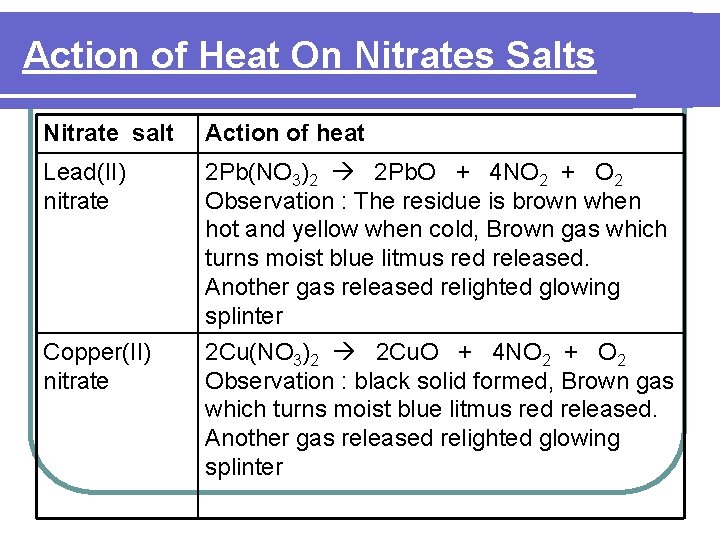
Action of Heat On Nitrates Salts Nitrate salt Action of heat Lead(II) nitrate 2 Pb(NO 3)2 2 Pb. O + 4 NO 2 + O 2 Observation : The residue is brown when hot and yellow when cold, Brown gas which turns moist blue litmus red released. Another gas released relighted glowing splinter 2 Cu(NO 3)2 2 Cu. O + 4 NO 2 + O 2 Observation : black solid formed, Brown gas which turns moist blue litmus red released. Another gas released relighted glowing splinter Copper(II) nitrate

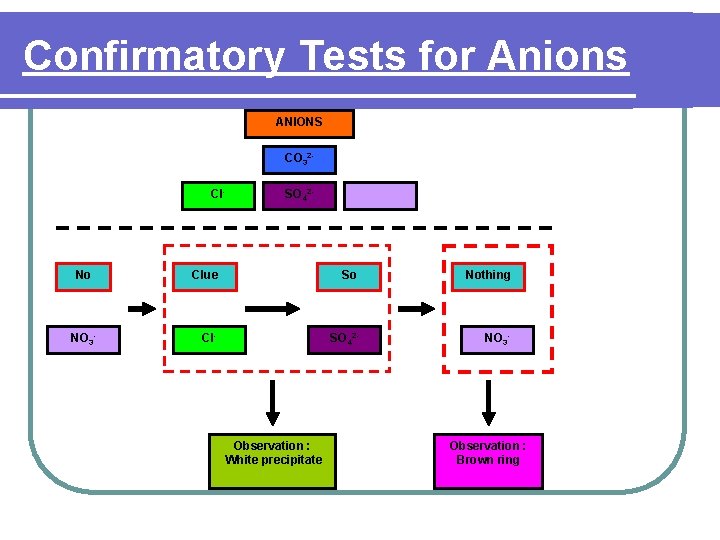
Confirmatory Tests for Anions ANIONS CO 32 Cl- No NO 3 - SO 42 - Clue So Nothing Cl- SO 42 - NO 3 - Observation : White precipitate Observation : Brown ring

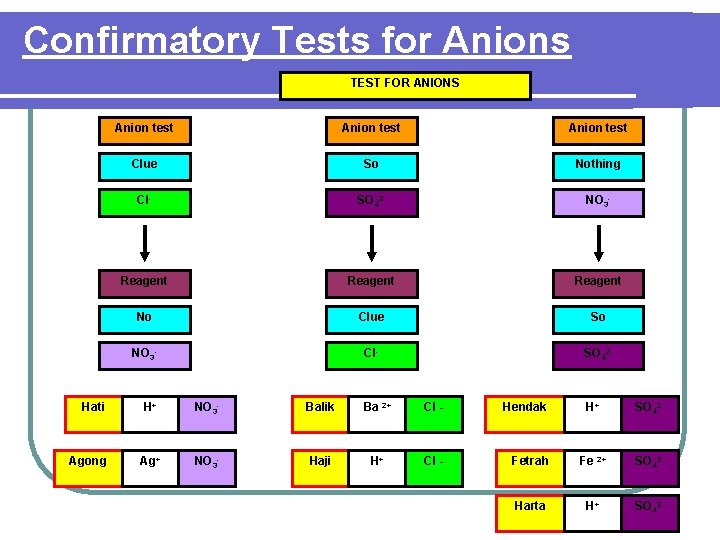
Confirmatory Tests for Anions TEST FOR ANIONS Hati Agong Anion test Clue So Nothing Cl- SO 42 - NO 3 - Reagent No Clue So NO 3 - Cl- SO 42 - H+ NO 3 - Balik Ba 2+ Cl - Hendak H+ SO 42 - Ag+ NO 3 - Haji H+ Cl - Fetrah Fe 2+ SO 42 - Harta H+ SO 42 -

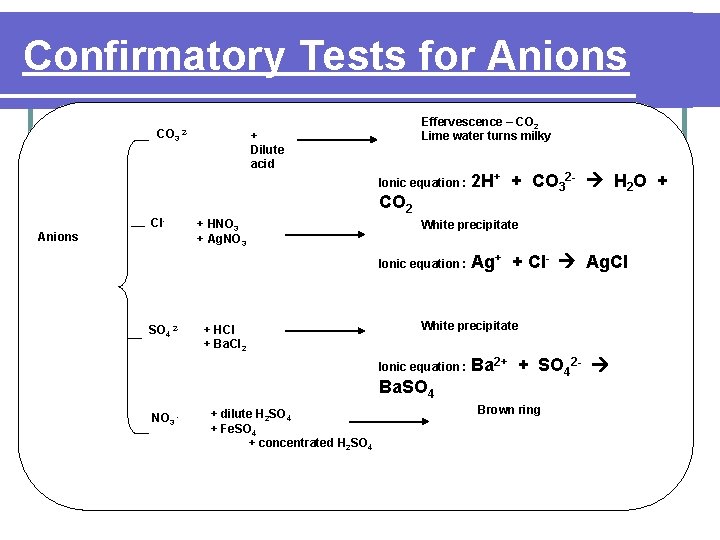
Confirmatory Tests for Anions CO 3 2 - Effervescence – CO 2 Lime water turns milky + Dilute acid Ionic equation : CO 2 Cl. Anions + HNO 3 + Ag. NO 3 White precipitate Ionic equation : SO 4 2 - + HCl + Ba. Cl 2 Ba. SO 4 + dilute H 2 SO 4 + Fe. SO 4 + concentrated H 2 SO 4 Ag+ + Cl- Ag. Cl White precipitate Ionic equation : NO 3 - 2 H+ + CO 32 - H 2 O + Ba 2+ + SO 42 - Brown ring

