Chapter 8 Notes Writing Ionic Formulas Compounds Up























































- Slides: 67

Chapter 8 Notes Writing Ionic Formulas

Compounds • Up until now, we have only looked at single elements. • There are just over 100 elements, so how do we account for there being so many different types of stuff?

Compounds • There are millions of different compounds —two or more elements that are combined chemically. • The two types of compounds we will discuss in this chapter are ionic compounds and covalent compounds.

A compound is a pure substance made up of two or more elements that are chemically joined in definite proportions. 2 oxygen atoms Ex. Carbon dioxide is CO 2 , while carbon monoxide (the poison) is CO. 1 oxygen atom

Compounds do not look or act like the elements that form them.

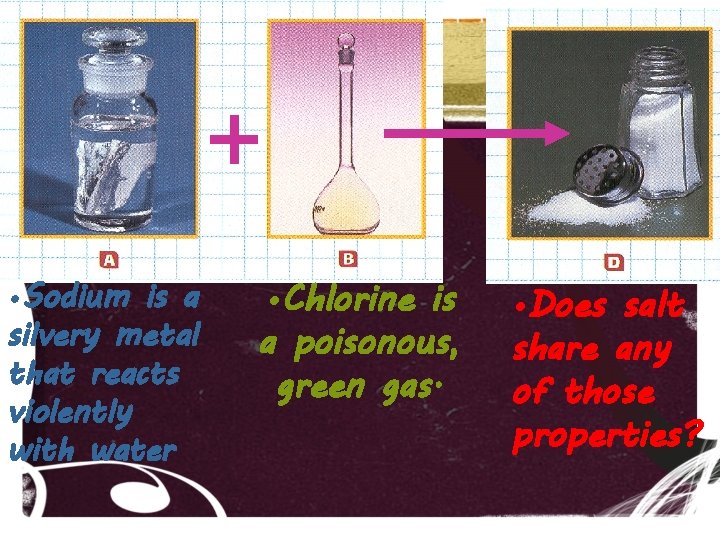
• Sodium is a silvery metal that reacts violently with water • Chlorine is a poisonous, green gas. • Does salt share any of those properties?

Oxygen + Hydrogen -----> Water Hydrogen is explosive! Is water explosive?

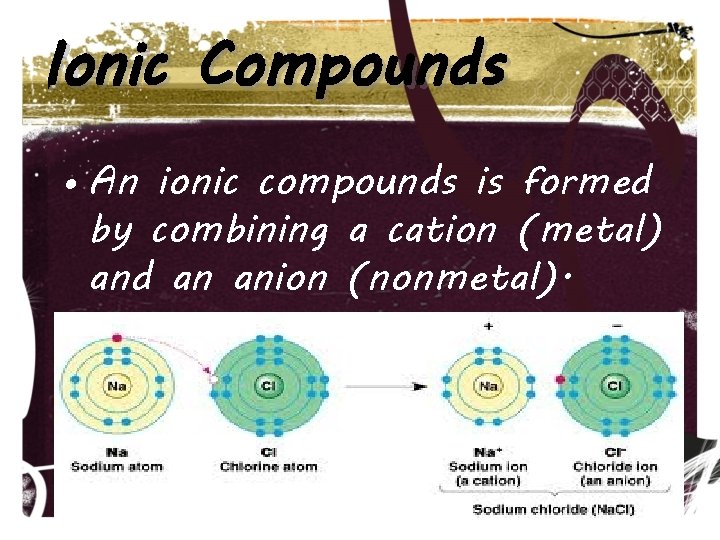
Ionic Compounds • Last chapter we discussed ions—elements that have a charge due to losing or gaining electrons. • Ionic compounds are made of ions.

Ionic Compounds • Metals lose electrons, giving them an overall (+) charge. These are called cations. • Nonmetals gain electrons, giving them an overall (-)charge. These are called anions.

Ionic Compounds • An ionic compounds is formed by combining a cation (metal) and an anion (nonmetal).

