CHAPTER 8 NOTES COVALENT BONDING Covalent Bonding Why

CHAPTER 8 NOTES COVALENT BONDING
Covalent Bonding • Why do atoms bond? • Atoms held together by sharing electrons • Molecules – a neutral group of atoms joined together by covalent bonds • Diatomic molecules – a molecule consisting of two atoms • Some elements exist in nature as diatoms • H, N, O, F, Cl, Br, I • Healthy Nerves Originate From Clear Brown Iodine • H O F Br I N Cl • Lucky 7 • Molecular compound – compound composed of molecules • Lower melting and boiling points than ionic compounds
Covalent Bonding • Molecular formula - the chemical formula of a molecular compound (how many atoms of each element a molecule contains) • Tells you nothing about the structure of the molecule • Structural formula • Space-filling molecular formula • Perspective drawing • Ball-and-stick molecular model
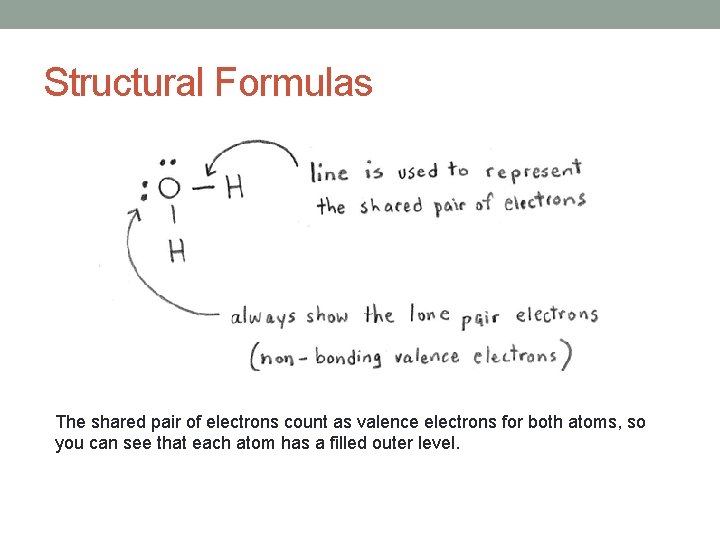
Structural Formulas The shared pair of electrons count as valence electrons for both atoms, so you can see that each atom has a filled outer level.

Covalent Bonding • Single covalent bond – 1 pair of electrons are being shared between two atoms or 2 shared electrons • Example: Methane (CH 4) • Double covalent bond – 2 pairs of electrons are being shared between two atoms • Example: Ethene (C 2 H 4) or Carbon dioxide • Triple covalent bond – 3 pairs of electrons are being shared between two atoms • Example: N 2 www. lookchem. com www. askiitians. com dkreutz. basd. k 12. wi. us
Covalent bonding • Covalent bond – bond in which electrons are shared • Atoms overlap their valence orbitals therefore leading to the “sharing” of valence electrons between the two atoms. • Key idea: How many electrons are shared between atoms? • # of electrons needed = # of electrons shared

Something to think about… • You’ve been told that molecular compounds generally have lower melting and boiling points than ionic compounds. What do you think causes this difference? • We will return to this question for the next few days to answer it completely.
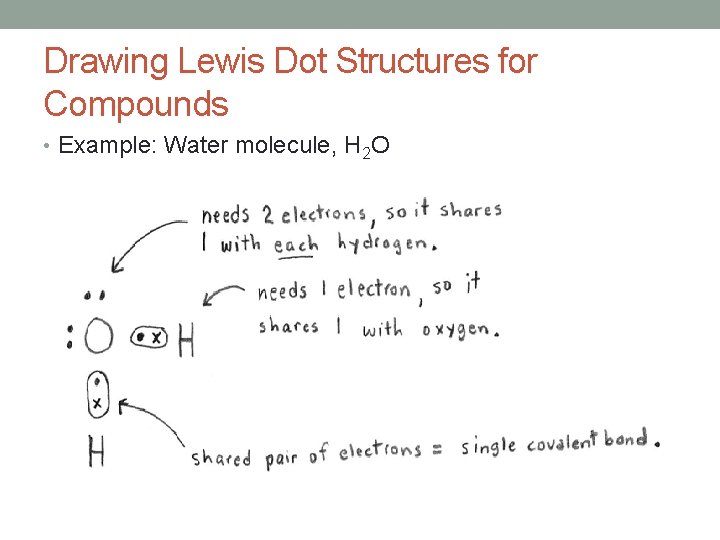
Drawing Lewis Dot Structures for Compounds • Example: Water molecule, H 2 O