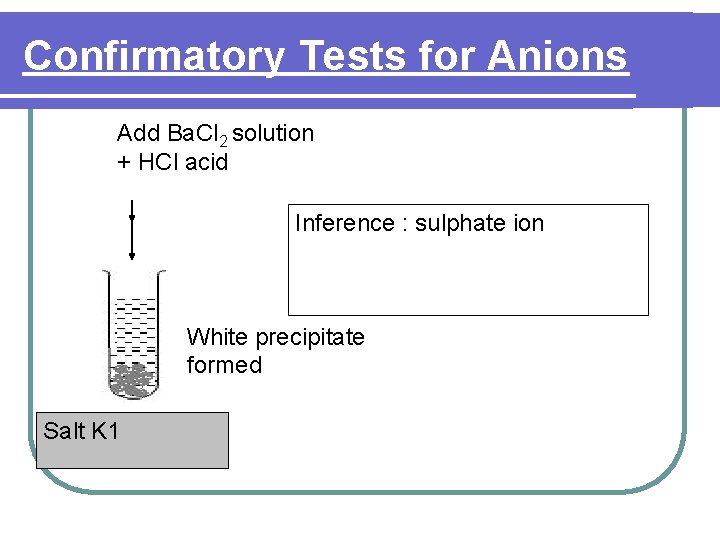
Confirmatory Tests for Anions Add Ba. Cl 2 solution + HCl acid Inference : sulphate ion White precipitate formed Salt K 1

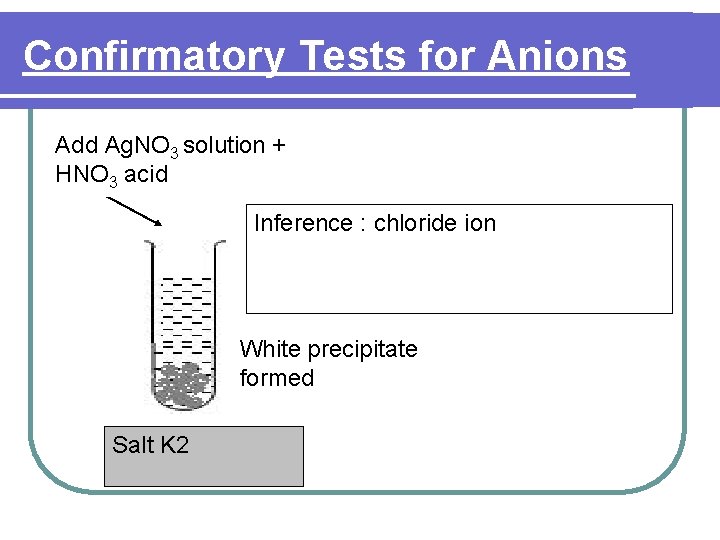
Confirmatory Tests for Anions Add Ag. NO 3 solution + HNO 3 acid Inference : chloride ion White precipitate formed Salt K 2

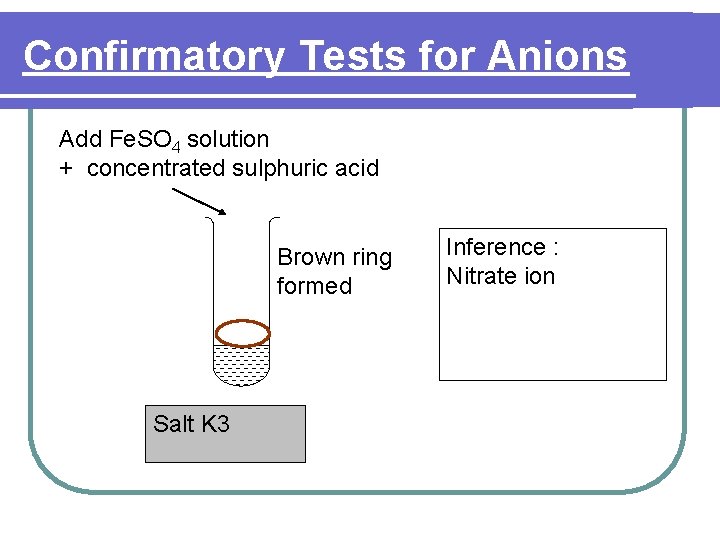
Confirmatory Tests for Anions Add Fe. SO 4 solution + concentrated sulphuric acid Brown ring formed Salt K 3 Inference : Nitrate ion

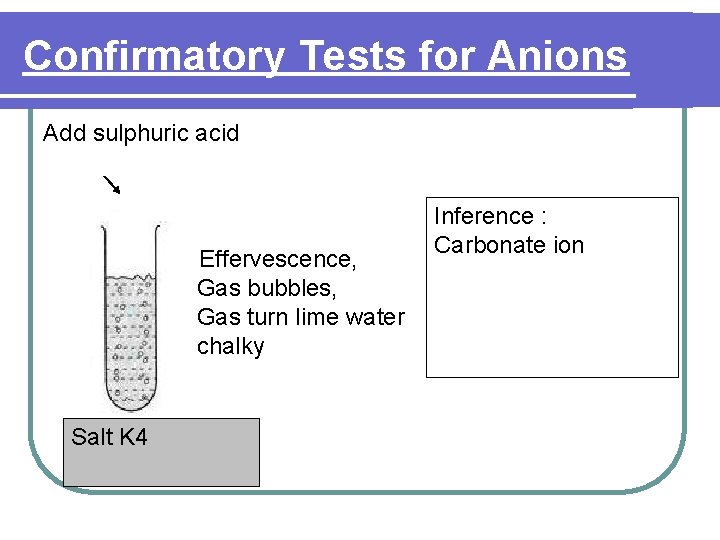
Confirmatory Tests for Anions Add sulphuric acid Effervescence, Gas bubbles, Gas turn lime water chalky Salt K 4 Inference : Carbonate ion

CONFIRMATORY TEST FOR CATIONS (SODIUM HYDROXIDE AS REAGENT)

