Chapter 8 Metabolism Energy and Enzymes AP Biology

Chapter 8: Metabolism, Energy, and Enzymes AP Biology
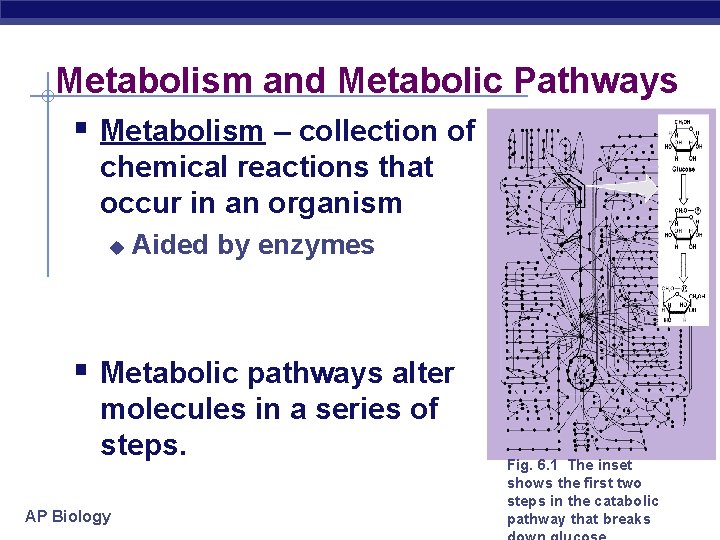
Metabolism and Metabolic Pathways § Metabolism – collection of chemical reactions that occur in an organism u Aided by enzymes § Metabolic pathways alter molecules in a series of steps. AP Biology Fig. 6. 1 The inset shows the first two steps in the catabolic pathway that breaks
Enzyme Role § Enzymes selectively accelerate each step. u The activity of enzymes is regulated to maintain an appropriate balance of supply and demand. § Catabolic pathways Break down complex molecules u Release energy u § Anabolic pathways u Build complex molecules § Energy released by catabolism is used to drive anabolism AP Biology
Organism Energy Transformation § Energy is the capacity to do work - to move matter against opposing forces. u Energy is also used to rearrange matter. § Kinetic energy Energy of motion u Ex. - Objects in motion, photons, and heat u § Potential energy Stored energy u Chemical energy - form of potential energy in molecules because of the arrangement of atoms. u AP Biology
Metabolic reactions & energy § Some chemical reactions release energy exergonic u breaking polymers u hydrolysis = catabolism u Ex. Cellular respiration u digesting molecules= less organization= lower energy state § Some chemical reactions require input of energy building molecules= more organization= higher energy state endergonic u building polymers u dehydration synthesis = anabolism u Ex. Photosynthesis u AP Biology
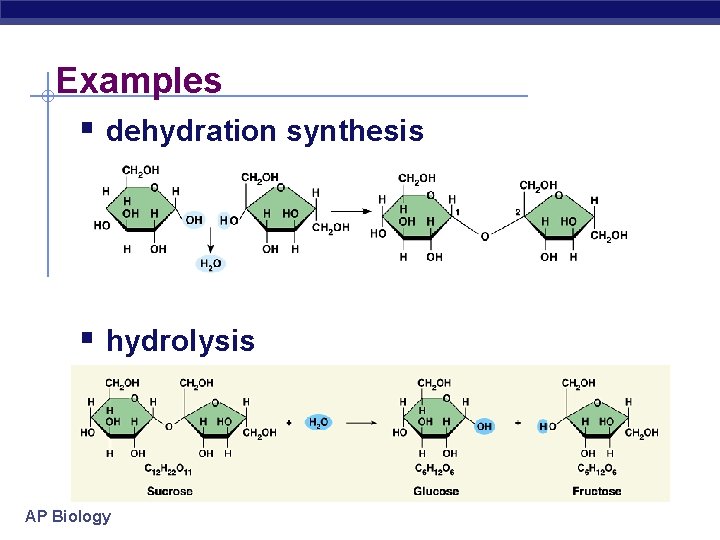
Examples § dehydration synthesis § hydrolysis AP Biology
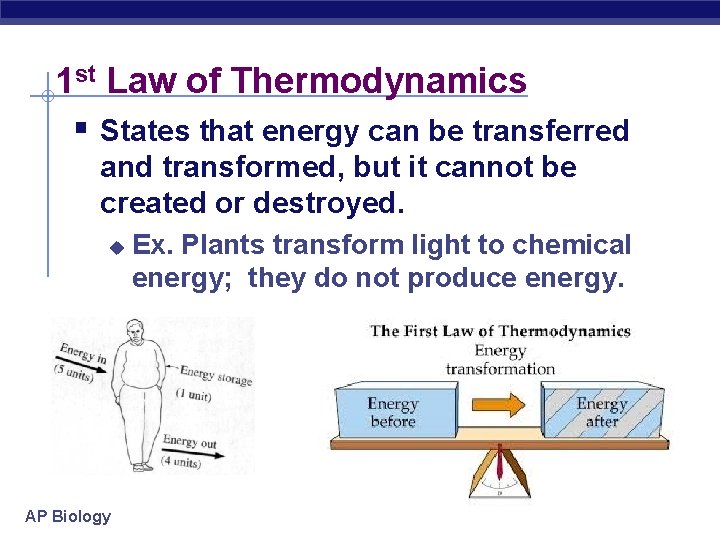
1 st Law of Thermodynamics § States that energy can be transferred and transformed, but it cannot be created or destroyed. u AP Biology Ex. Plants transform light to chemical energy; they do not produce energy.