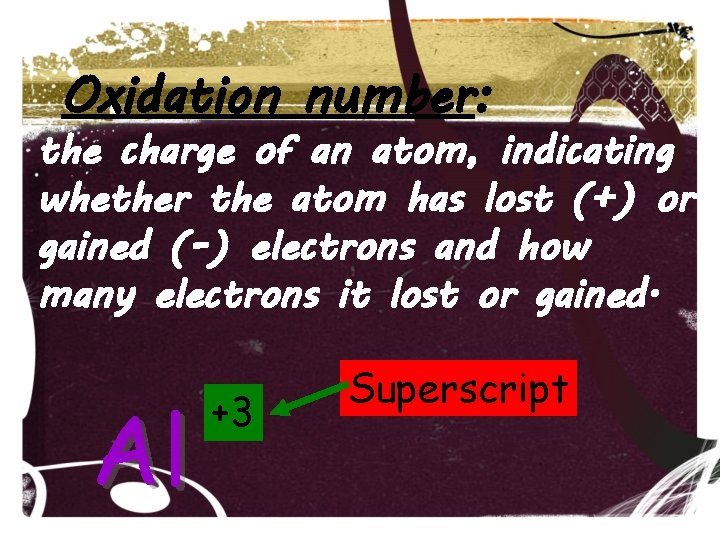
Oxidation number: the charge of an atom, indicating whether the atom has lost (+) or gained (-) electrons and how many electrons it lost or gained. Al +3 Superscript

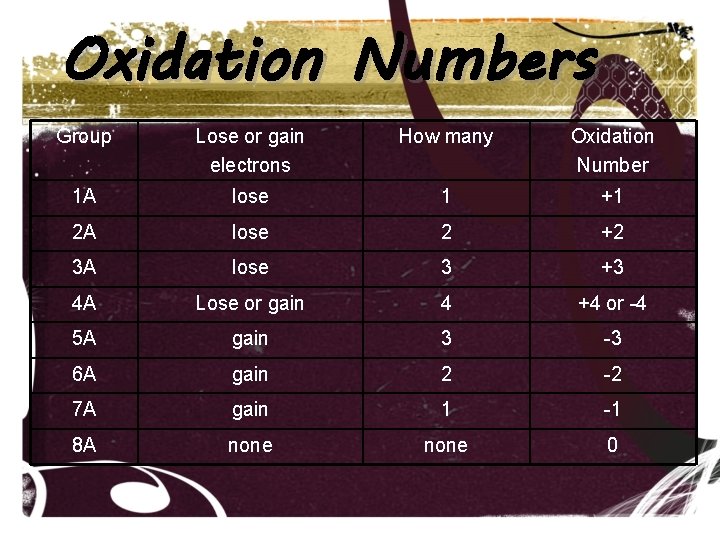
Oxidation Numbers Group Lose or gain electrons How many Oxidation Number 1 A lose 1 +1 2 A lose 2 +2 3 A lose 3 +3 4 A Lose or gain 4 +4 or -4 5 A gain 3 -3 6 A gain 2 -2 7 A gain 1 -1 8 A none 0

Ionic compounds consist of two oppositely charged ions. Li+1 F-1 Positive ion – metal Negative ion - nonmetal Li. Li+10 3 p+ 4 n 0 9 p+ 10 n 0 0 F-1

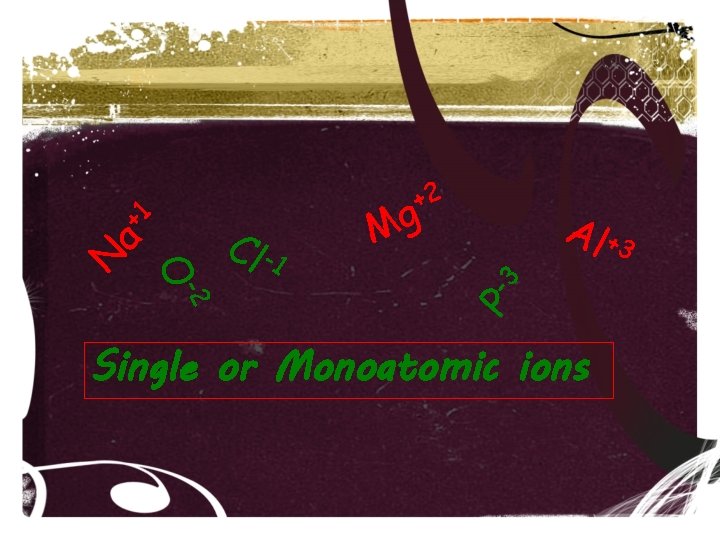
a -2 O N Cl -1 g M Al +3 P -3 +1 +2 Single or Monoatomic ions