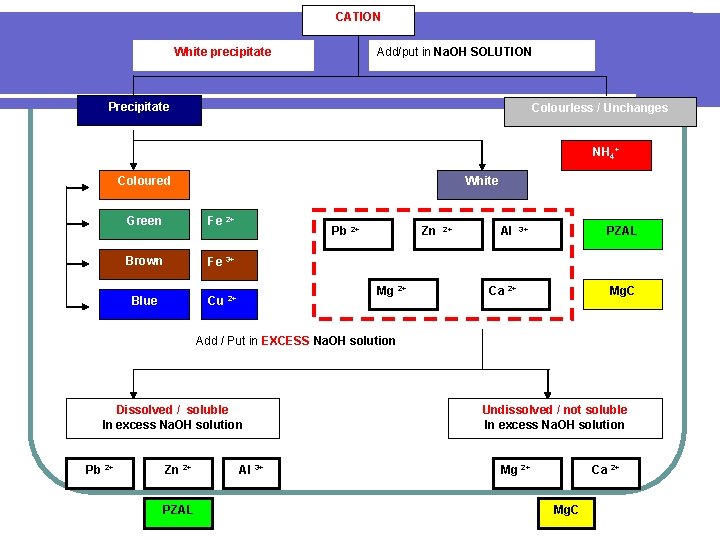
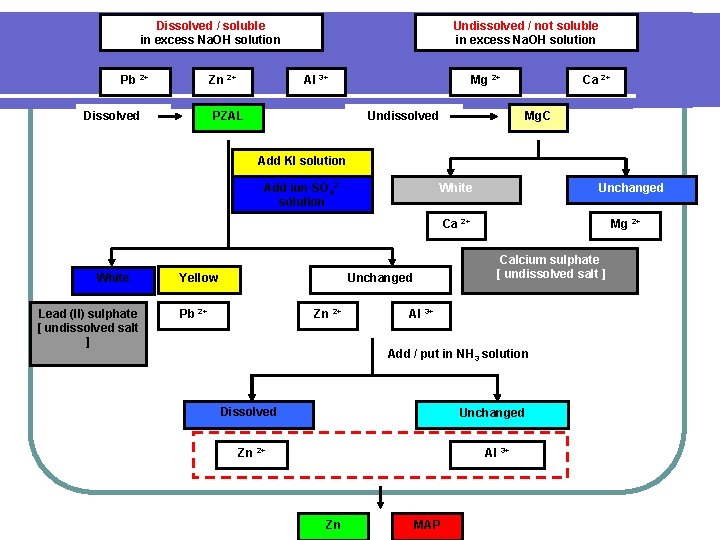
CATION White precipitate Add/put in Na. OH SOLUTION Precipitate Colourless / Unchanges NH 4+ Coloured White Green Fe 2+ Brown Fe 3+ Blue Cu Pb 2+ Zn Mg 2+ 2+ 2+ Al PZAL 3+ Ca 2+ Mg. C Add / Put in EXCESS Na. OH solution Dissolved / soluble In excess Na. OH solution Pb 2+ Zn 2+ PZAL Al 3+ Undissolved / not soluble In excess Na. OH solution Mg 2+ Ca 2+ Mg. C

Dissolved / soluble in excess Na. OH solution Pb 2+ Zn 2+ Dissolved Undissolved / not soluble in excess Na. OH solution Al 3+ PZAL Mg 2+ Undissolved Ca 2+ Mg. C Add KI solution Add ion SO 42 solution White Unchanged Ca 2+ White Lead (II) sulphate [ undissolved salt ] Yellow Calcium sulphate [ undissolved salt ] Unchanged Pb 2+ Zn 2+ Mg 2+ Al 3+ Add / put in NH 3 solution Dissolved Unchanged Zn 2+ Al 3+ Zn MAP

CONFIRMATORY TEST FOR CATIONS (AMMONIA AS REAGENT)

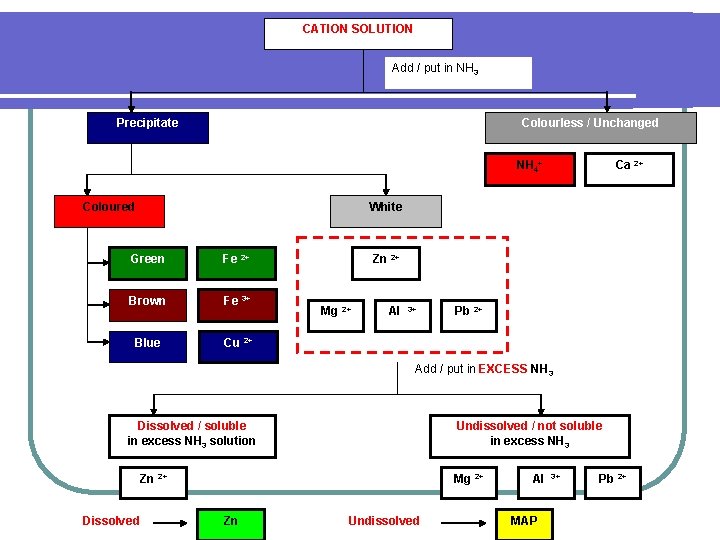
CATION SOLUTION Add / put in NH 3 Precipitate Colourless / Unchanged NH 4+ Coloured Ca 2+ White Green Fe 2+ Brown Fe 3+ Blue Cu 2+ Zn 2+ Mg 2+ Al 3+ Pb 2+ Add / put in EXCESS NH 3 Dissolved / soluble in excess NH 3 solution Undissolved / not soluble in excess NH 3 Zn 2+ Dissolved Mg 2+ Zn Undissolved Al MAP 3+ Pb 2+

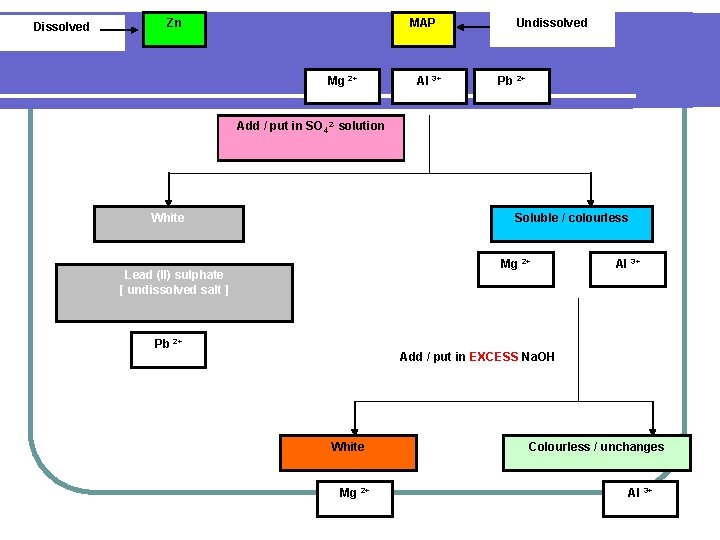
Dissolved Zn MAP Mg 2+ Al 3+ Undissolved Pb 2+ Add / put in SO 42 - solution White Soluble / colourless Mg 2+ Lead (II) sulphate [ undissolved salt ] Pb 2+ Al 3+ Add / put in EXCESS Na. OH White Mg 2+ Colourless / unchanges Al 3+