Model Building Activity • To learn how atoms covalently bond with each other, we will complete a model building lab with model kits. 1. Different elements are represented by different colored atoms. 2. Each atom has a different number of “holes” in it. The holes equal the number of bonds that atom makes (there are some exceptions so use the rule we learned about the # of bonds an atom makes. 3. Use the short springs for the bonds, unless you need to have 2 bonds going between 2 atoms, then use the longer, more flexible springs. 4. Identify the shape and molecular polarity. 5. Let’s do HBr together.
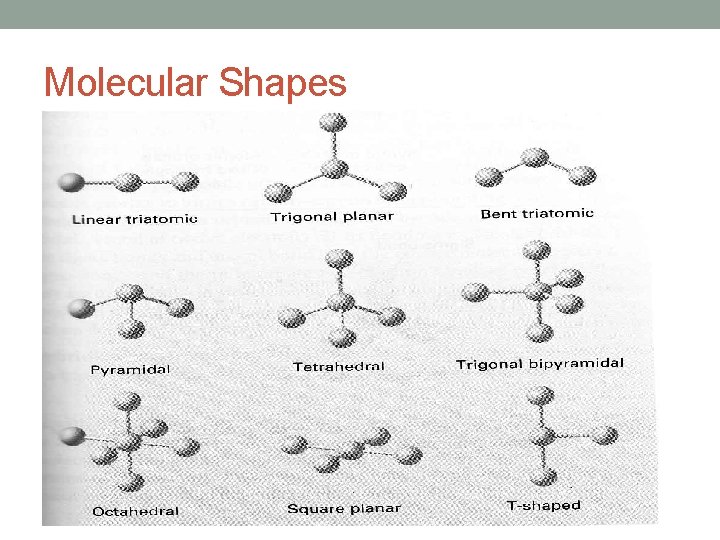
Molecular Shapes
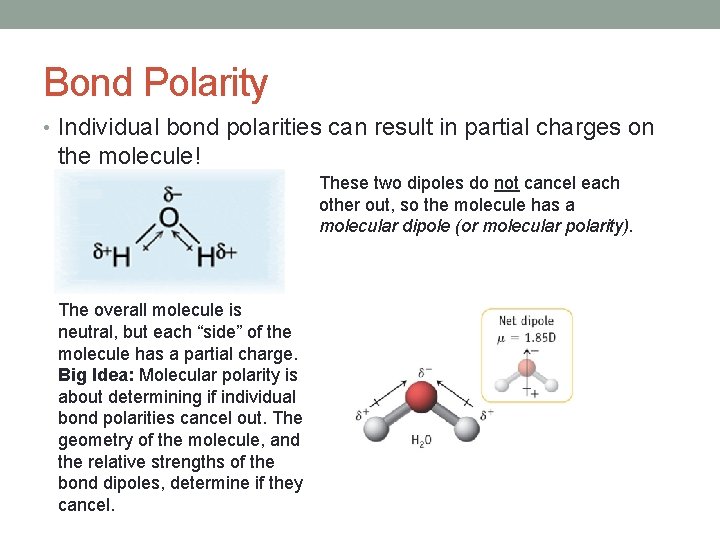
Bond Polarity • Individual bond polarities can result in partial charges on the molecule! These two dipoles do not cancel each other out, so the molecule has a molecular dipole (or molecular polarity). The overall molecule is neutral, but each “side” of the molecule has a partial charge. Big Idea: Molecular polarity is about determining if individual bond polarities cancel out. The geometry of the molecule, and the relative strengths of the bond dipoles, determine if they cancel.
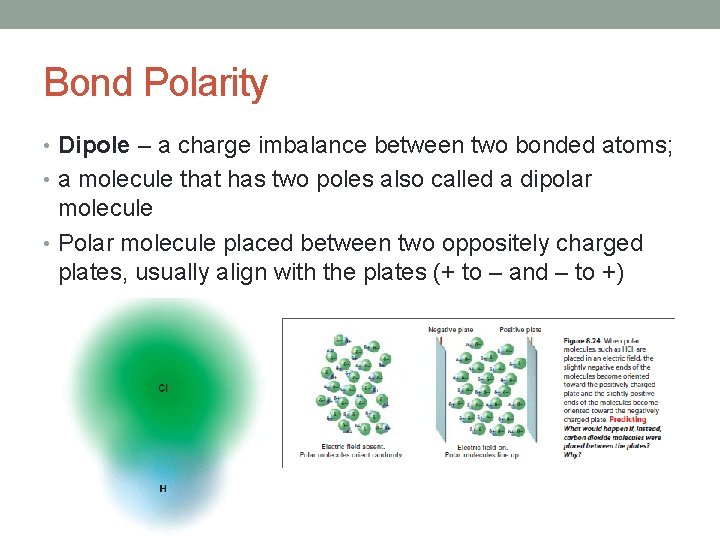
Bond Polarity • Dipole – a charge imbalance between two bonded atoms; • a molecule that has two poles also called a dipolar molecule • Polar molecule placed between two oppositely charged plates, usually align with the plates (+ to – and – to +)