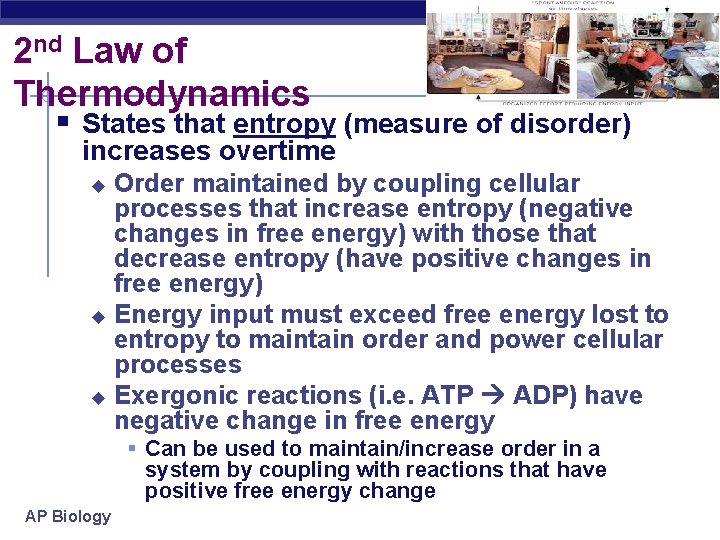
2 nd Law of Thermodynamics § States that entropy (measure of disorder) increases overtime Order maintained by coupling cellular processes that increase entropy (negative changes in free energy) with those that decrease entropy (have positive changes in free energy) u Energy input must exceed free energy lost to entropy to maintain order and power cellular processes u Exergonic reactions (i. e. ATP ADP) have negative change in free energy u § Can be used to maintain/increase order in a system by coupling with reactions that have positive free energy change AP Biology
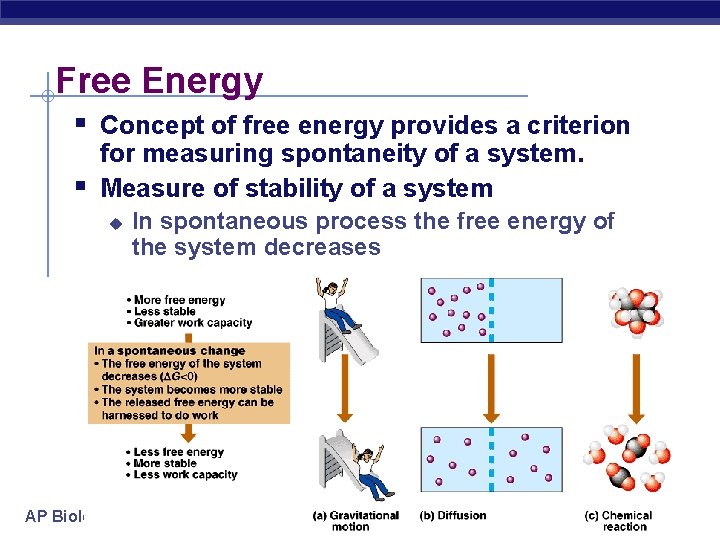
Free Energy § Concept of free energy provides a criterion § for measuring spontaneity of a system. Measure of stability of a system u AP Biology In spontaneous process the free energy of the system decreases
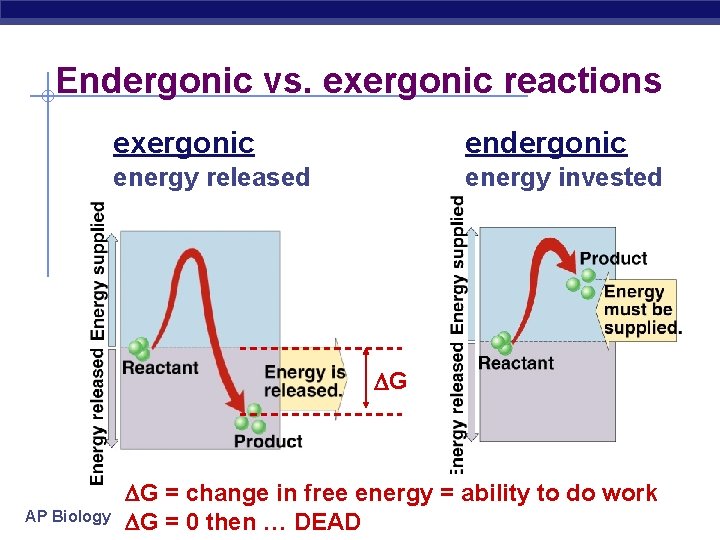
Endergonic vs. exergonic reactions exergonic endergonic energy released energy invested G AP Biology G = change in free energy = ability to do work 2005 -2006 G = 0 then … DEAD
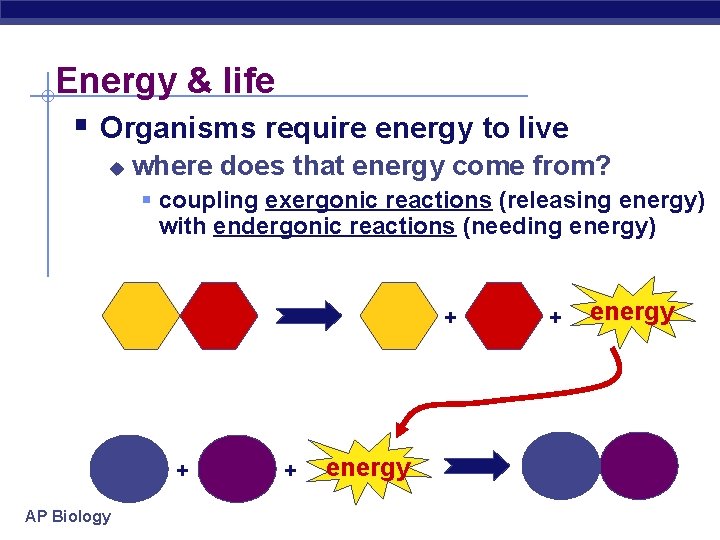
Energy & life § Organisms require energy to live u where does that energy come from? § coupling exergonic reactions (releasing energy) with endergonic reactions (needing energy) + + AP Biology + energy
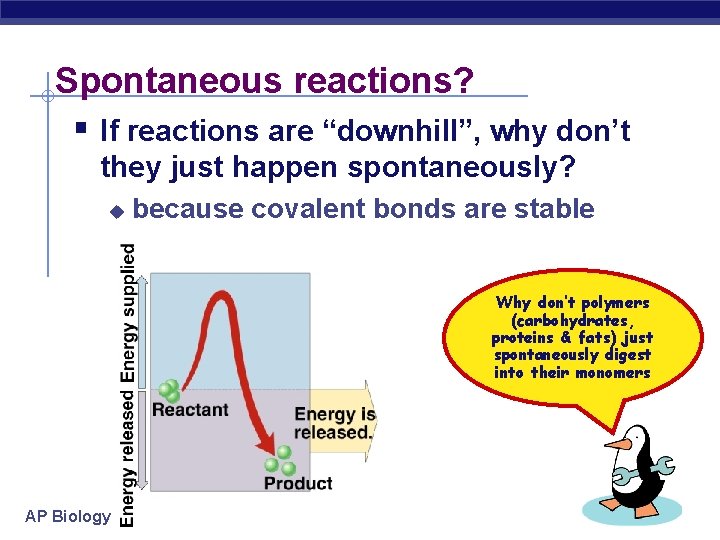
Spontaneous reactions? § If reactions are “downhill”, why don’t they just happen spontaneously? u because covalent bonds are stable Why don’t polymers (carbohydrates, proteins & fats) just spontaneously digest into their monomers AP Biology 2005 -2006
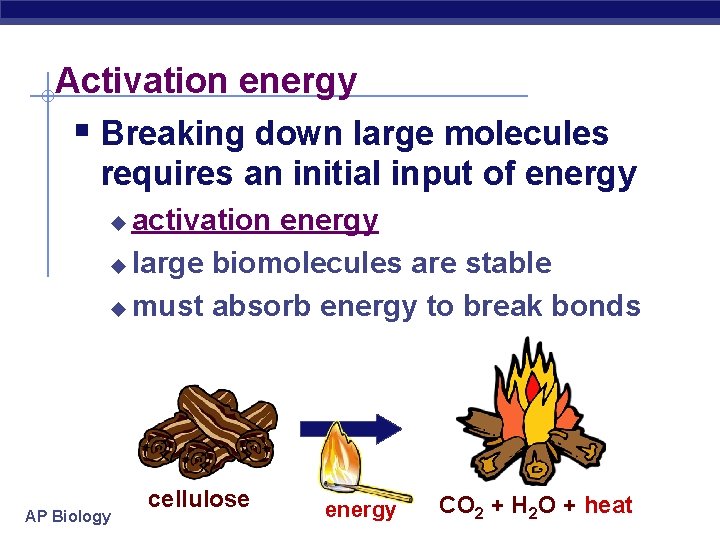
Activation energy § Breaking down large molecules requires an initial input of energy activation energy u large biomolecules are stable u must absorb energy to break bonds u AP Biology cellulose energy CO 2 + H 2 O + heat