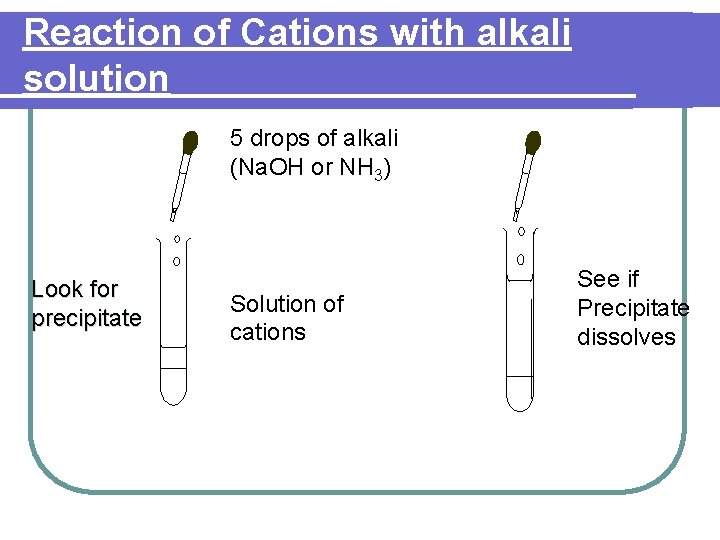
Reaction of Cations with alkali solution Positive ions are identified by their reactions with a. sodium hydroxide Na. OH solution b. Ammonia solution NH 3 2. In these reactions, the cations (positive metal ions) produce different coloured precipitate which may or may not be soluble in excess alkali. 1.

Reaction of Cations with alkali solution 5 drops of alkali (Na. OH or NH 3) Look for precipitate Solution of cations See if Precipitate dissolves

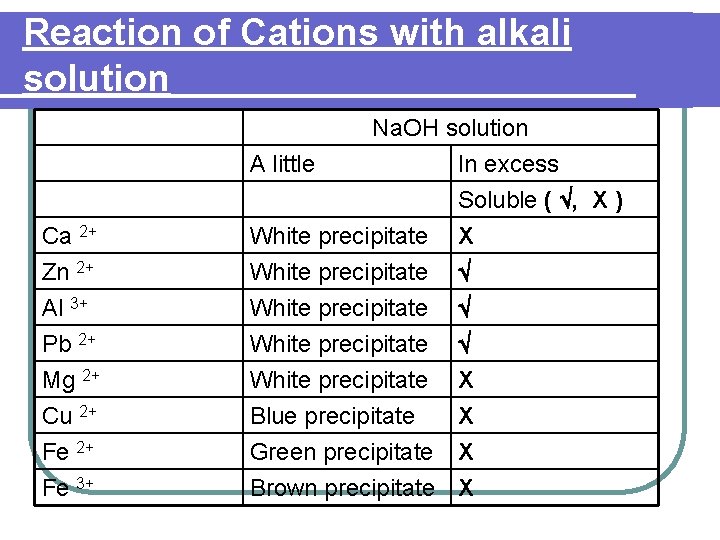
Reaction of Cations with alkali solution Ca 2+ Na. OH solution A little In excess Soluble ( , X ) White precipitate X Zn 2+ Al 3+ Pb 2+ Mg 2+ Cu 2+ Fe 3+ White precipitate Blue precipitate Green precipitate Brown precipitate X X

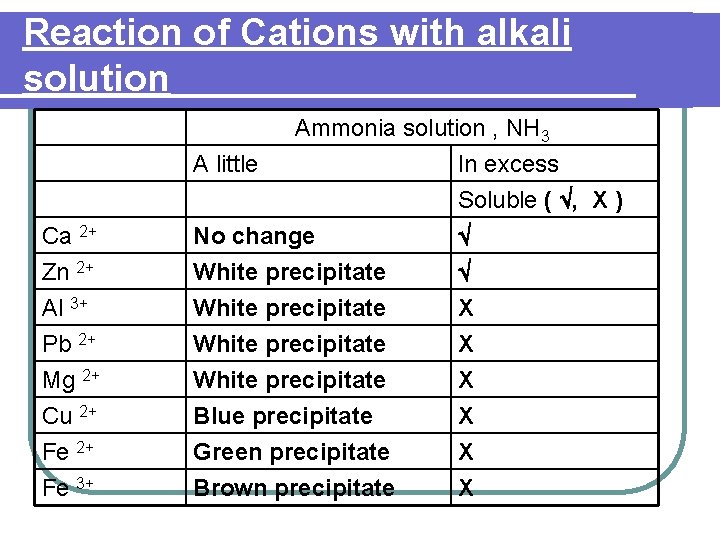
Reaction of Cations with alkali solution Ca 2+ Ammonia solution , NH 3 A little In excess Soluble ( , X ) No change Zn 2+ Al 3+ Pb 2+ Mg 2+ Cu 2+ Fe 3+ White precipitate Blue precipitate Green precipitate Brown precipitate X X X