Shapes and polarity Bent molecules – dipoles never cancel Pyramidal molecules – dipoles never cancel Dipoles in trigonal planar and tetrahedral molecules, do cancel out. No net dipole

Bond Angles • Linear – 180° • Trigonal planar - 120° • Tetrahedral -109. 5° • Pyramidal - 107° • Bent -105°
Intermolecular Attraction Forces • Weaker than ionic and covalent bonds • Why is water a liquid at room temperature, but CO 2 is a gas? • Why is I 2 a solid, but F 2 a gas? • The strength of the attractions between the molecules, determines the state! • Inter = between • Van der Waals forces – weakest attractions • Dipole Interactions • Dispersion Forces
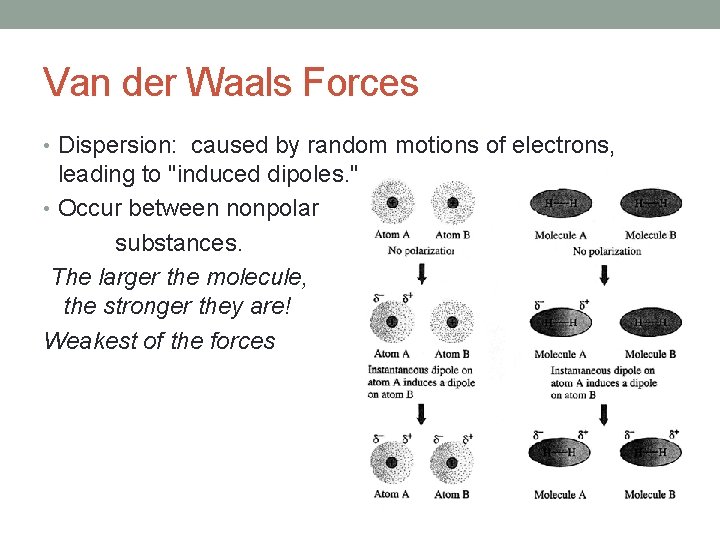
Van der Waals Forces • Dispersion: caused by random motions of electrons, leading to "induced dipoles. " • Occur between nonpolar substances. The larger the molecule, the stronger they are! Weakest of the forces
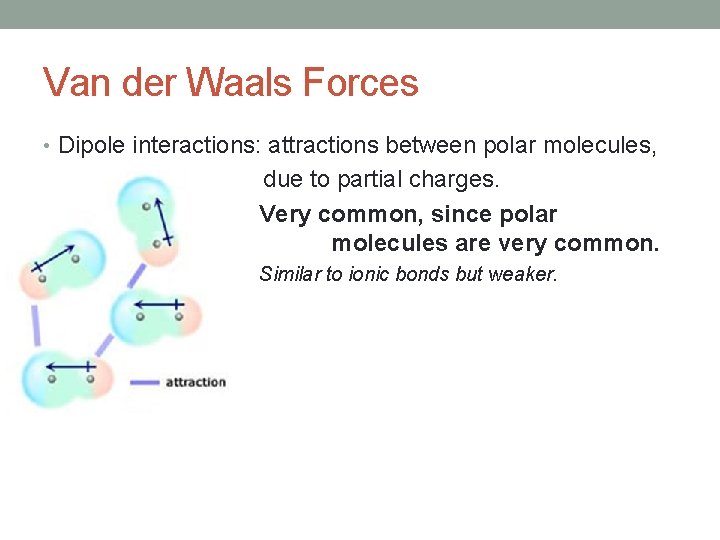
Van der Waals Forces • Dipole interactions: attractions between polar molecules, due to partial charges. Very common, since polar molecules are very common. Similar to ionic bonds but weaker.
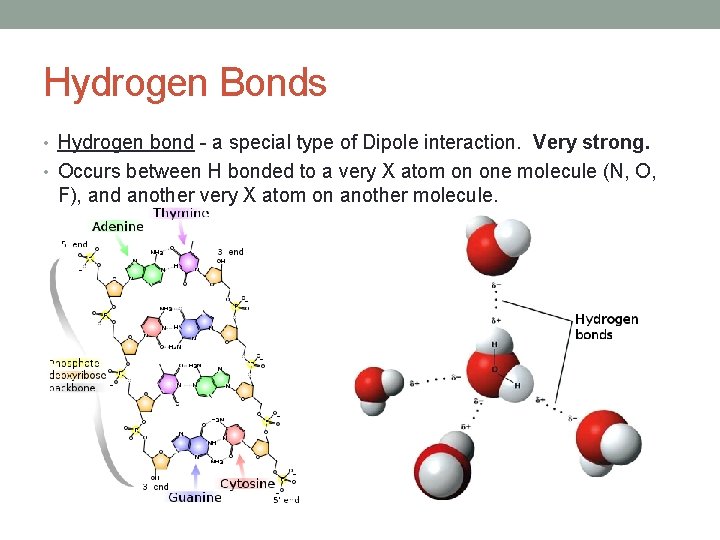
Hydrogen Bonds • Hydrogen bond - a special type of Dipole interaction. Very strong. • Occurs between H bonded to a very Χ atom on one molecule (N, O, F), and another very Χ atom on another molecule.