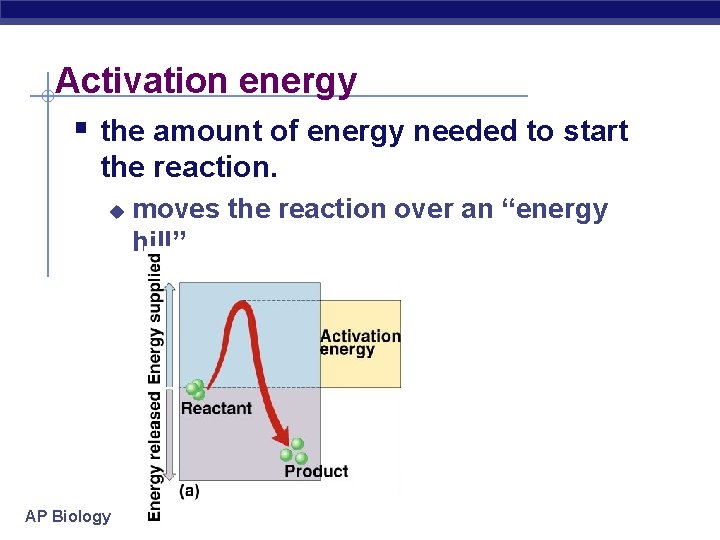
Activation energy § the amount of energy needed to start the reaction. u AP Biology moves the reaction over an “energy hill”
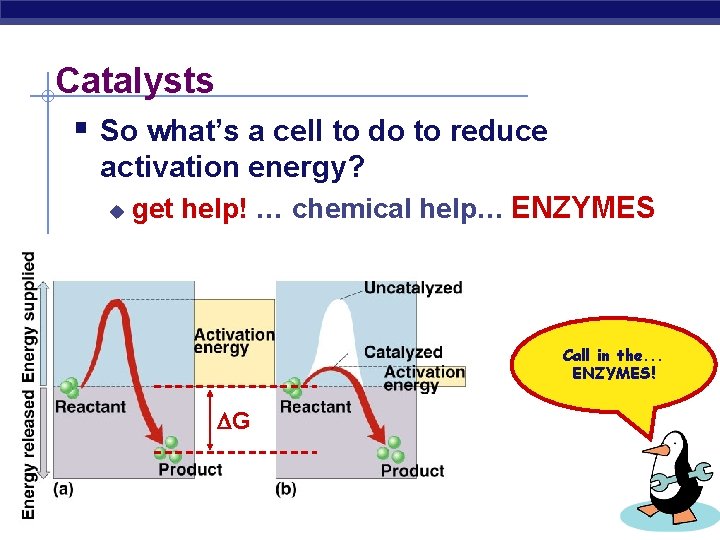
Catalysts § So what’s a cell to do to reduce activation energy? u get help! … chemical help… ENZYMES Call in the. . . ENZYMES! G AP Biology

Energy needs of life § Organisms are endergonic systems u What do we need energy for? § § § AP Biology synthesis (biomolecules) reproduction active transport movement temperature regulation 2005 -2006
Living economy § Fueling the economy u u u eat high energy organic molecules (food) break them down = catabolism (digest) capture energy in form cell can use § Need an energy currency u a way to pass energy around Whoa! Hot stuff! AP Biology ATP
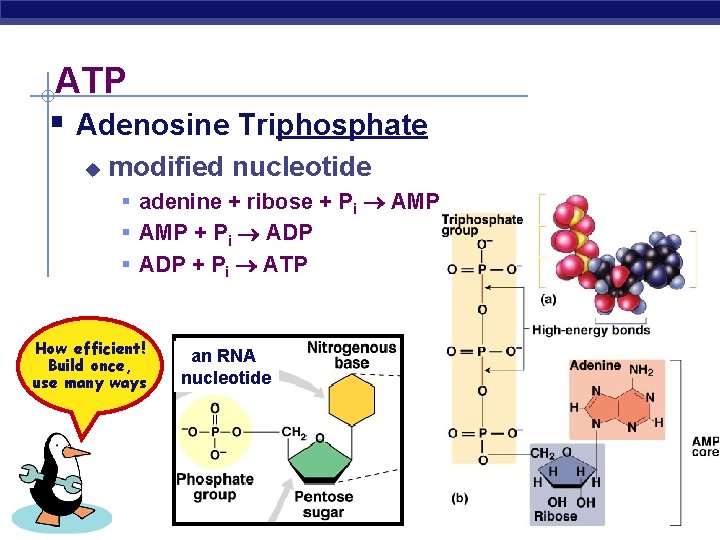
ATP § Adenosine Triphosphate u modified nucleotide § adenine + ribose + Pi AMP § AMP + Pi ADP § ADP + Pi ATP How efficient! Build once, use many ways AP Biology an RNA nucleotide 2005 -2006
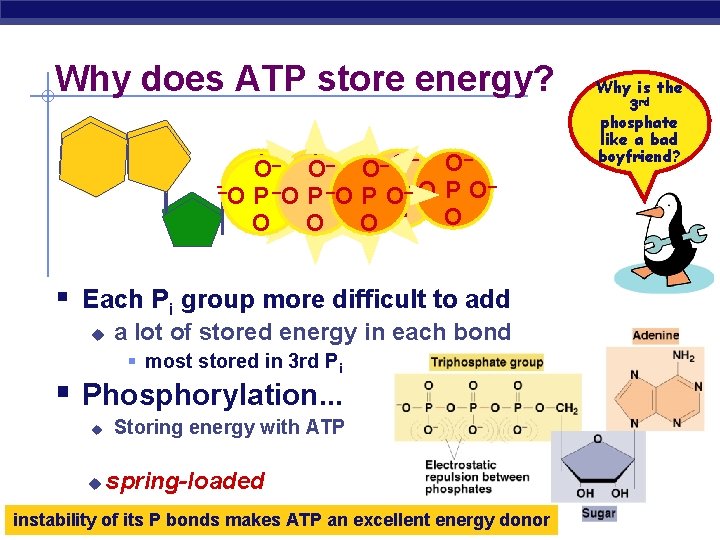
Why does ATP store energy? – – – – O O O O – –O– ––O P––O – – –O OP P –O O O– P O OPO O O O Why is the 3 rd phosphate like a bad boyfriend? § Each Pi group more difficult to add u a lot of stored energy in each bond § most stored in 3 rd Pi § Phosphorylation. . . u u Storing energy with ATP spring-loaded AP Biology instability of its P bonds makes ATP an excellent energy donor 2005 -2006
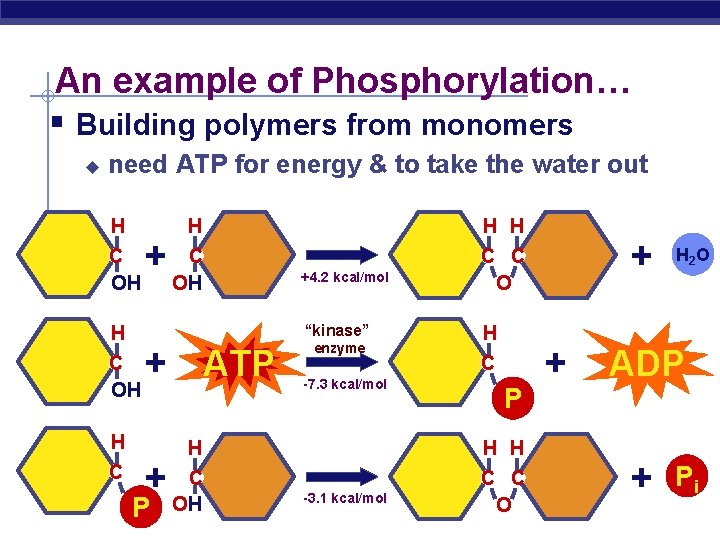
An example of Phosphorylation… § Building polymers from monomers u need ATP for energy & to take the water out H C OH + C OH C C O + ATP H AP Biology H H +4. 2 kcal/mol “kinase” H C H + P enzyme -7. 3 kcal/mol H C P H H H C OH C C O -3. 1 kcal/mol + + H 2 O ADP + 2005 -2006 Pi
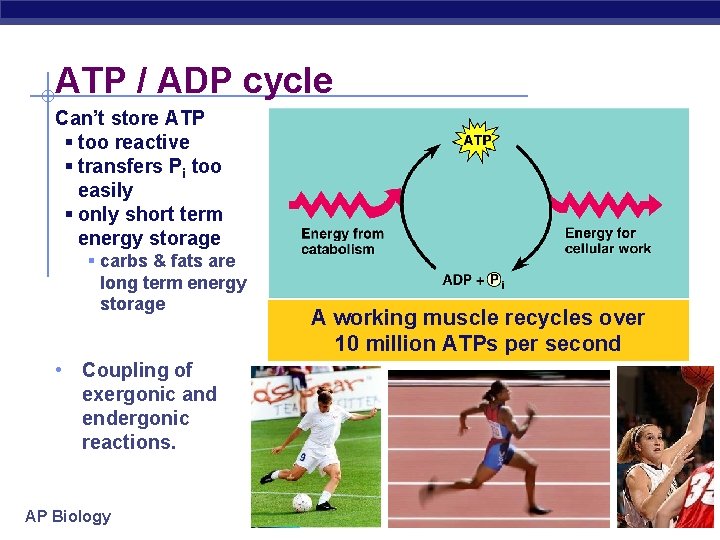