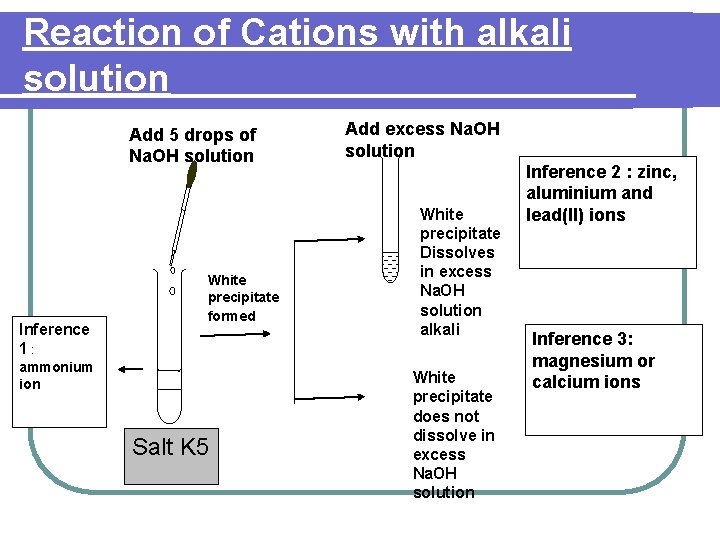
Reaction of Cations with alkali solution Add 5 drops of Na. OH solution Inference 1: White precipitate formed ammonium ion Salt K 5 Add excess Na. OH solution White precipitate Dissolves in excess Na. OH solution alkali White precipitate does not dissolve in excess Na. OH solution Inference 2 : zinc, aluminium and lead(II) ions Inference 3: magnesium or calcium ions

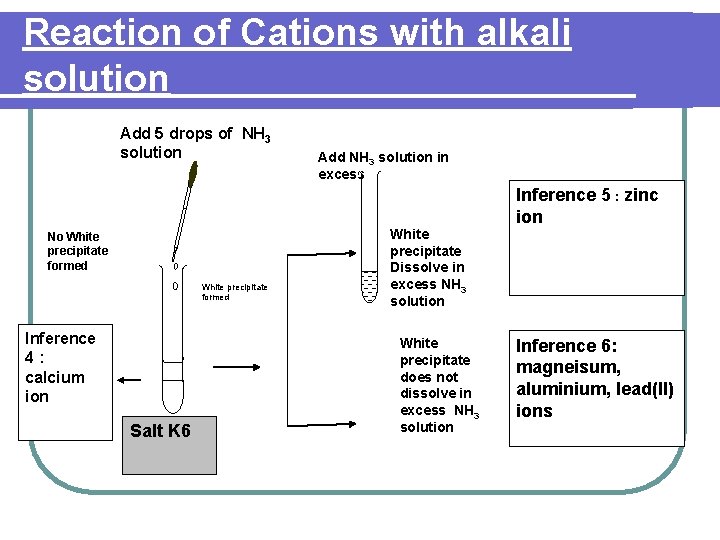
Reaction of Cations with alkali solution Add 5 drops of NH 3 solution No White precipitate formed Inference 4: calcium ion Salt K 6 Add NH 3 solution in excess White precipitate Dissolve in excess NH 3 solution White precipitate does not dissolve in excess NH 3 solution Inference 5 : zinc ion Inference 6: magneisum, aluminium, lead(II) ions

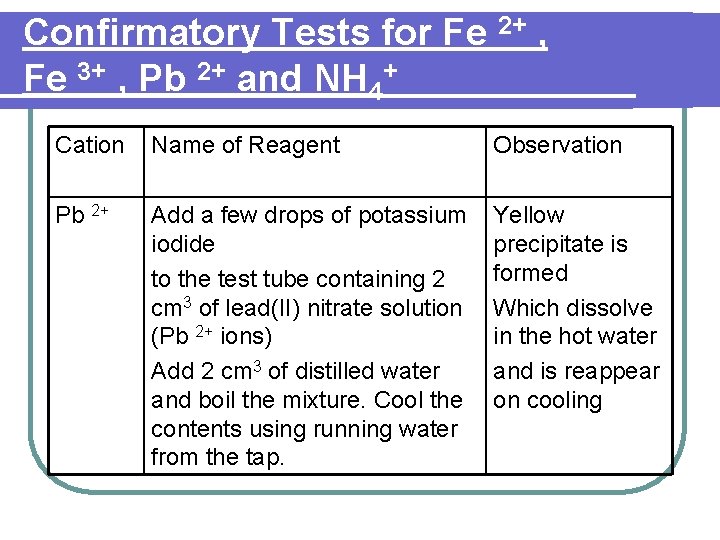
Confirmatory Tests for Fe 2+ , Fe 3+ , Pb 2+ and NH 4+ Cation Name of Reagent Observation Pb 2+ Add a few drops of potassium iodide to the test tube containing 2 cm 3 of lead(II) nitrate solution (Pb 2+ ions) Add 2 cm 3 of distilled water and boil the mixture. Cool the contents using running water from the tap. Yellow precipitate is formed Which dissolve in the hot water and is reappear on cooling

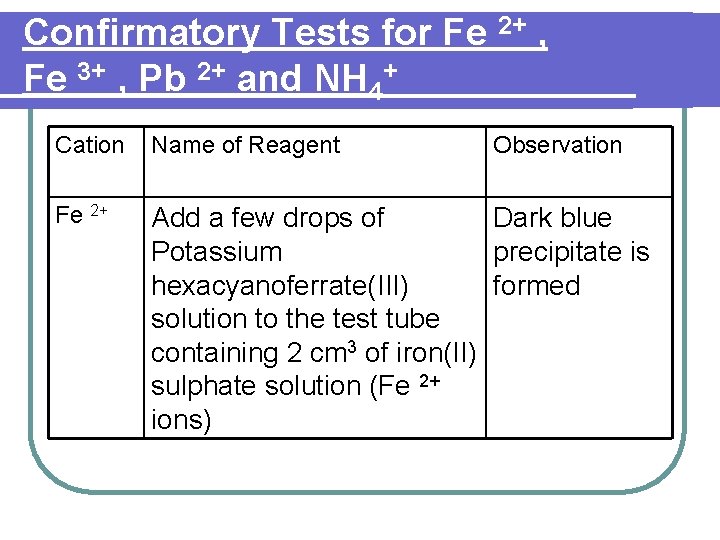
Confirmatory Tests for Fe 2+ , Fe 3+ , Pb 2+ and NH 4+ Cation Name of Reagent Observation Fe 2+ Add a few drops of Dark blue Potassium precipitate is hexacyanoferrate(III) formed solution to the test tube containing 2 cm 3 of iron(II) sulphate solution (Fe 2+ ions)