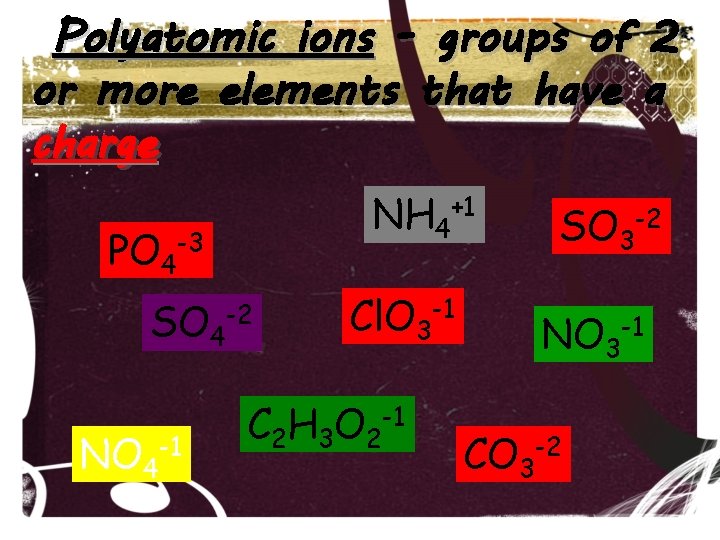
Polyatomic Ions • Anions and cations can also be made up of a group of elements bonded together that carry an overall charge. • These groups of elements are called polyatomic ions.

Polyatomic ions - groups of 2 or more elements that have a charge NH 4+1 PO 4 -3 SO 4 NO 4 -1 -2 Cl. O 3 -1 C 2 H 3 O 2 -1 SO 3 -2 NO 3 -1 CO 3 -2

Chemical Formulas • A chemical formula tells you the type and number of each element in the compound. (like the recipe of a compound) Na 2 SO 4 Ba 3(PO 4)2

Formulas consist of a positive ion and a negative ion. +2 g A M l +3 1 st Thea positive Cl -1 ion always comes N +1 -2 P -3 O Na. Cl Mg. O Al. P

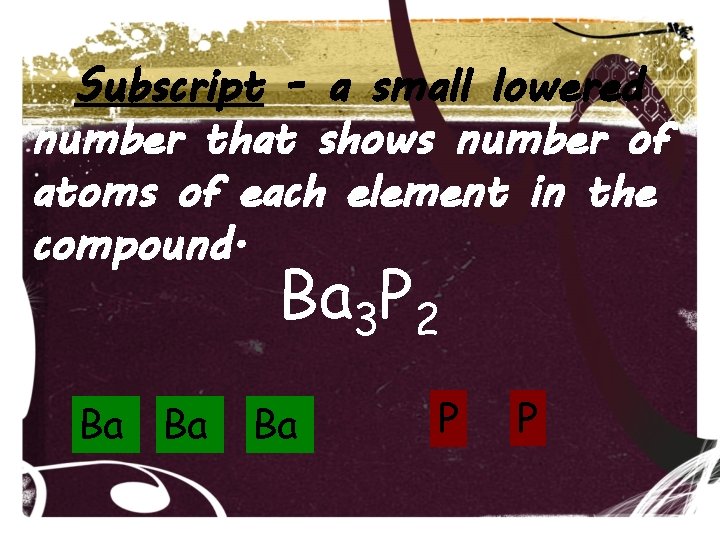
Subscript - a small lowered number that shows number of atoms of each element in the compound. Ba 3 P 2 Ba Ba Ba P P

A is subscript of (1) NEVER written. Na 1 Cl 1 NO!!!

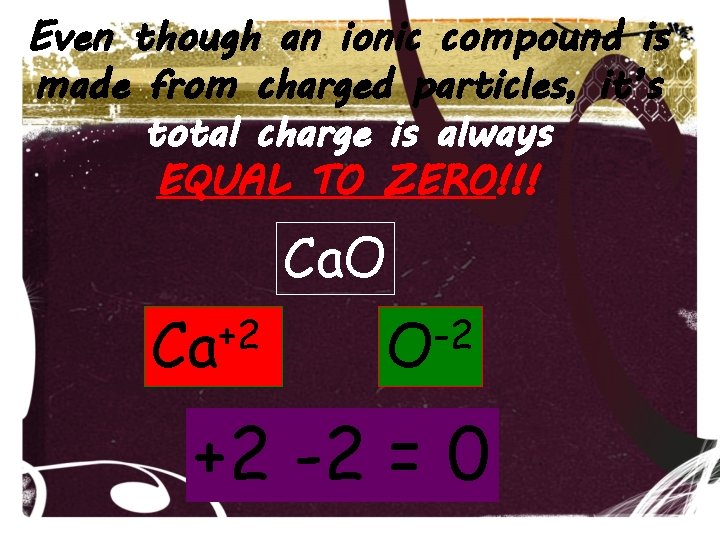
Even though an ionic compound is made from charged particles, it’s total charge is always EQUAL TO ZERO!!! Ca. O +2 Ca -2 O +2 -2 = 0