ATP / ADP cycle Can’t store ATP § too reactive § transfers Pi too easily § only short term energy storage § carbs & fats are long term energy storage • Coupling of exergonic and endergonic reactions. AP Biology A working muscle recycles over 10 million ATPs per second
What’s the point? § Cells spend a lot of time making ATP! “The point is to make ATP!” For chemical, mechanical, and transport work Make ATP! That’s all I do all day. And no one even notices! AP Biology I didn’t HEAR you!
Enzymes AP Biology
Enzymes § Biological catalysts u u proteins (& RNA) facilitate chemical reactions § increase rate of reaction without being consumed § reduce activation energy § don’t change free energy ( G) released or required u u required for most biological reactions highly specific § thousands of different enzymes in cells u AP Biology control reactions of life
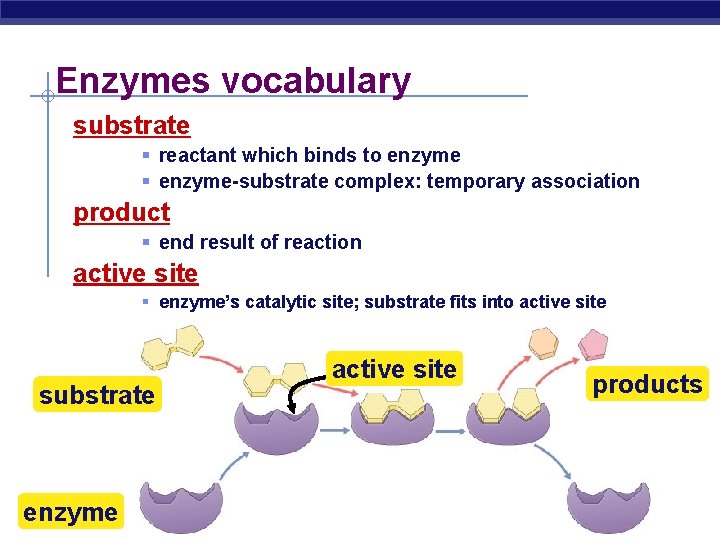
Enzymes vocabulary substrate § reactant which binds to enzyme § enzyme-substrate complex: temporary association product § end result of reaction active site § enzyme’s catalytic site; substrate fits into active site substrate enzyme AP Biology active site products
Properties of enzymes § Reaction specific u each enzyme works with a specific substrate § chemical fit between active site & substrate w H bonds & ionic bonds § Not consumed in reaction u single enzyme molecule can catalyze thousands or more reactions per second § enzymes unaffected by the reaction § Affected by cellular conditions u any condition that affects protein structure § temperature, p. H, salinity AP Biology
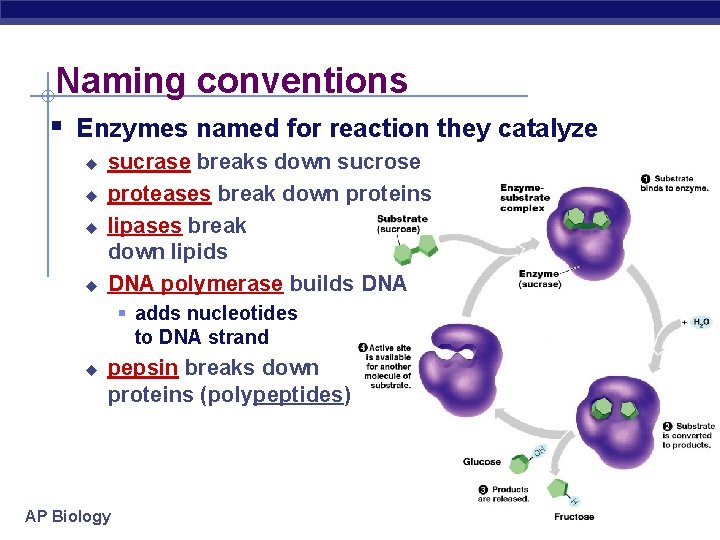
Naming conventions § Enzymes named for reaction they catalyze u u sucrase breaks down sucrose proteases break down proteins lipases break down lipids DNA polymerase builds DNA § adds nucleotides to DNA strand u pepsin breaks down proteins (polypeptides) AP Biology
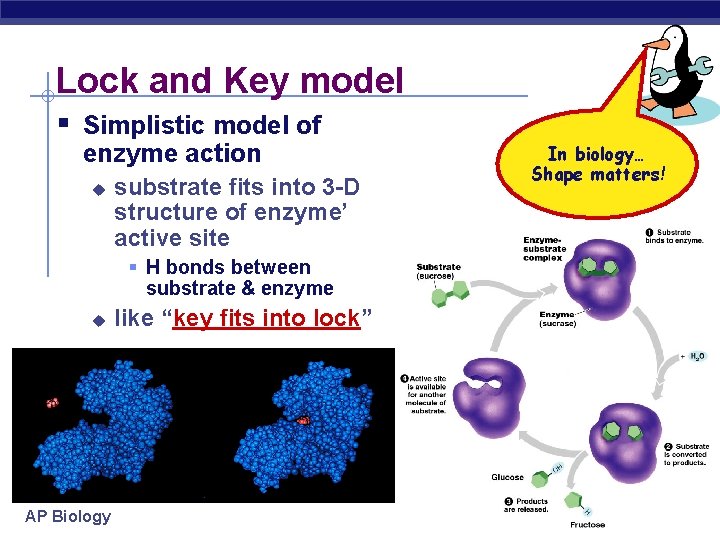