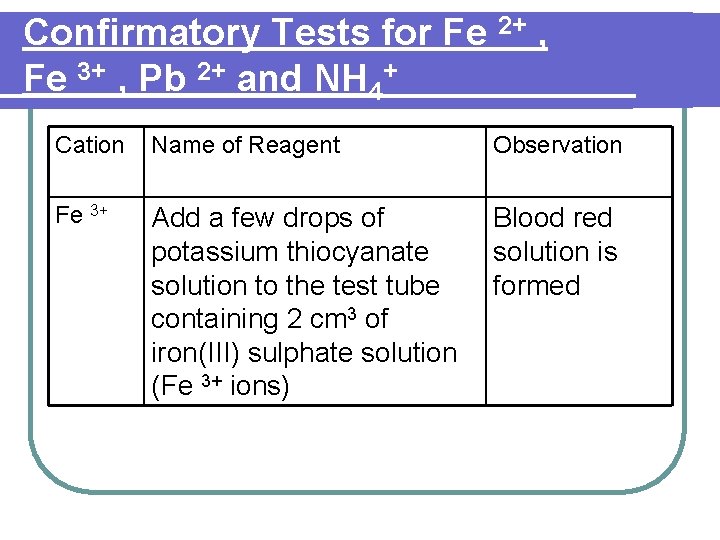
Confirmatory Tests for Fe 2+ , Fe 3+ , Pb 2+ and NH 4+ Cation Name of Reagent Observation Fe 3+ Add a few drops of potassium thiocyanate solution to the test tube containing 2 cm 3 of iron(III) sulphate solution (Fe 3+ ions) Blood red solution is formed

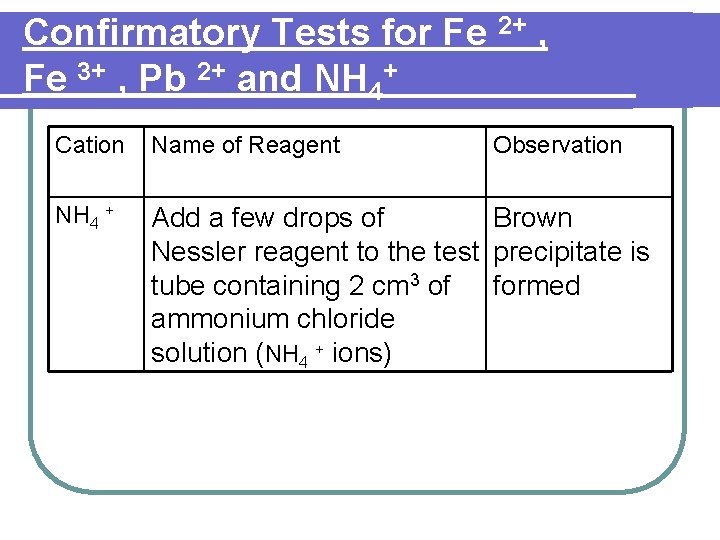
Confirmatory Tests for Fe 2+ , Fe 3+ , Pb 2+ and NH 4+ Cation Name of Reagent Observation NH 4 + Add a few drops of Brown Nessler reagent to the test precipitate is tube containing 2 cm 3 of formed ammonium chloride solution (NH 4 + ions)

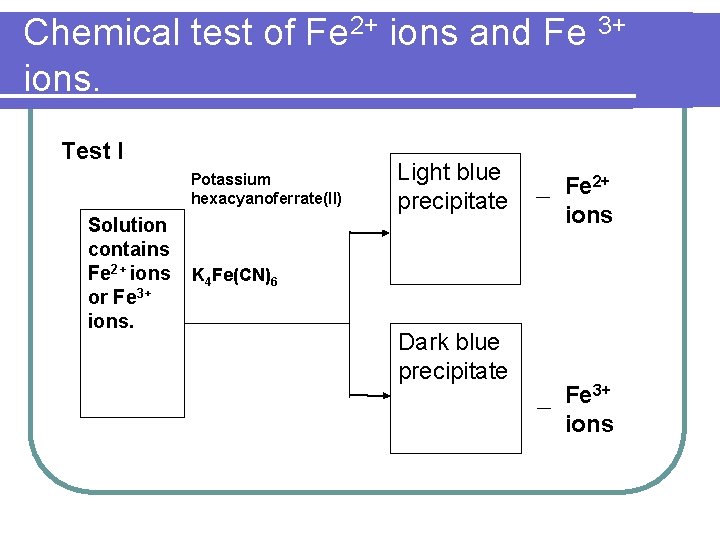
Chemical test of Fe 2+ ions and Fe 3+ ions. Test I Potassium hexacyanoferrate(II) Solution contains Fe 2+ ions or Fe 3+ ions. Light blue precipitate Fe 2+ ions K 4 Fe(CN)6 Dark blue precipitate Fe 3+ ions

Chemical test of Fe 2+ ions and Fe 3+ ions. (i) Pour 2 cm 3 of iron(II) sulphate solution and 2 cm 3 of iron(III) chloride solution into two test tubes respectively. Then add a few drops of potassium hexacyanoferrate(II) solution to two test tubes, Fe 2+ ions solution will form light blue precipitate whereas Fe 3+ ions solution will form dark blue precipitate

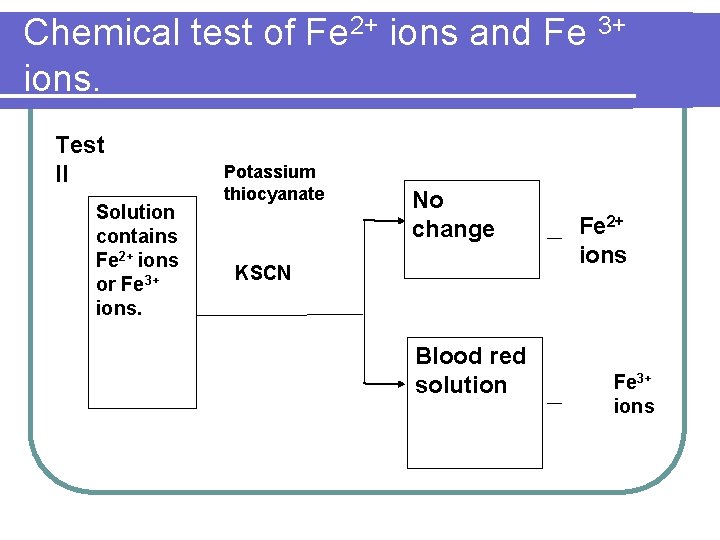
Chemical test of Fe 2+ ions and Fe 3+ ions. Test II Solution contains Fe 2+ ions or Fe 3+ ions. Potassium thiocyanate No change KSCN Blood red solution Fe 2+ ions Fe 3+ ions

Chemical test of Fe 2+ ions and Fe 3+ ions. (i) Pour 2 cm 3 of iron(II) sulphate solution and 2 cm 3 of iron(III) chloride solution into two test tube respectively. Then add a few drops of potassium thiocyanate solution to two test tubes, there is no change in Fe 2+ ions solution whereas Fe 3+ ions solution will form blood red solution.