Hydrogen Bonding • Has about 5% the strength of an average covalent bond • Strongest of the intermolecular forces

Check for Understanding • Place the intermolecular attractions in order of increasing strength. Dispersion forces < dipole interactions< hydrogen bonding

Covalent Network Solids • Covalent Network Solids - a covalently-bonded crystal. Very strong! (all atoms are covalently bonded to each other. Carbon (diamond allotrope) Si. O 2 (quartz) • Diamond vaporizes at 3500 degrees C (does not melt) • Quartz melts at 2700 degrees C

Ionic Forces in a Crystal • Attractions between formula units in an ionic crystal are very strong! This explains why ionic compounds are solids at room temperature. Na. Cl – table salt

Bond Dissociation Energy • The energy required to break the bond between two covalently bonded atoms • Large BDE = a strong covalent bond. • Expressed as the energy needed to break 1 mole of bonds (6. 02 x 1023 bonds) Units - k. J/mol
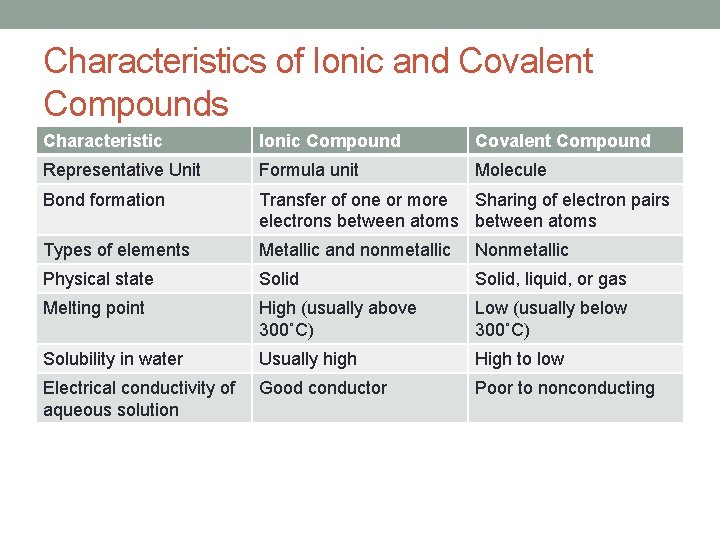
Characteristics of Ionic and Covalent Compounds Characteristic Ionic Compound Covalent Compound Representative Unit Formula unit Molecule Bond formation Transfer of one or more Sharing of electron pairs electrons between atoms Types of elements Metallic and nonmetallic Nonmetallic Physical state Solid, liquid, or gas Melting point High (usually above 300˚C) Low (usually below 300˚C) Solubility in water Usually high High to low Electrical conductivity of aqueous solution Good conductor Poor to nonconducting