Lock and Key model § Simplistic model of enzyme action u substrate fits into 3 -D structure of enzyme’ active site § H bonds between substrate & enzyme u AP Biology like “key fits into lock” In biology… Shape matters!
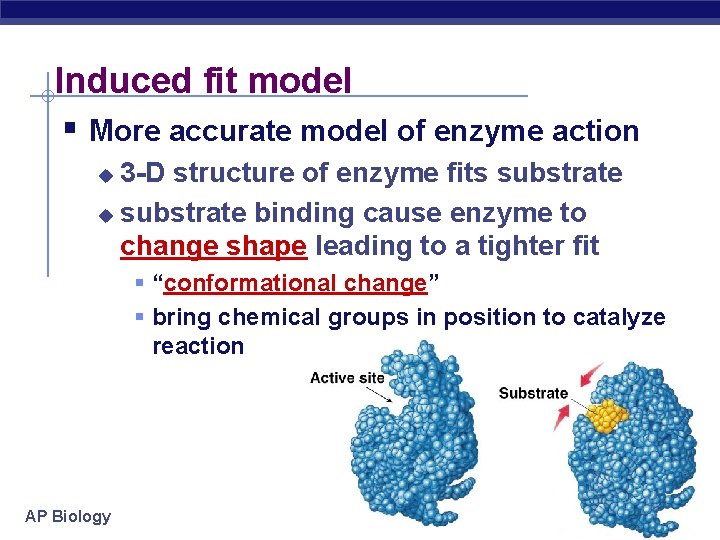
Induced fit model § More accurate model of enzyme action 3 -D structure of enzyme fits substrate u substrate binding cause enzyme to change shape leading to a tighter fit u § “conformational change” § bring chemical groups in position to catalyze reaction AP Biology
How does it work? § Variety of mechanisms to lower activation energy & speed up reaction u synthesis § active site orients substrates in correct position for reaction w enzyme brings substrate closer together u digestion § active site binds substrate & puts stress on bonds that must be broken, making it easier to separate molecules AP Biology
Factors Affecting Enzyme Function § Enzyme concentration § Substrate concentration § Temperature § p. H § Salinity § Activators § Inhibitors AP Biology catalase
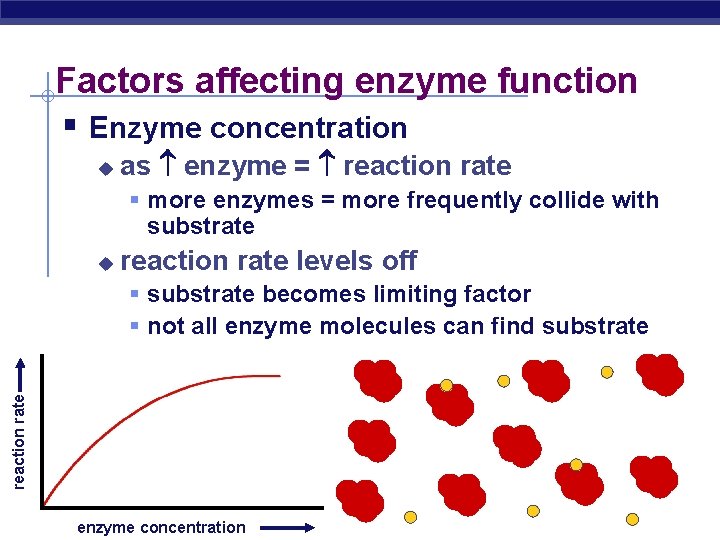
Factors affecting enzyme function § Enzyme concentration u as enzyme = reaction rate § more enzymes = more frequently collide with substrate u reaction rate levels off reaction rate § substrate becomes limiting factor § not all enzyme molecules can find substrate AP Biology enzyme concentration
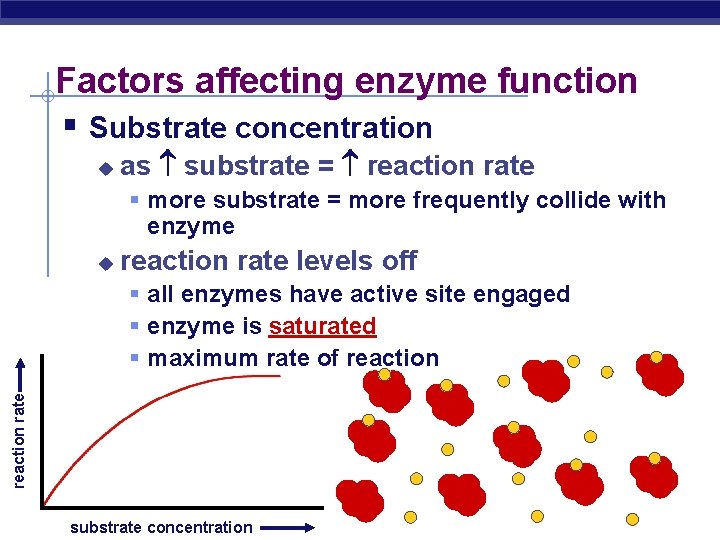
Factors affecting enzyme function § Substrate concentration u as substrate = reaction rate § more substrate = more frequently collide with enzyme u reaction rate levels off reaction rate § all enzymes have active site engaged § enzyme is saturated § maximum rate of reaction AP Biology substrate concentration
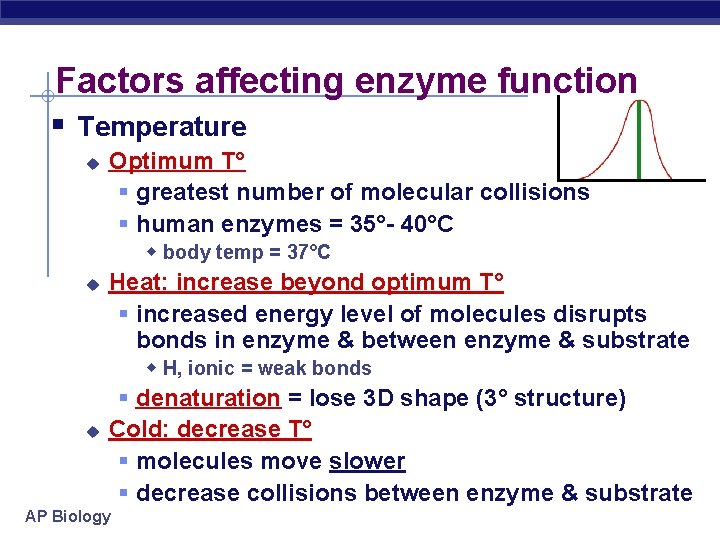