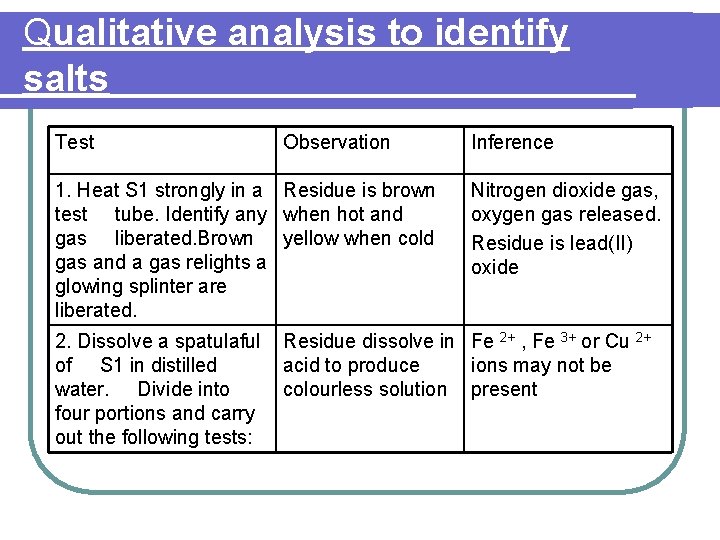
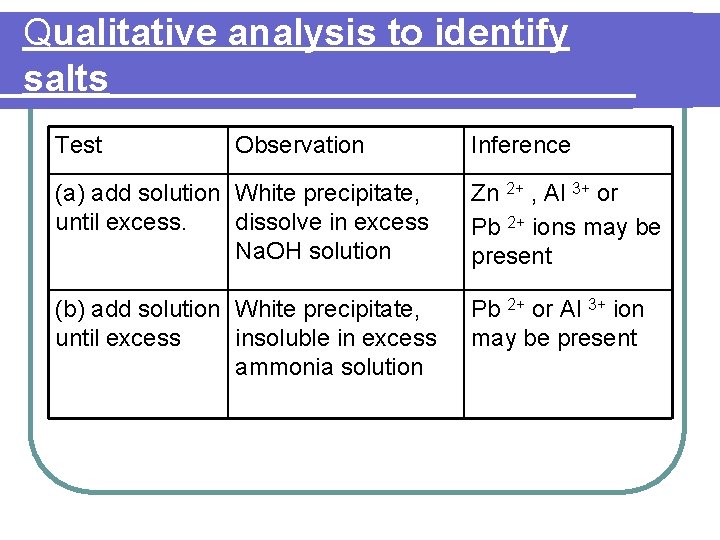
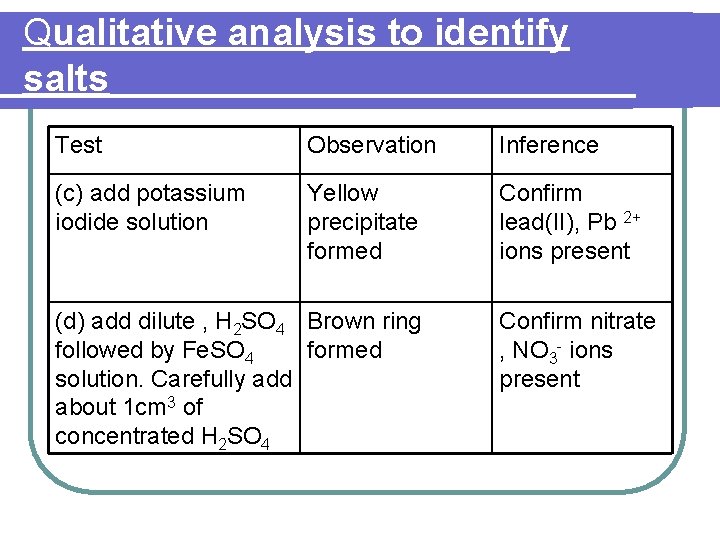
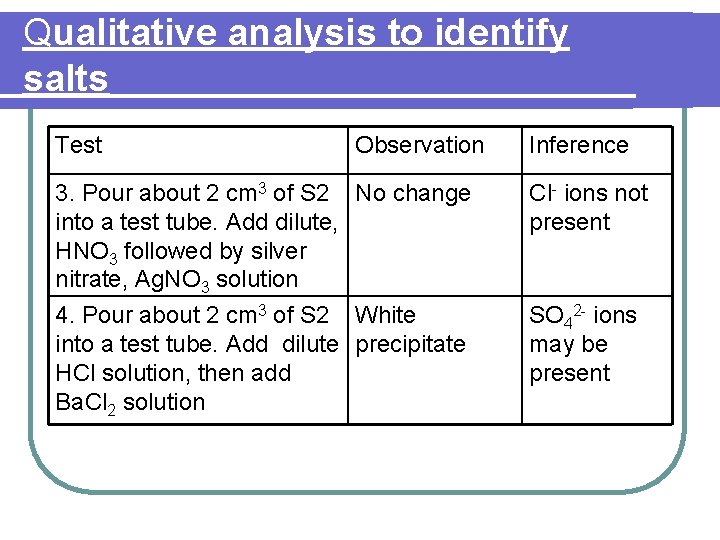
Qualitative analysis to identify salts Identify the salt S 1 The following tests were carried out to identify salt S 1. Based on the observations given for each test, state its inference. Finally, identify salt S 1

Qualitative analysis to identify salts Test Observation 1. Heat S 1 strongly in a Residue is brown test tube. Identify any when hot and gas liberated. Brown yellow when cold gas and a gas relights a glowing splinter are liberated. 2. Dissolve a spatulaful of S 1 in distilled water. Divide into four portions and carry out the following tests: Inference Nitrogen dioxide gas, oxygen gas released. Residue is lead(II) oxide Residue dissolve in Fe 2+ , Fe 3+ or Cu 2+ acid to produce ions may not be colourless solution present

Qualitative analysis to identify salts Test Observation Inference (a) add solution White precipitate, until excess. dissolve in excess Na. OH solution Zn 2+ , Al 3+ or Pb 2+ ions may be present (b) add solution White precipitate, until excess insoluble in excess ammonia solution Pb 2+ or Al 3+ ion may be present