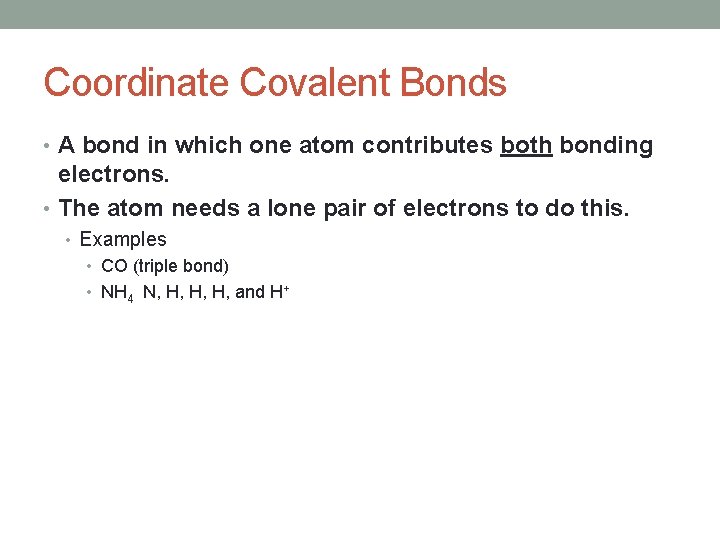
Coordinate Covalent Bonds • A bond in which one atom contributes both bonding electrons. • The atom needs a lone pair of electrons to do this. • Examples • CO (triple bond) • NH 4 N, H, H, H, and H+

Polyatomic Ions • A tightly bound group of atoms that has a positive or negative charge and behaves as one unit • Most polyatomic have both covalent and coordinate covalent bonds
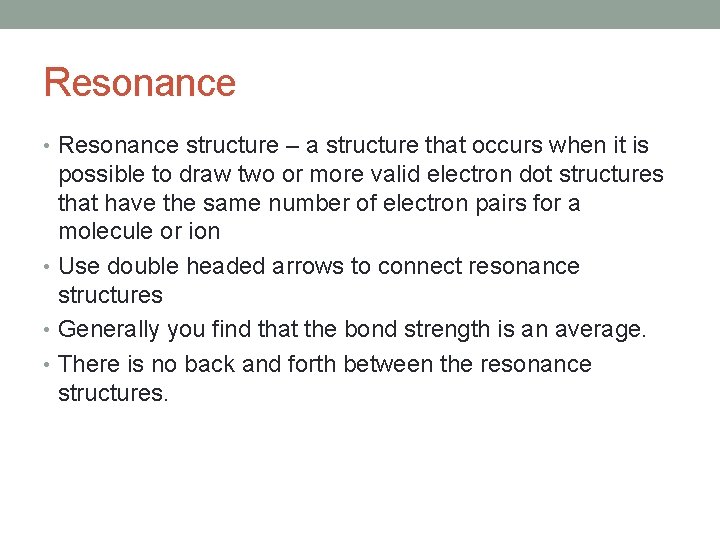
Resonance • Resonance structure – a structure that occurs when it is possible to draw two or more valid electron dot structures that have the same number of electron pairs for a molecule or ion • Use double headed arrows to connect resonance structures • Generally you find that the bond strength is an average. • There is no back and forth between the resonance structures.
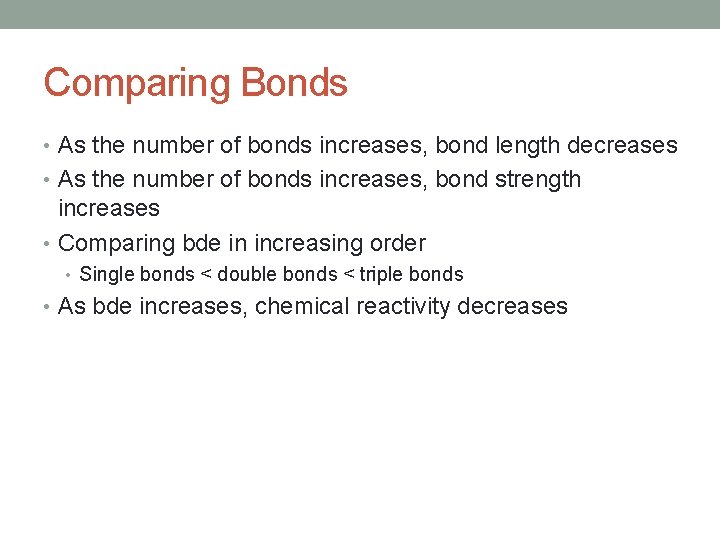
Comparing Bonds • As the number of bonds increases, bond length decreases • As the number of bonds increases, bond strength increases • Comparing bde in increasing order • Single bonds < double bonds < triple bonds • As bde increases, chemical reactivity decreases