Factors affecting enzyme function § Temperature u Optimum T° § greatest number of molecular collisions § human enzymes = 35°- 40°C w body temp = 37°C u Heat: increase beyond optimum T° § increased energy level of molecules disrupts bonds in enzyme & between enzyme & substrate w H, ionic = weak bonds u § denaturation = lose 3 D shape (3° structure) Cold: decrease T° § molecules move slower § decrease collisions between enzyme & substrate AP Biology
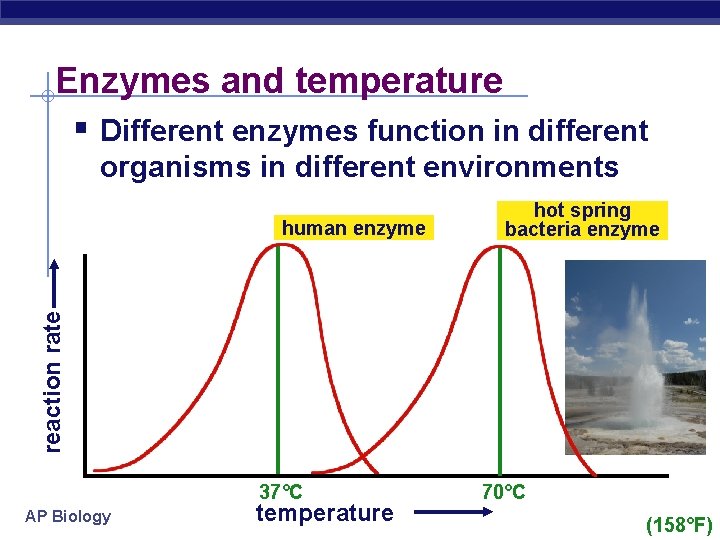
Enzymes and temperature § Different enzymes function in different organisms in different environments reaction rate human enzyme hot spring bacteria enzyme 37°C AP Biology temperature 70°C (158°F)
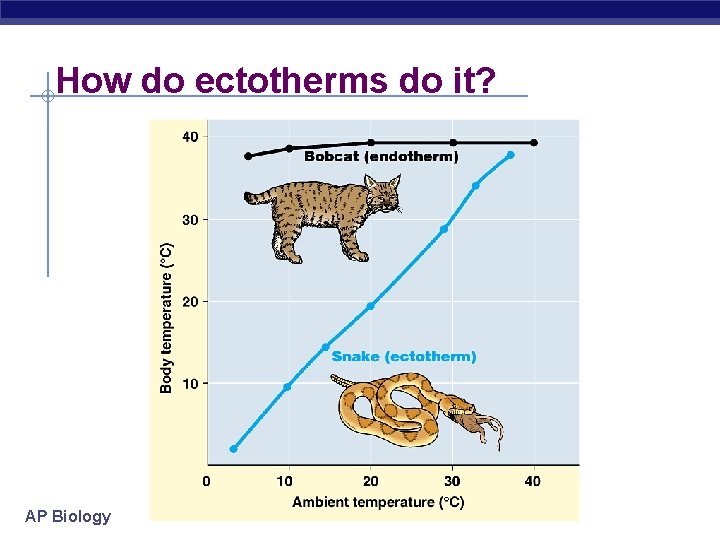
How do ectotherms do it? AP Biology
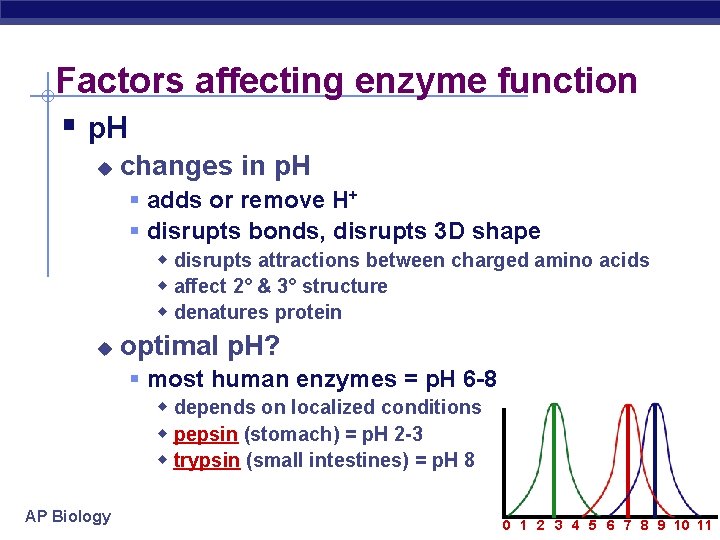
Factors affecting enzyme function § p. H u changes in p. H § adds or remove H+ § disrupts bonds, disrupts 3 D shape w disrupts attractions between charged amino acids w affect 2° & 3° structure w denatures protein u optimal p. H? § most human enzymes = p. H 6 -8 w depends on localized conditions w pepsin (stomach) = p. H 2 -3 w trypsin (small intestines) = p. H 8 AP Biology 0 1 2 3 4 5 6 7 8 9 10 11
Factors affecting enzyme function § Salt concentration u changes in salinity § adds or removes cations (+) & anions (–) § disrupts bonds, disrupts 3 D shape w disrupts attractions between charged amino acids w affect 2° & 3° structure w denatures protein u enzymes intolerant of extreme salinity § Dead Sea is called dead for a reason! AP Biology
Factors affecting enzyme function § Change in function can be interpreted from data regarding: u Concentration of product or substrate as a function of time § Too much substrate graph will level off unless more enzymes are added § Lots of product many enzymes present § Representations demonstrate relationship between enzyme’s activity, disappearance of substrate, and/or presence of competitive inhibitor AP Biology