Binary compounds: composed of only two elements. (Look for 2 capital letters!) Ex. Na. Cl sodium chloride Ex. Mg 3 N 2 magnesium nitride

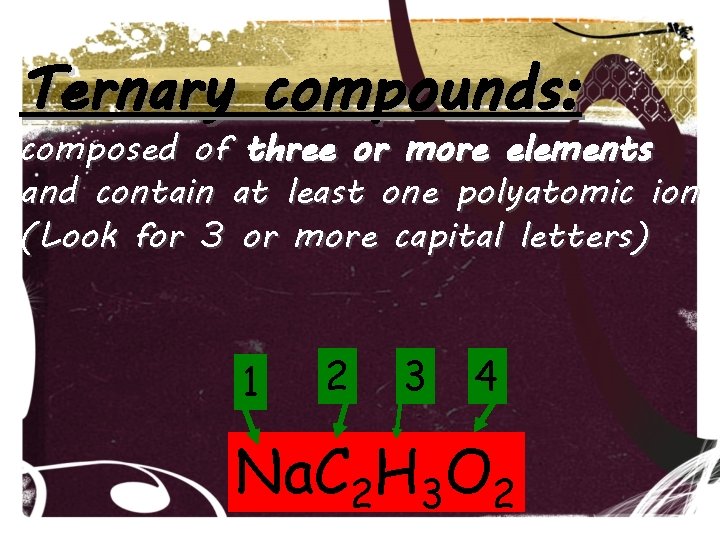
Ternary compounds: composed of three or more elements and contain at least one polyatomic ion (Look for 3 or more capital letters) 1 2 3 4 Na. C 2 H 3 O 2

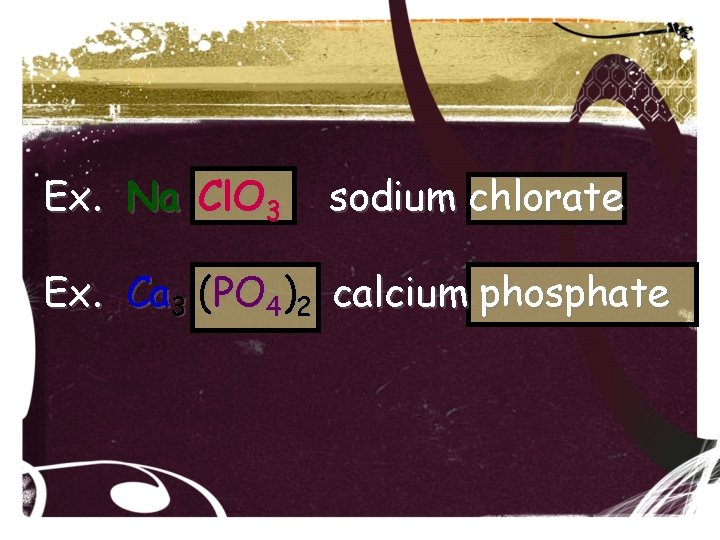
Ex. Na Cl. O 3 sodium chlorate Ex. Ca 3 (PO 4)2 calcium phosphate

Variable vs Non-variable Metals VARIABLE Look at the chart on the back of periodic table. If the metal is on the back of the periodic table with more than one oxidation number listed it is variable. EX: Cr= +2, +3, or +6 Fe= +2, or +3

NON VARIABLE If it is not on the back of the periodic table OR Only one choice of oxidation number listed. EX: Ag+1, Zn+2, or Ni+2 Then it is non variable

Roman Numerals • Used when a metal has more than one potential oxidation number • The name of the metal must tell you the valence of the metal

Chromium III chloride The Roman numeral III three tells you that the oxidation number of the metal is +3.

Chromium VI VI chlorid The Roman numeral six tells you that the oxidation number of the metal is +6.