Exceptions to the Octet Rule • The octet rule will not be satisfied in molecules whose total number of valence electrons is an odd number. • Some molecules will have fewer or more than a complete octet • Example NO 2 – two possible structures • Remember the number of bonds formed will equal the number of electrons needed • There is an unpaired electron on nitrogen no matter how the structure is drawn • Some with even numbers will also be exceptions to the octet rule. • Example BF 3 • Expanded octets • PCl 5 and SF 6

Bonding Theories • VSEPR • Valence-Shell Electron-Pair Repulsion Theory • The repulsion between electron pairs causes molecular shapes to adjust so that the valence-electron pairs stay as far apart as possible. • Bond angles • Tetrahedral angle– 109. 5º • Bent - 105º • Linear - 180º
Molecular Orbitals • Quantum mechanic model • Produced when two atoms combine their atomic orbitals overlap to produce molecular orbitals • Bonding orbital • A molecular orbital that can be occupied by two electrons of a covalent bond • Sigma bonds • Two atomic orbitals combine – the resulting molecular structure is symmetrical around the bond axis

Sigma Bonds
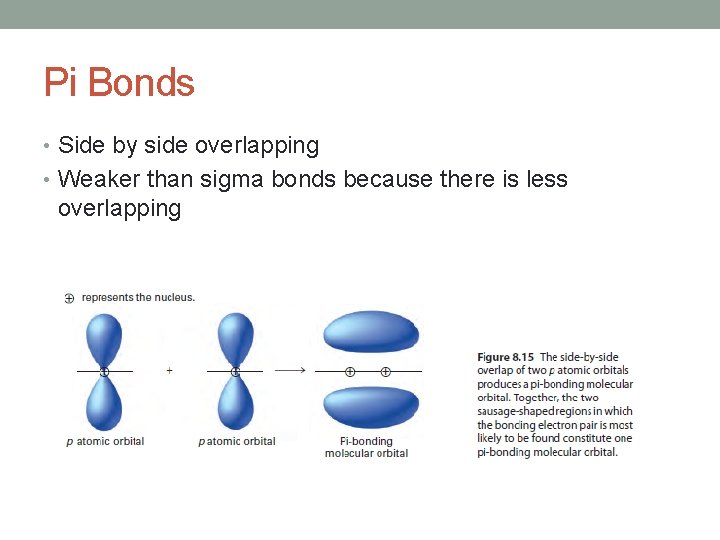
Pi Bonds • Side by side overlapping • Weaker than sigma bonds because there is less overlapping
Hybridization • Occurs when several atomic orbitals mix to form the same number of hybrid orbitals • Gives information about molecular bonding and molecular shape
- Slides: 34