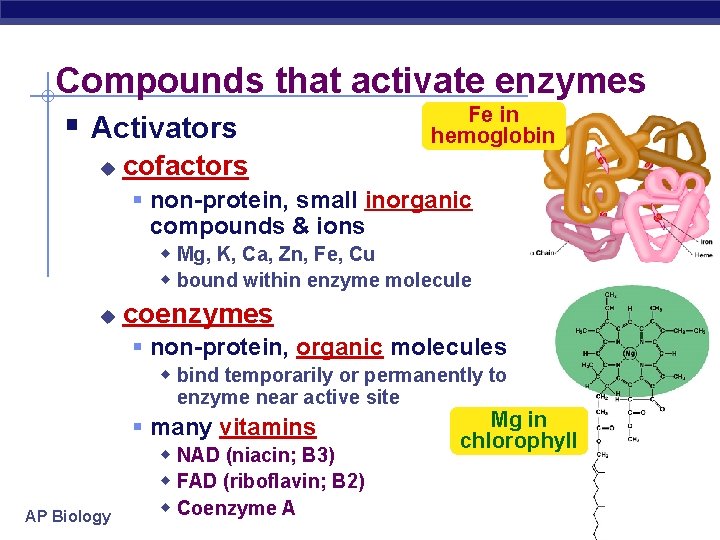
Compounds that activate enzymes Fe in § Activators hemoglobin u cofactors § non-protein, small inorganic compounds & ions w Mg, K, Ca, Zn, Fe, Cu w bound within enzyme molecule u coenzymes § non-protein, organic molecules w bind temporarily or permanently to enzyme near active site AP Biology § many vitamins w NAD (niacin; B 3) w FAD (riboflavin; B 2) w Coenzyme A Mg in chlorophyll
Compounds which inhibit enzymes § Inhibitors molecules that reduce enzyme activity u Bind to active or allosteric site change activity of enzyme u competitive inhibition u noncompetitive inhibition u irreversible inhibition u feedback inhibition u AP Biology
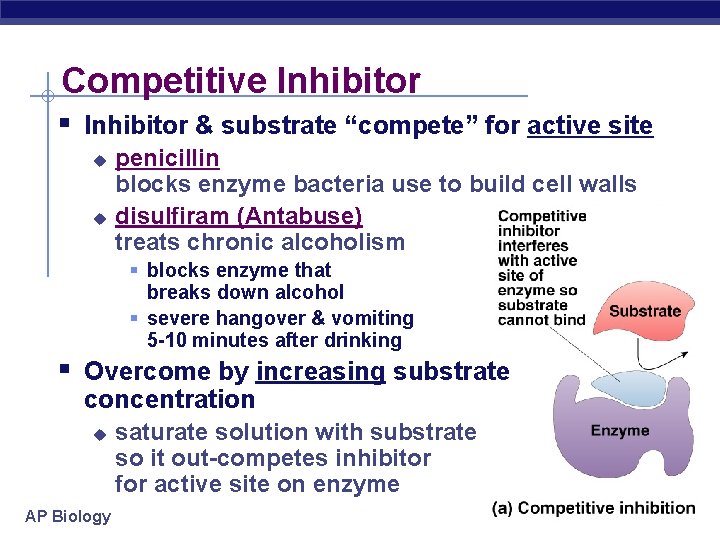
Competitive Inhibitor § Inhibitor & substrate “compete” for active site u u penicillin blocks enzyme bacteria use to build cell walls disulfiram (Antabuse) treats chronic alcoholism § blocks enzyme that breaks down alcohol § severe hangover & vomiting 5 -10 minutes after drinking § Overcome by increasing substrate concentration u AP Biology saturate solution with substrate so it out-competes inhibitor for active site on enzyme
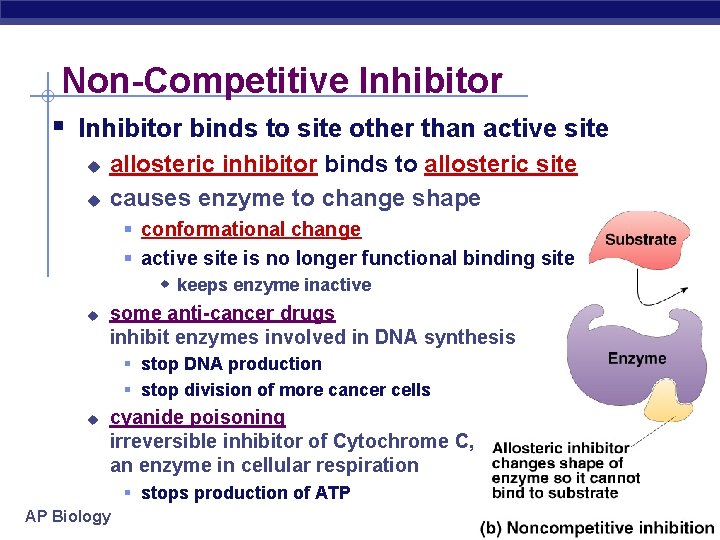
Non-Competitive Inhibitor § Inhibitor binds to site other than active site u u u allosteric inhibitor binds to allosteric site causes enzyme to change shape § conformational change § active site is no longer functional binding site w keeps enzyme inactive some anti-cancer drugs inhibit enzymes involved in DNA synthesis § stop DNA production § stop division of more cancer cells u cyanide poisoning irreversible inhibitor of Cytochrome C, an enzyme in cellular respiration § stops production of ATP AP Biology