Writing and Naming Ionic Compounds

Writing Chemical Formulas 1) Write the symbol for the elements and their oxidation number, positive ion first (the metal). EX: For a compound of Magnesium and Chlorine +2 Mg Cl -1

Writing Chemical Formulas 2) Criss cross the numbers and write them as subscripts without the signs. +2 2 Mg Cl -1 1

Writing Chemical Formulas 3) If there is a one charge, do not write it. Mg 1 Cl 2

Writing Chemical Formulas 4) If you have subscripts that are multiples, reduce them down. +4 4 1 -2 2 Pb O 2

Naming Ionic Compounds For ionic compounds where the metal always has the same oxidation number, 1) Name the metal. EX: Ba. Br 2 Barium

Naming Ionic Compounds For ionic compounds where the metal always has the same oxidation number, 2) Write the name of the non-metal, EX: Ba. Br 2 and change the end to –ide. Barium brom ine ide

Naming Binary Non variable Compounds 1. Name the positive ion 2. Name the negative ion changing the ending to IDE. Al. Br 3 Aluminum bromide No Roman numeral is needed

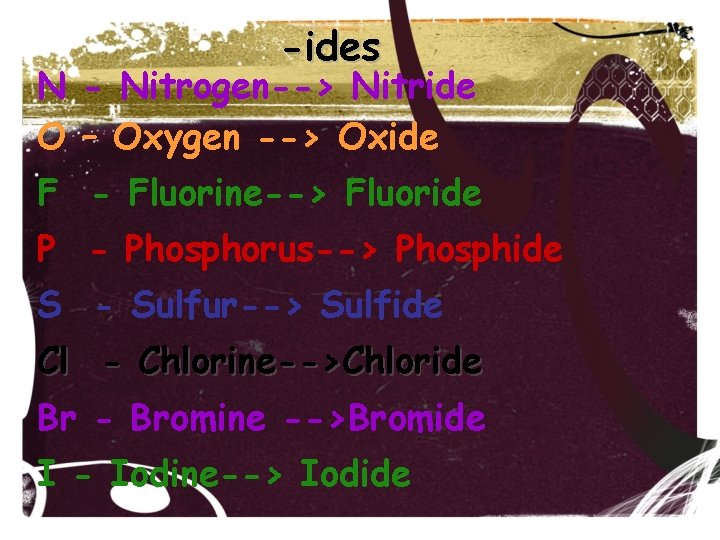
-ides N - Nitrogen--> Nitride O – Oxygen --> Oxide F - Fluorine--> Fluoride P - Phosphorus--> Phosphide S - Sulfur--> Sulfide Cl - Chlorine-->Chloride Br - Bromine -->Bromide I - Iodine--> Iodide

Naming Polyatomic Non variable Compounds 1. Name the positive ion 2. Name the negative ion changing the ending to -ate. Al. PO 4 Aluminum Phosphate No Roman numeral is needed

Naming Ionic Compounds If there is a polyatomic anion, then you do not change the ending: EX: Ca(NO 3)2 Calcium nitrate

Name These: Na 2 O Mg. Cl 2 Na 2 CO 3

Naming binary compounds of variable metals Determine the oxidation number of the variable metal using the crisscross method Cr 2 O 3 Cr+ OThe oxidation number of the chromium is +3 The name is Chromium III oxide

Naming Ionic Compounds 3)If a positive ion (a metal) can have more than one oxidation number, you have to designate its charge in the name! We do this by putting the charge as a roman numeral in parenthesis between the positive and negative

Naming Ionic Compounds Why do we need to do that? Name: Fe 2 O 3 Fe. O These both exist in nature, so we have to show which one we mean.

Naming Ionic Compounds If. Reverse this is true, then what +3 criss was iron to begin with? Fe-22 O 3 cross to find the Name it: charge of the iron: Iron (III)oxygen ide

Naming Ionic Compounds this is not true, the +2 -2 Ifnumbers Reverse criss must have been Fe-1 O cross reduced. to find the Name it: charge of the iron: Iron ( II )oxygen ide

Naming Ionic Compounds Metals that don’t need parentheses: Group I, II and IIIA Zn, Cd (always +2) and Ag (always +1) Which means transition, inner