Qualitative analysis to identify salts Test Observation Inference (c) add potassium iodide solution Yellow precipitate formed Confirm lead(II), Pb 2+ ions present (d) add dilute , H 2 SO 4 Brown ring followed by Fe. SO 4 formed solution. Carefully add about 1 cm 3 of concentrated H 2 SO 4 Confirm nitrate , NO 3 - ions present

l. Conclusion for salt S 1 : Lead(II) nitrate

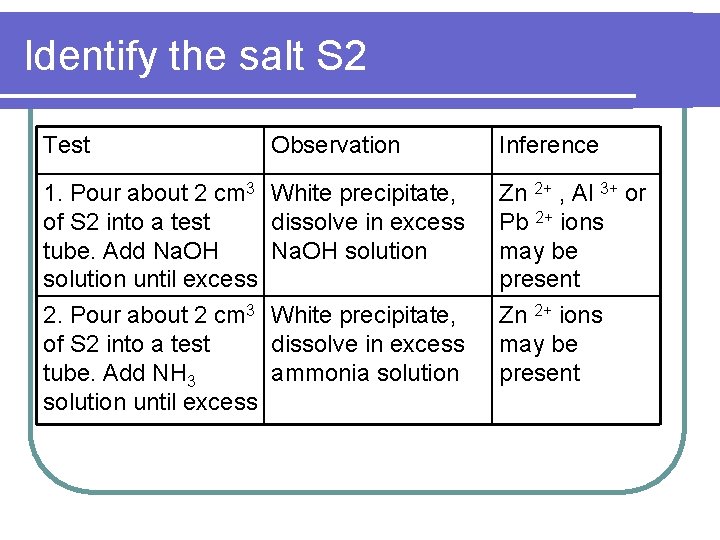
Identify the salt S 2 Test Observation Inference 1. Pour about 2 cm 3 White precipitate, of S 2 into a test dissolve in excess tube. Add Na. OH solution until excess Zn 2+ , Al 3+ or Pb 2+ ions may be present 2. Pour about 2 cm 3 White precipitate, of S 2 into a test dissolve in excess tube. Add NH 3 ammonia solution until excess Zn 2+ ions may be present

Qualitative analysis to identify salts Test Observation Inference 3. Pour about 2 cm 3 of S 2 No change into a test tube. Add dilute, HNO 3 followed by silver nitrate, Ag. NO 3 solution Cl- ions not present 4. Pour about 2 cm 3 of S 2 White into a test tube. Add dilute precipitate HCl solution, then add Ba. Cl 2 solution SO 42 - ions may be present

l. Conclusion for salt S 2 : Zinc sulphate

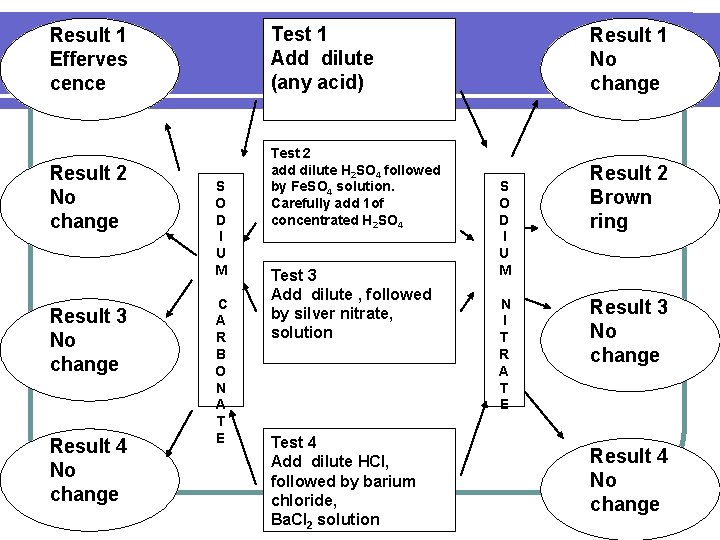
SODIUM CARBONATE AND SODIUM NITRATE

Result 1 Efferves cence Test 1 Add dilute (any acid) Result 2 No change Test 2 add dilute H 2 SO 4 followed by Fe. SO 4 solution. Carefully add 1 of concentrated H 2 SO 4 Result 3 No change Result 4 No change S O D I U M C A R B O N A T E Test 3 Add dilute , followed by silver nitrate, solution Test 4 Add dilute HCl, followed by barium chloride, Ba. Cl 2 solution Result 1 No change S O D I U M N I T R A T E Result 2 Brown ring Result 3 No change Result 4 No change

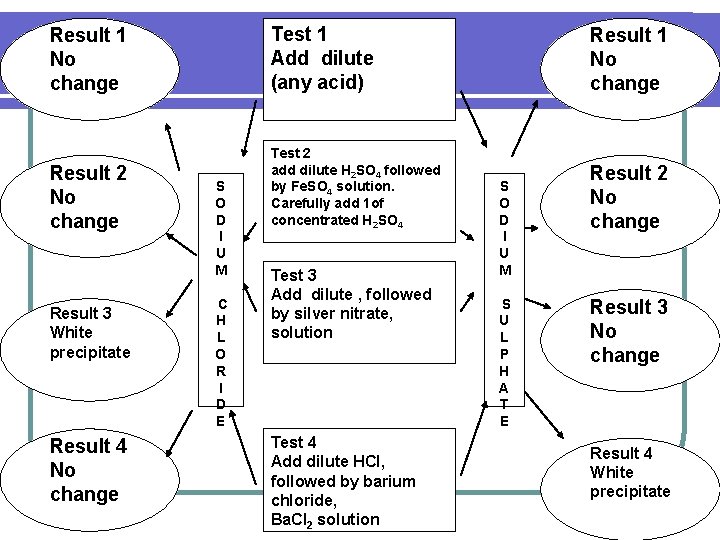
SODIUM CHLORIDE AND SODIUM SULPHATE

Result 1 No change Test 1 Add dilute (any acid) Result 2 No change Test 2 add dilute H 2 SO 4 followed by Fe. SO 4 solution. Carefully add 1 of concentrated H 2 SO 4 Result 3 White precipitate Result 4 No change S O D I U M C H L O R I D E Test 3 Add dilute , followed by silver nitrate, solution Test 4 Add dilute HCl, followed by barium chloride, Ba. Cl 2 solution Result 1 No change S O D I U M S U L P H A T E Result 2 No change Result 3 No change Result 4 White precipitate

Exercise : Page 144: Quick Review A Page 147: Quick Review B