Irreversible inhibition § Inhibitor permanently binds to enzyme u competitor § permanently binds to active site u allosteric § permanently binds to allosteric site § permanently changes shape of enzyme § nerve gas, sarin, many insecticides (malathion, parathion…) w cholinesterase inhibitors n AP Biology doesn’t breakdown the neurotransmitter, acetylcholine
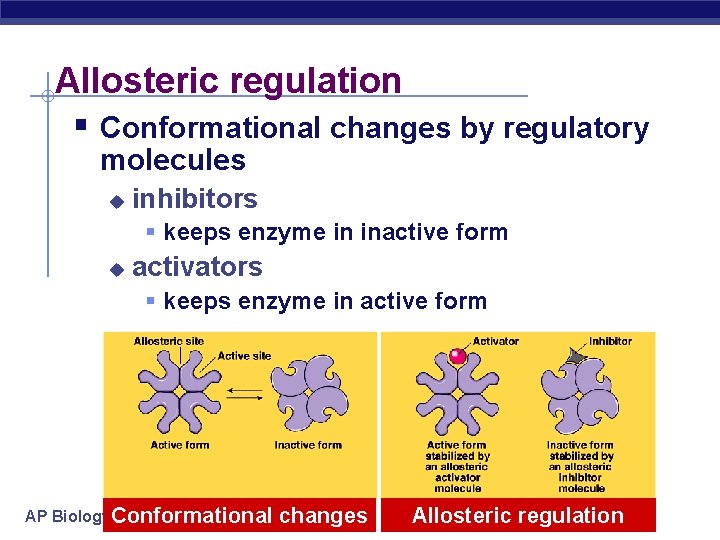
Allosteric regulation § Conformational changes by regulatory molecules u inhibitors § keeps enzyme in inactive form u activators § keeps enzyme in active form AP Biology. Conformational changes Allosteric regulation
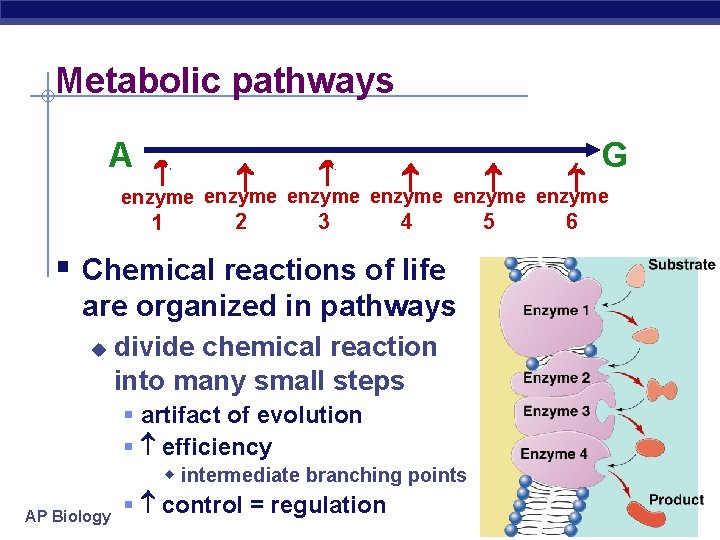
Metabolic pathways A B C D E F G 1 2 3 4 5 6 enzyme enzyme § Chemical reactions of life are organized in pathways u AP Biology divide chemical reaction into many small steps § artifact of evolution § efficiency w intermediate branching points § control = regulation

Feedback Inhibition § Regulation & coordination of production u u product is used by next step in pathway final product is inhibitor of earlier step § allosteric inhibitor of earlier enzyme § feedback inhibition u no unnecessary accumulation of product A B C D E F G 1 2 3 4 5 6 X enzyme enzyme AP Biology allosteric inhibitor of enzyme 1
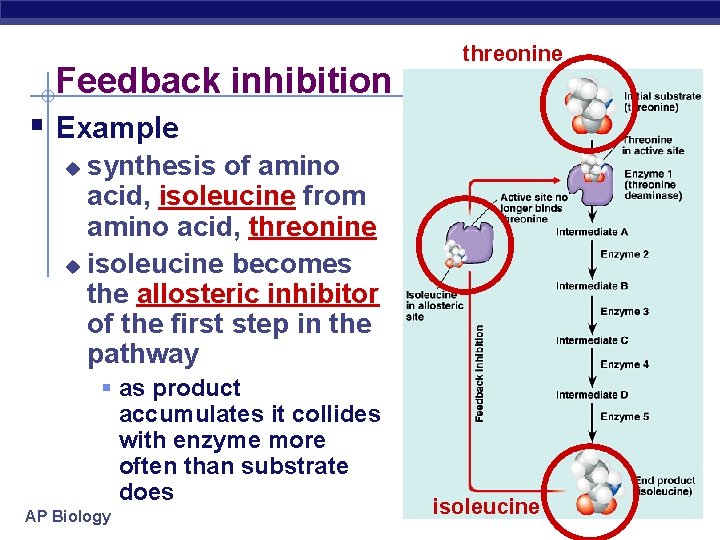
Feedback inhibition threonine § Example synthesis of amino acid, isoleucine from amino acid, threonine u isoleucine becomes the allosteric inhibitor of the first step in the pathway u § as product accumulates it collides with enzyme more often than substrate does AP Biology isoleucine
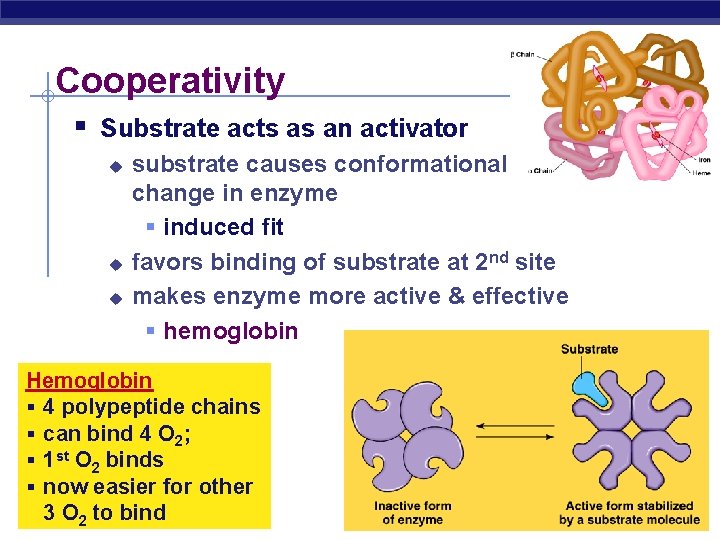
Cooperativity § Substrate acts as an activator u u u substrate causes conformational change in enzyme § induced fit favors binding of substrate at 2 nd site makes enzyme more active & effective § hemoglobin Hemoglobin § 4 polypeptide chains § can bind 4 O 2; § 1 st O 2 binds § now easier for other O 2 to bind AP 3 Biology
Don’t be inhibited! Ask Questions! AP Biology 2007 -2008
- Slides: 50