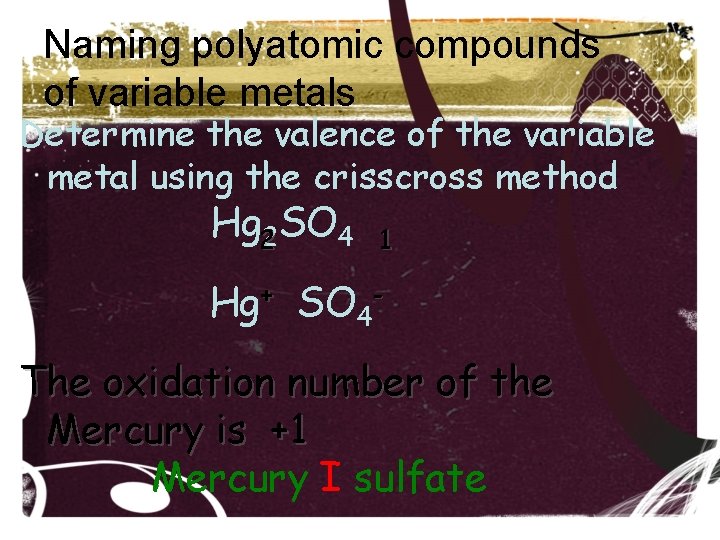
Naming polyatomic compounds of variable metals Determine the valence of the variable metal using the crisscross method Hg 22 SO 4 1 Hg+ SO 4 The oxidation number of the Mercury is +1 Mercury I sulfate

Naming Ionic C ompounds Final flowchart of how to name: Name the positive ion. Does it need a roman numeral? If so, reverse criss cross, if not, ignore. Name the negative ion and: If a nonmetal end in -ide; if not, end normally

Name these: Na 2 S Cu. Cl 2 K 2 SO 4 Pb(NO 3)3

Finding formulas 1. Determine the charge of each side of the formula. Nonvariables: Aluminum oxide: Al+3 O-2 Potassium Chloride K+1 Cl-1 Calcium nitrate Ca +2 NO 3 -1 2. Add the oxidation numbers together. +3 -2 = +1 +1 -1 = 0 +2 -1 = +1

2. Add the oxidation numbers together. Al+3 O-2 K+1 Cl-1 +3 -2 = +1 +1 -1 = 0 3. If the total = zero the formula is balanced with one ion each ex: KCl

If the total does not equal zero use the crisscross method to determine the number of ions needed for each side of the formula. 3 Al+3 O-22 Al O

If the total does not equal zero use the crisscross method to determine the number of ions needed for each side of the formula. 3 2 +3 -2 Al SO 4 Al (SO 4)

Writing Chemical Formulas 5) If using polyatomic ions, put parentheses if there are more than one. +2 2 -1 1 Ca (NO 3)

Writing Chemical Formulas Here’s an example of a polyatomic that doesn’t have parentheses. +1 1 -3 3 Na PO 4

Practice These: Barium and chlorine Ba. Cl 2 Rubidium and nitrogen Rb 3 N Lithium and phosphate Li 3 PO 4 Iron (III) and nitrate. Fe(NO 3)3 Manganese (IV) and sulfur Mn. S 2

Chapter 8 Notes: Part III Bonding in Metals

Metallic Bonds • Metallic bonds consist of metal cations with a freefloating “sea of electrons” • This explains many physical properties—why metals are good conductors, and why they are malleable and

Malleablilty/Ductility • Ductile – the ablity to be drawn into wires • Malleable – the ablity to be hammered into

Malleablilty/Ductility • Metals display these characteristics because when subjected to pressure, cations can easily slide past one another (unlike ionic solids, which have very strong attractive

Alloys • Most metals you use everyday are a mixture of two or more elements, for example brass, bronze or steel.

Alloys • The importance of alloys are that often they have superior properties than the elements they are made of.

Oxyanions An oxyanion is a polyatomic ion composed of an element, usually a nonmetal, bonded to one or more oxygen atoms. Many oxyaions contain the same nonmetal and have the same charges but differ in the number of oxygen atoms.

These ions are easily named using the following conventions. • The ion with more oxygen atoms is named using the root of the nonmetal plus the suffix-ate. • The ion with fewer oxygen atoms is named using the root of the nonmetal plus the suffix-ite.

Halogens form four oxyanions • The ion with the greatest number of oxygen atoms is named using the prefix per-, the root of the nonmetal, and the suffix –ate. • The ion with one less oxygen atom is named with the root of the nonmetal and the suffix –ate. • The ion two fewer oxygen atoms is named using the root of the nonmetal plus the suffix –ite.

• The ion with three fewer oxygen atoms is named using the prefix hypo-, the root of the nonmetal, and the suffix –ite.